
Case Report
Ann Hematol Oncol. 2025; 12(1): 1472.
Ruxolitinib Efficacy in a Complex Case of TAFRO-Associated Idiopathic Multicentric Castleman Disease
Tosoni L¹*, Lazzarotto D¹, Filì C¹, Simeone E¹, Zannier ME¹, Battaglia G¹, Callegari C¹, Fanin M¹, Morelli GL¹, Bergnach M¹, Damiani D1,2, Fanin R1,2 and Tiribelli M1,2
1Division of Hematology and Bone Marrow Transplant, Azienda Sanitaria Universitaria Friuli Centrale, Udine, Italy
2Department of Medicine, University of Udine, Italy
*Corresponding author: Luca Tosoni, MD, Division of Hematology and Bone Marrow Transplant, Azienda Sanitaria Universitaria Friuli Centrale, Udine, Italy P.le S.M. Misericordia 15 – 33100 – Udine, Italy Tel: +39 (0)432 559662; Email: tosoni.luca.1@spes.uniud.it
Received: January 28, 2025; Accepted: February 13, 2025; Published: February 17, 2025
Abstract
Severe TAFRO-associated idiopathic multicentric Castleman Disease (iMCD-TAFRO) is burdened by adverse prognosis, as systemic inflammation might lead to multi-organ failure and death. Relapsed/refractory cases management remains challenging, as few therapeutic options exist for siltuximab non-responders. Recent findings on JAK-STAT involvement in iMCD-TAFRO pathogenesis promoted JAK inhibitors as a possible new chemo-free salvage option, with very few cases described in literature. We report on a 47-year-old male patient with severe relapsed/refractory iMCD-TAFRO successfully treated with ruxolitinib as salvage therapy in a unique combination setting alongside tocilizumab. Ruxolitinib was effective and excellently well tolerated. More, our case provides insights into new targeted therapy combination strategies in iMCD-TAFRO, suggesting JAK-STAT3 and IL-6 simultaneous blockade might be beneficial. Future studies are needed to determine the role of JAK inhibitors in the treatment of iMCD-TAFRO.
Keywords: Castleman disease; iMCD-TAFRO; Ruxolitinib; JAK inhibitor; Case report.
Introduction
Castleman Disease (CD) delineates a rare group of lymphoproliferative disorders that share specific histopathological features, with a wide range of etiologies and clinical presentations [1]. Thrombocytopenia, ascites, reticulin fibrosis, renal dysfunction, organomegaly (TAFRO) syndrome-associated idiopathic multicentric CD (iMCD-TAFRO) describes an aggressive subtype whose clinical course is affected by severe life-threatening cytokine storm, organ failure, and death [2]. Interleukin-6 (IL-6) pathway dysregulation is known to play a crucial role in disease pathogenesis, so that consensus guidelines recommend its blockade using target agent siltuximab as first-line therapy alongside high-dose corticosteroids [3]. However, less than half iMCD and iMCD-TAFRO cases respond to initial treatment, and disease recurrence within 1 to 2 years is quite common [3]. Salvage therapies include immunosuppressive and immunomodulatory agents, while chemotherapy is reserved to specific cases [4]. Despite disease pathogenesis is not yet fully elucidated, recent research identified JAK-STAT3 signaling pathway as strictly connected with mTOR signaling and crucial in iMCD [5]. Based on this rationale, JAK inhibitor ruxolitinib emerged as a new possible therapeutic agent for iMCD-TAFRO, with very few reports described in literature [6-8]. Here we present a case of severe relapsed/ refractory iMCD-TAFRO successfully treated with ruxolitinib in a unique combination setting.
Case Presentation
A 47-year-old male was admitted to the emergency room on November 29th, 2023, due to fever, abdominal pain and severe weight gain (18 kg from baseline of 70 kg) over the previous two weeks. Complete blood count (CBC) revealed severe thrombocytopenia (11 x109/l) and neutropenia (0,45 x109/l), blood tests showed increased ferritin (3540 μg/l), creatinine (2,0 mg/dl), C-reactive protein (32 mg/l, CRP), and procalcitonin (9,5 μg/l, PCT). CT scan revealed hepatosplenomegaly and diffuse serosal effusion. Bone marrow evaluation showed macrophage activation, hemophagocytic focal aspects, dysmegakaryopoiesis, T cell interstitial mild excess, in absence of fibrosis. Patient’s conditions rapidly worsened with acute respiratory failure requiring non-invasive ventilation (NIV) in intensive care unit (ICU). In suspicion of secondary hemophagocytic lymphohistiocytosis in differential diagnosis with adult-onset Still disease, therapy with steroids (dexamethasone 20 mg QD) plus anakinra (100 mg QD) was started on December 1st, 2023 (D1) obtaining a rapid laboratory (platelet count 37 x109/l, creatinine 1,7 mg/dl, PCT 5,4 μg/l), and clinical (4 kg weight loss) improvement within the first 10 days. On D12, a CT-PET scan showed multiple adenopathies (maximal SUV 3.5), and a lymph node biopsy allowed the diagnosis of CD with both hypervascular and plasmacytic features. Diagnostic workup finally led to iMCD-TAFRO diagnosis [2]. During steroid tapering, fever, weight gain, and renal impairment recurred, needing diuretic therapy and the increase of steroids dose, obtaining first complete response (CR1) on D43, assessed via CDCN response criteria [3]. Anakinra was then stopped on D45. Rapidly, the patient experienced another clinical and laboratory relapse (fever, weight gain, CRP, PCT and creatinine increase), therefore conventional therapy with siltuximab plus dexamethasone was started on D75 in association with a short course of anakinra, obtaining second CR (CR2) on D100. On D105 patient experienced a further, more severe clinical (fever, anasarca) and laboratory (platelet count 58 x109/l; creatinine 3,8 mg/dl, CRP 440 mg/l) relapse, that required ICU admission with NIV and continuous veno-venous hemofiltration support. Patient’s critical clinical conditions and severe renal failure hampered a polychemotherapy salvage approach along with immunosuppressive agents more frequently used in severe relapsed cases. Therefore, a single dose (1000 mg) cyclophosphamide was administered on D115, and a long-lasting chemo-free approach was then preferred aiming to disrupt cytokine storm molecular drivers while sparing organ toxicity. Based on this rationale and the few cases reported in literature, ruxolitinib 10 mg BID was introduced on D120 in combination with tocilizumab 8 mg/kg/week, in addition to ongoing dexamethasone and anakinra, used during the acute phase. At the time of ruxolitinib introduction, hemoglobin was 9,0 g/dl, platelet count was 20 x109/l, and leukocytes were normal. After clinical stabilization, tocilizumab was progressively delayed until 8 mg/kg/ month, ruxolitinib was maintained at 10 mg BID, while steroids were tapered without rebound of the symptoms. Clinical course was complicated by probable fungal pneumonia on D145, treated with isavuconazole, a stroke with multiple convulsive events requiring short course ICU on D160, and calcinosis cutis on D185 treated with bisphosphonates. Hemodialysis was stopped on D176, and third CR (CR3) was finally assessed on D194. Ruxolitinib, tocilizumab and low dose prednisone were continued due to clinical benefit and excellent tolerance. Main clinical and laboratory data and treatments from D1 to CR3 are summarized in Figure 1. A CT-PET performed on D280 resulted negative, and after six months of ruxolitinib therapy (D300), ideal body weight (70 kg) was restored, CBC, creatinine, PCT and CRP were normal. At the latest contact (D360), complete response was confirmed.
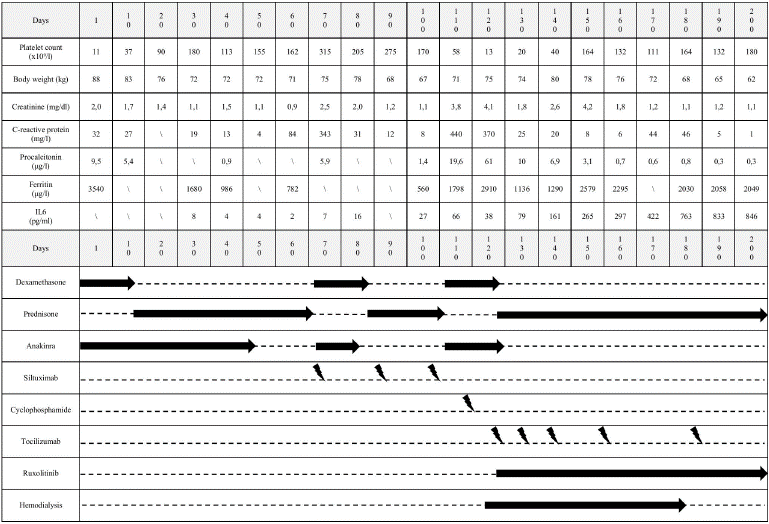
Figure 1: Timeline table displaying clinical and laboratory assessment (upper part) and therapeutic management (lower part) during from D1 to CR3.
Discussion
Because of its rarity, iMCD-TAFRO diagnosis is complex, and treatment often requires highly personalized approaches. Salvage therapy is best individualized and mainly based on expert opinions, retrospectively analysis of registry data and case reports, even though concerning more than half iMCD and iMCD-TAFRO that underwent corticosteroids and IL-6 blocker first line therapy [3,9]. International evidence-based consensus treatment guidelines suggest lymphoma- or myeloma-like chemotherapy use in severe disease salvage [4]. Despite having the highest overall response rate, cytotoxic chemotherapy use requires meticulous risk/benefit assessment due to its considerable organ toxicity, and consistent data on salvage setting are limited [3]. Further chemo-free approaches include T-cells suppressors as calcineurin and mTOR inhibitors, thalidomide, bortezomib, and biological response modifiers as IL1R blocker anakinra [3,4]. Recent findings on JAK-STAT3 involvement in iMCD-TAFRO pathogenesis promoted JAK inhibitors as a new possible chemo-free salvage option. In support of this, a proteomic study on iMCD found that JAK-STAT3 signaling was significantly enriched among IL-6 blockers responders and non-responders, suggesting a potential use of JAK inhibitors in the latter group [10]. Ruxolitinib has been widely used to treat myelofibrosis over the last decade exhibiting potent anti-inflammatory and immunosuppressive effects in this setting [11]. More recently, ruxolitinib was shown to effectively reduce the functional effect of proinflammatory cytokines (such as IL-6) implied in different models of hyperinflammatory syndrome, empowering its wide range of applicability [12]. Ruxolitinib effectiveness as iMCD-TAFRO salvage therapy can be also accounted for its ability to downregulate T-cell activation, as IL-6 blockade-refractory cases showed increased CD8+ and decreased CD4+/CD8+ T cell levels during disease flares [13].
Besides enlarging the few iMCD-TAFRO cases treated with ruxolitinib reported, our specific case proved effectiveness and excellent tolerability in a unique combination salvage setting alongside tocilizumab, suggesting JAK-STAT3 and IL-6 simultaneous blockade might be beneficial in long lasting disease control. More, limited organ toxicity profile suggests ruxolitinib as a valid alternative in patients not eligible to a polychemotherapy canonical immunosuppressive salvage approach. Alongside, anakinra short course addition to highdose glucocorticoids resulted feasible and exhibited effectiveness in controlling cytokine storm both at disease onset and relapses.
In conclusion, ruxolitinib salvage therapy resulted effective and tolerable in our iMCD-TAFRO case and proved as a valid alternative to polychemotherapy in specific severe relapsed-refractory cases. Future studies are needed to better elucidate the range of applicability of ruxolitinib in the treatment of iMCD-TAFRO.
Declarations
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate: Informed consent was obtained from the patient included in the case report.
Data availability statement: Data available upon reasonable request.
Conflict of interest: Authors have no conflict of interest to declare.
Author contributions: LT, DL and MT conceived the study, analyzed data and wrote the paper; CF, ES, MEZ, GB, CG, MF, GLM and MB collected data; DD and RF critically revised the paper.
References
- Dispenzieri A, Fajgenbaum DC. Overview of castleman disease. Blood. 2020; 135: 1353–1364.
- Nishimura Y, Fajgenbaum DC, Pierson SK, et al. Validated International Definition of the TAFRO clinical subtype of idiopathic multicentric Castleman disease. Am J Hematol. 2021; 96: 1241–1252.
- Van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric castleman disease. Blood. 2018; 132: 2115–2124.
- Fajgenbaum DC. Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease. Blood. 2018; 132: 2323–2330.
- Sumiyoshi R, Koga T, Kawakami A. Biomarkers and Signaling Pathways Implicated in the Pathogenesis of Idiopathic Multicentric Castleman Disease/ Thrombocytopenia, Anasarca, Fever, Reticulin Fibrosis, Renal Insufficiency, and Organomegaly (TAFRO) Syndrome. Biomedicines. 2024; 12: 1141.
- Lust H, Gong S, Remiker A, et al. Idiopathic multicentric Castleman disease with TAFRO clinical subtype responsive to IL-6/JAK inhibition: A pediatric case series. Pediatr Blood Cancer. 2021; 68: e29261.
- Killian M, Viel S, Chalayer E, et al. JAK1/2 Inhibition in Severe TAFRO Syndrome: A Case Report. Ann Intern Med. 2021; 174: 719–721.
- Kakutani T, Nunokawa T, Chinen N, et al. Treatment-resistant idiopathic multicentric Castleman disease with thrombocytopenia, anasarca, fever, reticulin fibrosis, renal dysfunction, and organomegaly managed with Janus kinase inhibitors: A case report. Medicine (Baltimore). 2022; 101: e32200.
- Pierson SK, Lim MS, Srkalovic G, et al. Treatment consistent with idiopathic multicentric Castleman disease guidelines is associated with improved outcomes. Blood Adv. 2023; 7: 6652–6664.
- Pierson, SK, Shenoy S, Oromendia AB, et al. Discovery and validation of a novel subgroup and therapeutic target in idiopathic multicentric Castleman disease. Blood Adv. 2021; 5: 3445–3456.
- Elli EM, Baratè C, Mendicino F, et al. Mechanisms Underlying the Antiinflammatory and Immunosuppressive Activity of Ruxolitinib. Front Oncol. 2019; 9: 1186.
- Huarte E, Peel MT, Verbist K, et al. Ruxolitinib, a JAK1/2 Inhibitor, Ameliorates Cytokine Storm in Experimental Models of Hyperinflammation Syndrome. Front Pharmacol. 2021; 12: 650295.
- Fajgenbaum DC, Langan RA, Japp AS, et al. Identifying and targeting pathogenic PI3K/AKT/ mTOR signaling in IL-6 blockade-refractory idiopathic multicentric Castleman disease. J Clin Invest. 2019; 129: 4451–4463.