
Research Article
Ann Hematol Onco. 2024; 11(6): 1470.
Prevalence of Myeloproliferative Neoplasms (MPNs) and its Molecular Biomarkers in Saudi Population in Al-Madinah Region
Sana S Alqarni1*, Raghad Y Alghazy 1,2,3,4, Haifa M Al Nafea1, Khalid K Alharbi1, Sara F Alsobaie1, Maher M Aljohani 2,3,4,5, and Abdulaziz M Aljohani2,3,4
1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
2Department of Pathology and Laboratory Medicine, Ministry of the National Guard –Health Affairs, Medina, Saudi Arabia
3King Abdullah International Medical Research Center (KAIMRC), Jeddah, Saudi Arabia
4King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
5Department of Pathology, College of Medicine, Taibah University, Medina, Saudi Arabia
*Corresponding author: Sana S Alqarni, Department of Clinical laboratory science, College of Applied Medical Sciences, King Saud University, Saudi Arabia, Riyadh 11451P.BOX 145111 ZIP 4545, Saudi Arabia. Email: saalqarni@ksu.edu.sa
Received: November 20, 2024; Accepted: December 11, 2024; Published: December 18, 2024
Abstract
Myeloproliferative Neoplasms (MPNs) are hematological disorders characterized by increased production of myeloid lineage blood cells. MPNs are categorized as Philadelphia (Ph) chromosome-positive, including Chronic Myeloid Leukemia (CML), Ph chromosome-negative, Polycythemia Vera (PV), Essential Thrombocythemia (ET), and Primary Myelofibrosis (PMF). Limited data exist on the frequency of MPNs and their molecular markers in the Saudi population. This study aimed to identify the common MPN subtypes and their associated molecular markers in Saudi citizens residing in the Al-Madinah Region.
We retrospectively analyzed the clinical data of 60 patients between 2014 and 2023. Bone marrow samples were analyzed for mutations in the BCR-ABL, JAK2, CALR, and MPL genes using karyotyping, specific FISH panels, and various mutation detection methods, including Sanger sequencing.
Our findings revealed that MPNs were more prevalent (78%) than Acute Myeloid Leukemia (AML; 11.6%) and Acute Lymphoblastic Leukemia (ALL; 10%) in the study population. Among MPNs, CML was the most common (34%), followed by equal rates of PV and ET (27.6% each), with PMF showing the lowest incidence (10.6%). Molecular biomarker analysis demonstrated BCR-ABL-positive mutations in all CML cases, JAK2-positive mutations in all PV cases, and the most frequent mutation in PMF cases. ET and PMF cases exhibited various mutation patterns, with triple-negative status for JAK2, CALR, and MPL being the most frequent molecular alterations in ET.
This study represents the first estimation of Ph chromosome-negative MPN incidence and identification of common molecular biomarkers used for diagnosis in Saudi Arabia. Further studies with larger sample sizes and broader regional coverage are required to confirm these findings and to provide a more comprehensive understanding of MPNs in the Saudi population.
Keywords: Myeloproliferative neoplasms; Saudi Arabia; Al-Madinah Region; Molecular markers; BCR-ABL; JAK2; CALR; MPL
Introduction
Myeloproliferative Neoplasms (MPNs) are a group of disorders that affect the bone marrow through a significant increase in the myeloid blood cell line [1]. These disorders result from abnormal proliferation of one or more terminal myeloid cell lines in Peripheral Blood (PB). Laboratory diagnosis of MPNs can be classified into two groups: detection through BCR-ABL rearrangement (Philadelphia chromosome-positive, suspected of Chronic Myeloid Leukemia or CML) and the identification of driver genes in Philadelphia chromosome-negative forms (MPN Ph neg, suspected of polycythemia vera or PV, essential thrombocythemia or ET, and Primary Myelofibrosis or PMF) [2]. A high incidence rate of Philadelphia chromosome-positive MPNs is observed in CML, accounting for approximately 30% of leukemia cases in adults [3], affecting 1–2 individuals per 100,000 individuals with a median age of 52 to 64 years [4-6]. Philadelphia chromosome-negative MPNs are, however, very rare, with annual incidence rates of 2.7 and 3.1 cases per 100,000 people reported in Europe and the United States [7,8]. Literature shows that men aged =50 years have a higher risk of developing MPNs [9]. CML is characterized by the presence of the Philadelphia chromosome and a balanced translocation between chromosomes 9 and 22 [t(9;22)]. This translocation results in the fusion of the Breakpoint Cluster Region (BCR) gene on chromosome 22q11.2 with the Abelson gene (ABL1) from chromosome 9q34, creating the BCR-ABL1 fusion oncogene [11]. The resulting BCR-ABL1 oncoprotein acts as a constitutively active tyrosine kinase that induces leukemogenesis through cytokineindependent cell cycle activation and abnormal apoptotic signals [11]. CML is more common in males than in females [12]. Polycythemia Vera (PV) accounts for approximately 45% of all MPN cases [13]. It is characterized by marrow hypercellularity, megakaryocyte hyperplasia, and hypertrophy. The primary molecular aberration in PV is the JAK2 mutation present in 95% of cases [10]. JAK2, located on chromosome 9p24, typically exhibits a gain-of-function mutation in exon 14, involving the substitution of phenylalanine for valine at position 617 [14]. Additional mutations in exon 12 of JAK2 have been described [15]. These mutations result in constitutive activation of JAK2, leading to cytokine hypersensitivity and erythrocytosis [15]. The PV incidence is reported to be higher in males than in females [16].
Essential Thrombocythemia (ET) accounts for approximately 25% of all MPN cases [17]. It is characterized by megakaryocyte hyperplasia in the BM without evidence of fibrosis or persistent thrombocytosis in the PB [10]. Common mutations in ET include JAK2, CALR, and MPL, with JAK2 being the most frequent mutation [18]. MPL, located on chromosome 1p34, encodes the Thrombopoietin (TPO) receptor. Mutations in this gene lead to ligand-independent intracellular signaling activation [18]. CALR, located on chromosome 19p13.2, functions as an endoplasmic reticulum chaperone. CALR mutations, primarily insertions and/or deletions in exon 9, result in a frameshift that alters amino acid configuration [19]. Recent studies have suggested a higher incidence of ET in females than in males [18,20].
Primary Myelofibrosis (PMF) is characterized by Bone Marrow (BM) fibrosis and atypical megakaryocytic hyperplasia. JAK2 and MPL mutations are involved in 50% and 11% of the cases, respectively [21]. PMF typically presents with leukoerythroblastosis, leukopenia, and thrombocytosis or thrombocytopenia in PB [10]. The myeloproliferative phenotype in PMF results from mutations in JAK2, CALR, or MPL, with additional mutations affecting DNA methylation, chromatin modification, RNA splicing, and DNA repair in some cases [19]. PMF has been reported to be more predominant in males than in females [9].
Cancer epidemiology in Saudi Arabia shows significant regional variations that are potentially attributable to differences in etiological factors [22,23]. However, knowledge regarding the prevalence of MPNs and their molecular biomarkers in the Saudi population is lacking. Therefore, this study aimed to assess the prevalence and molecular biomarkers of MPN subtypes in the Saudi population in the Al-Madinah Region. Given the limited sample size, this study is exploratory in nature and serves as a pilot study, providing preliminary findings to form a foundation for future large-scale investigations.
Methodology
We conducted a retrospective analysis of the clinical data of patients with MPN, ALL, and AML. The data of 60 patients were collected, representing all AML, ALL, and MPN diagnosed cases at Prince Mohammed bin Abdulaziz Hospital from 2014 to 2023 using karyotyping, specific FISH panels, and variety-specific mutation detection methods such as Sanger sequencing. Data were analyzed using Excel software. Normally distributed quantitative data were expressed as percentages, means, standard deviations, and ranges. Given its small sample size, this study was categorized as a pilot study aimed at exploring preliminary trends and methodologies for future research. The project was approved by the local ethics research committee of King Abdullah International Medical Research Center (KAIMRC). IRB Approval No: IRB/1503/23.
Data Collection
The data used in this study were collected from patients registered at Prince Mohammed bin Abdulaziz Hospital. The Data were composed of records of patients admitted for MPNs, AML, and ALL based on the World Health Organization guidelines for diagnosis. All patients were screened by karyotyping and molecular genetic analysis, including FISH panels and Sanger sequencing.
Result
Myeloproliferative Neoplasms (MPNs)
This pilot study investigated the prevalence of MPNs and active molecular biomarkers of Myeloproliferative Neoplasms (MPNs) in the Saudi population of the Al-Madinah region. A total of 60 Bone Marrow (BM) samples were obtained and analyzed, including 47 cases (78.3%) diagnosed with MPNs, with an average patient age of 50 years (median 50.5 years). Among these, 21 patients were adult males, and 26 were adult females, showing no significant difference in MPN incidence between the sexes. In addition to MPN cases, seven cases (11.6%) were diagnosed with Acute Myeloid Leukemia (AML), predominantly in males (six males and one female), with an average age of 38 years (median, 44 years). Six cases (10%) were classified as precursor B-Acute Lymphoblastic Leukemia (ALL), affecting both the adult and pediatric populations. This included two pediatric females, two adult females, and two adult males, with an average age of 23.3 years (median, 25 years). These findings suggest a higher incidence of ALL in females compared to males (Table 1).
Diagnosis
Cases (n)
Average Age (Years)
Median Age (Years)
Male (%)
Female (%)
Mean Survival (Months)
Median Survival (Months)
MPN (Total)
47
50
50.5
44.7
55.3
N/A
N/A
AML
7
38
44
85.7
14.3
24
21
ALL
6
23.3
25
50
50
47.143
42
CML
16
46.8
46.5
50
50
82.286
84
PV
13
57.6
61
61.5
38.5
63
60
ET
13
41.4
40
30.8
69.2
112.5
108
PMF
5
61
68
20
80
37.5
36
Table 1: Summary of the myeloproliferative neoplasm (MPN) cases and related hematologic disorders, including Acute Myeloid Leukemia (AML), Acute Lymphoblastic Leukemia (ALL), Chronic Myeloid Leukemia (CML), Polycythemia Vera (PV), Essential Thrombocythemia (ET), and Primary Myelofibrosis (PMF).
CML is the most prevalent cause of MPNs in the Saudi population in Al- the Madinah region
CML was the most common MPN diagnosis, affecting 16 cases (34%), with an average age of 46.8 years (median 46.5 years). PV and ET were each diagnosed in 13 patients (27.6%), with average ages of 57.6 and 41.4 years, respectively. PMF accounted for five cases (10.6%), with an average age of 61 years (median, 68 years). Molecular analysis of MPN cases revealed distinct mutation patterns. All CML cases were BCR-ABL-positive, whereas all PV cases were JAK2-positive and BCR-ABL-negative. In ET cases, several mutation patterns were observed, including triple-negative mutations in JAK2, MPL, and CALR, as well as cases with JAK2-positive mutations. PMF cases exhibit a range of mutations, with JAK2 mutations being the most frequent. Overall, triple-negative cases for JAK2, CALR, and MPL were most common in ET, whereas JAK2 mutations were prevalent in PMF (Table 1).
Survival Analysis of MPN Subtypes
Survival time analysis revealed significant differences in survival across the various MPN subtypes. Chronic Myeloid Leukemia (CML) had the highest mean survival of 82.3 months (median, 84 months), whereas ET had a mean survival of 112.5 months (median, 108 months). AML patients exhibited the lowest mean survival of 24 months (median, 21 months).
Primary Myelofibrosis (PMF) and Polycythemia Vera (PV) had mean survival times of 37.5 and 63 months, respectively (Figure 1). Overall, the mean survival across all diagnoses was 65.7 months (median, 60 months), with statistical tests confirming significant differences in survival distributions across the different types of MPNs (p < .001).
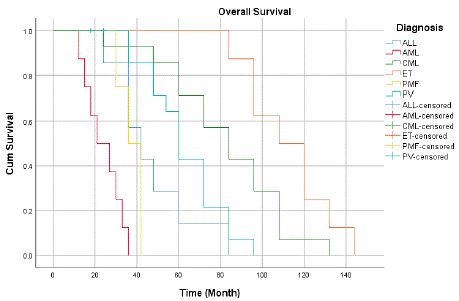
Figure 1: Overall Survival of the MPN patients.
Common Symptoms and Treatment Approaches
Common symptoms among the patients included fatigue (85%), pruritus (40%), and emotional distress (60%) (Figure 2). Treatment approaches varied, with all patients receiving Tyrosine Kinase Inhibitors (TKIs), 80% undergoing phlebotomy, 70% receiving cytoreductive therapy, and 50% treated with JAK2 inhibitors (Figure 3).
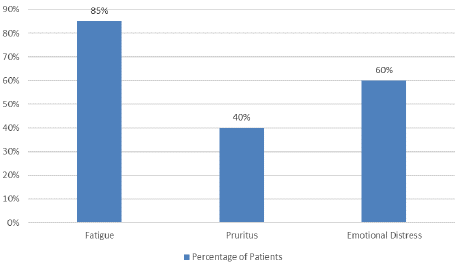
Figure 2: Common Symptoms in MPN patients.
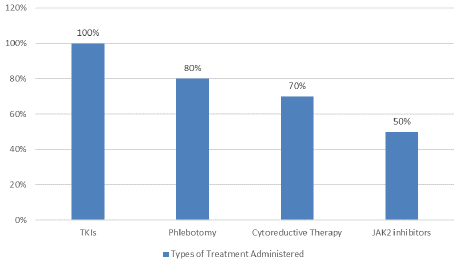
Figure 3: Types of Treatment Administered on MPN Patients.
Molecular Analysis for MPN Cases
The MPN cases were found to have different genetic mutations at different frequencies (Table 3). All 16 CML cases were analyzed to determine the presence of mutations in the BCR-ABL gene. All CML cases showed BCR-ABL-positive mutations in both female and male patients (Table 2).
MPN classification
Frequency
Gender
Type of mutation
Male
Female
CML
16
8
8
- BCR-ABL1
PV
13
8
5
- JAK2 positive / BCR-ABL1 negative
ET
13
4
9
- One female case, JAK2 negative. MPL and CALR results are not available. BCR-ABL1 negative mutation
- six cases (4 female and two male) of Triple-negative
- three female cases, JAK2 positive / CALR and MPL negative. BCR-ABL1 negative mutation
- two cases (1 male and 1 female), CALR positive/ JAK2 and MPL negative, BCR-ABL1 negative mutation
- one male case, JAK2 and CALR positive, BCR-ABL1 negative mutation
PMF
5
1
4
- One female case, MPL positive and CALR negative, BCR-ABL1 negative mutation.
- One female case, Triple negative
- Two female cases, JAK2 positive and CALR negative, BCR-ABL1 negative mutation
- One male case, ASXL1, JAK2, TET2 and U2AF1, BCR-ABL1 negative mutation
Table 2: Mutations’ frequency of MPN cases.
All 13 PV cases were analyzed to test for JAK2 and BCR-ABL mutations. All PV cases showed JAK2 positive mutation and BCRABL- negative mutations, as described in (Table 2).
All 13 ET cases were analyzed to test for the presence of mutations in BCR-ABL, JAK2, CALR, or MPL. Several mutation patterns were identified in all cases, as shown in (Table 3). Molecular analysis of the female patients revealed one female with JAK2 negative mutation; however, there were no available results for CALR and MPL genes in the patient's medical record.
All four patients were triple-negative for JAK2, MPL, and CALR mutations. In addition, three cases had JAK2 positive mutation and MPL- and CALR-negative mutations. On the other hand, male patients were analyzed, and one patient had a CALR-positive mutation, whereas JAK2 and MPL mutations were negative. Two cases were negative for JAK2, MPL, and CALR mutations (triple negative). One case appeared to have positive mutations in the JAK2 and CALR genes. These data suggest that the triple negativity of JAK2, CALR, or MPL is the most common molecular alteration in ET. The frequencies of these mutations are shown in (Table 2).
All five PMF cases were analyzed to determine the presence of mutations in BCR-ABL, JAK2, CALR, and MPL. Several mutation patterns were identified in all cases, as shown in (Table 3). One female patient had a MPL-positive mutation and a negative CALR mutation. However, the patient's condition progressed to AML. One patient harbored a triple-negative mutation. Two patients tested positive for JAK2 and CALR mutations. However, one male patient had mutations in ASXL1, JAK2, TET2, and U2 small nuclear RNA auxiliary factor 1 (U2AF1) genes. Moreover, both male and female patients had BCRABL- negative mutations (Table 2). These data suggest that JAK2 mutations may be the most common finding in PMF cases.
Discussion
The analysis of cancer epidemiology in Saudi Arabia reveals notable regional variations potentially related to differences in etiological factors [22,23]. Recent studies have shown an increase in leukemia incidence rates, particularly in the central, eastern, and northern regions [24]. However, knowledge regarding the prevalence and molecular biomarkers of Myeloproliferative Neoplasms (MPNs) in Saudi Arabia is lacking. This study aimed to identify the prevalence of MPNs in the Saudi population in the Western Region (Al- Madinah) and identify the molecular biomarkers available for clinical approaches to these neoplasms.
Analysis of survival times across different diagnoses revealed significant variations in both the mean and median survival estimates. For Acute Lymphoblastic Leukemia (ALL), the mean survival time is estimated at 47.143 months, with a median of 42.000 months, indicating that patients typically survive for just over three and a half years. Acute Myeloid Leukemia (AML) patients, on the other hand, had a median survival of 21.000 months and a mean survival of 24.000 months, indicating a shorter survival period for this diagnosis. Compared to ALL and AML, Chronic Myeloid Leukemia (CML) exhibits a significantly higher mean survival of 82.286 months and a median of 84.000 months, indicating superior outcomes. With a median survival of 108.000 months and the longest mean survival of 112.500 months, patients with Essential Thrombocythemia (ET) have an outstanding prognosis. Polycythemia Vera (PV) has a mean survival of 63.000 months and a median of 60.000 months, both of which indicate moderate survival results in comparison to other diagnoses. Primary Myelofibrosis (PMF) has a mean survival of 37.500 months and a median of 36.000 months.
The results demonstrated that among the disorders under investigation, ET had the greatest prognosis, while AML had the lowest odds of survival, with CML and PV in the middle. This cohort's general trends in patient outcomes are highlighted by the reported overall mean survival of 65.716 months across all diagnoses, with a median of 60.000 months. Statistical analyses utilizing the Breslow (Generalized Wilcoxon), Tarone-Ware, and Log Rank (Mantel-Cox) tests were performed to assess the equality of survival distributions across various diagnoses to further corroborate these findings. Chisquare values of 82.698 (p <.001), 72.627 (p <.001), and 77.668 (p <.001) indicated significant differences in the results. These results support the observed patterns in the mean and median survival estimates for each diagnosis by confirming that there are statistically significant disparities in survival times among the various hematologic diseases evaluated. For patients to receive complete patient care, it is essential to understand the Quality of Life (QoL) of patients with MPNs. Research has shown that symptoms including tiredness, pruritus, and mental distress might lead to a worse Quality of Life (QoL) in MPN patients [32]. Improved QoL outcomes can result from effective management of MPNs, which includes therapies such as TKIs for CML and phlebotomy with low-dose aspirin for PV [33]. However, insufficient adherence to medication might have a detrimental effect on the quality of life and survival of patients with MPNs. [34].
This study found that MPNs were more prevalent (78%) than AML (11.6%) or Acute Lymphoblastic Leukemia (ALL) (10%) in the Saudi population living in the Al-Madinah region. This is consistent with a recent study in the southern region of Saudi Arabia [35] but inconsistent with studies conducted in the northern [36] and central [37] regions. The high prevalence of CML in the Al-Madinah and Aseer regions compared to the Northern and Central regions could be linked to local health system factors and possible etiological factors such as genetic or environmental causes [39]. CML (34%) was the most prevalent MPN in the Saudi population in the Al-Madinah region, followed by PV (27.6%) and ET (27.6%), with PMF (10.6%) showing the lowest incidence rates. This distribution differs from studies conducted in other countries [41-44], possibly because of genetic variation between populations and the small sample size in this study. Regarding sex distribution, our findings indicate no statistically significant difference in the incidence rate between males and females, which is consistent with some previous studies [46-48] but differs from others [49]. These differences may be due to the low prevalence of the disease, necessitating larger sample sizes for an accurate incidence rate determination.
Molecular analysis revealed that BCR-ABL1 mutation was the most common mutation encountered during diagnosis, followed by JAK2 mutation. This is consistent with previous studies [51-53]. Our study suggests that the lack of all three driver mutations (JAK2, MPL, and CALR), known as triple-negative MPN, is the most frequent molecular alteration in ET. This finding differs slightly from previous literature [19,54] and highlights the need for further investigation using advanced techniques, such as whole exome sequencing [55-57].
The main limitation of this study is the small sample size, which constrains the generalizability of the results. Although this pilot study provides useful early insights into the prevalence and molecular indicators of MPNs in the Al-Madinah area, larger studies are necessary to corroborate these preliminary patterns. Future studies should include a broader and more heterogeneous population to validate the identified trends and further explore the molecular landscape of MPNs in Saudi Arabia.
Conclusion
In conclusion, this study provides valuable initial data on the prevalence and molecular characteristics of MPNs in the Al-Madinah region of Saudi Arabia. These findings may aid in the diagnosis, prognosis, and treatment of patients. However, further investigations with larger sample sizes and involving other regions in Saudi Arabia are needed to confirm and expand upon these results. Multicenter collaboration could significantly contribute to the accurate determination of the prevalence of MPNs in different regions of Saudi Arabia and add to the body of knowledge in the field.
Author Statements
Author Contributions
Conceptualization: S.A.; validation: H.A.; investigation: S. A., R., and M. A Data analysis, R. A. Writing an original draft, S.A. and R.A.; Review and editing, A.A, SA, and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the King Abdullah International Medical Research Center (KAIMRC); (project no. JED-23-427780- 83614). Jeddah, Saudi Arabia.
Institutional Review Board Statement
The project was approved by the local ethics research committee of King Abdullah International Medical Research Center (KAIMRC). IRB Approval No: IRB/1503/23.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data related to this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors extend their appreciation to the King Saud University, Riyadh, Saudi Arabia.
References
- Döhner H. Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood. 2022; 140: 1345–1377.
- Moncada A, Pancrazzi A. Lab Tests for MPN. Int Rev Cell Mol Biol. 2022; 366: 187–220.
- Miranda-Filho A, Piñeros M, Ferlay J, Soerjomataram I, Monnereau A, Bray F. Epidemiological Patterns of Leukemia in 184 Countries: A Population-Based Study. Rev Epidemiol Sante Publique. 2018; 66: S285.
- Wan Z, Chen X, Gao X, Dong Y, Zhao Y, Wei M, et al. Chronic Myeloid Leukemia-derived Exosomes Attenuate Adipogenesis of Adipose Derived Mesenchymal Stem Cells via Transporting MiR-92a-3p. J Cell Physiol. 2019; 234: 21274–21283.
- Meenakshi Sundaram DN, Jiang X, Brandwein JM, Valencia-Serna J, Remant KC, Uludag H. Current Outlook on Drug Resistance in Chronic Myeloid Leukemia (CML) and Potential Therapeutic Options. Drug Discov Today. 2019; 24: 1355–1369.
- Belohlavkova P, Steinerova K, Karas M, Skoumalova I, Rohon P, Indrak K, et al. First-Line Imatinib in Elderly Patients with Chronic Myeloid Leukaemia from the CAMELIA Registry: Age and Dose Still Matter. Leuk Res. 2019; 81: 67–74.
- Visser O, Trama A, Maynadié M, Stiller C, Marcos-Gragera R, De Angelis R, et al. Incidence, Survival and Prevalence of Myeloid Malignancies in Europe. Eur J Cancer. 2012; 48: 3257–3266.
- Noone AM, HNKM, et al. SEER Cancer Statistics Review. National Cancer Institute.
- Karantanos T, Chaturvedi S, Braunstein EM, Spivak J, Resar L, Karanika S, et al. Sex Determines the Presentation and Outcomes in MPN and Is Related to Sex-Specific Differences in the Mutational Burden. Blood Adv. 2020; 4: 2567.
- Fowlkes S, Murray C, Fulford A, de Gelder T, Siddiq N. Myeloproliferative Neoplasms (MPNs) – Part 1: An Overview of the Diagnosis and Treatment of the “Classical” MPNs. Canadian Oncology Nursing Journal / Revue canadienne de soins infirmiers en oncologie. 2018; 28: 262–268.
- Jabbour E, Kantarjian H. Chronic Myeloid Leukemia: 2018 Update on Diagnosis, Therapy and Monitoring. Am J Hematol. 2018; 93: 442–459.
- Lin Q, Mao L, Shao L, Zhu L, Han Q, Zhu H, et al. Global, Regional, and National Burden of Chronic Myeloid Leukemia, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Front Oncol. 2020; 10: 580759.
- Waggoner MS, PA-C, M. Polycythemia Vera: Thinking Beyond the Hematocrit. J Adv Pract Oncol. 2023; 14: 405–413.
- James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, et al. A Unique Clonal JAK2 Mutation Leading to Constitutive Signalling Causes Polycythaemia Vera. Nature. 2005; 434: 1144–1148.
- Pillai AA, Fazal S, Mukkamalla SKR, Babiker HM. Polycythemia. Treasure Island (FL): StatPearls Publishing. 2023.
- Palandri F, Mora B, Gangat N, Catani L. Is There a Gender Effect in Polycythemia Vera? Ann Hematol. 2021; 100: 11-25.
- Accurso V, Santoro M, Mancuso S, Napolitano M, Carlisi M, Mattana M, et al. The Essential Thrombocythemia in 2020: What We Know and Where We Still Have to Dig Deep. Clinical Medicine Insights: Blood Disorders. SAGE Publications Ltd. 2020.
- Accurso V, Santoro M, Mancuso S, Napolitano M, Carlisi M, Mattana M, et al. The Essential Thrombocythemia in 2020: What We Know and Where We Still Have to Dig Deep. Clin Med Insights Blood Disord. 2020: 13.
- Rumi E, Trotti C, Vanni D, Casetti IC, Pietra D, Sant’antonio E. The Genetic Basis of Primary Myelofibrosis and Its Clinical Relevance. Int J Mol Sci. 2020; 21: 1–14.
- Barraco D, Mora B, Guglielmelli P, Rumi E, Maffioli M, Rambaldi A, et al. Gender Effect on Phenotype and Genotype in Patients with Post-Polycythemia Vera and Post-Essential Thrombocythemia Myelofibrosis: Results from the MYSEC Project. Blood Cancer J. 2018; 8: 89.
- Malara A, Abbonante V, Zingariello M, Migliaccio A, Balduini A. Megakaryocyte Contribution to Bone Marrow Fibrosis: Many Arrows in the Quiver. Mediterr J Hematol Infect Dis. 2018; 10: e2018068.
- Jastaniah W, Alsultan A, Al Daama S, Ballourah W, Bayoumy M, Al-Anzi F, et al. Treatment Results in Children with Myeloid Leukemia of Down Syndrome in Saudi Arabia: A Multicenter SAPHOS Leukemia Group Study. Leuk Res. 2017; 58: 48–54.
- Bazarbashi S, Al Eid H, Minguet J. Cancer Incidence in Saudi Arabia: 2012 Data from the Saudi Cancer Registry. Asian Pac J Cancer Prev. 2017; 18: 2437–2444.
- Alghamdi IG, Hussain II, Alghamdi MS, Dohal AA, El-Sheemy MA. The Incidence of Leukemia in Saudi Arabia. Descriptive Epidemiological Analysis of Data from the Saudi Cancer Registry 2001-2008. Saudi Med J. 2014; 35: 674–683.
- Bower, H., Björkholm, M., Dickman, P.W., Höglund, M., Lambert, P.C. and Andersson, T.M.L., 2016. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. Journal of Clinical Oncology. 2016; 34: 2851-2857.
- Breccia M. Imatinib improved the overall survival of chronic myeloid leukemia patients in low-and middle-income countries: A therapeutic goal has been reached. E Clinical Medicine. 2020; 19: 100277.
- Kröger N, Bacigalupo A, Barbui T, Ditschkowski M, Gagelmann N, Griesshammer M, et al. Indication and management of allogeneic haematopoietic stem-cell transplantation in myelofibrosis: updated recommendations by the EBMT/ELN International Working Group. The Lancet Haematology. 2024; 11: e62-e74.
- Tefferi A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. American journal of hematology. 2023; 98: 801-821.
- Büyükasik Y, Ali R, AR MC, Turgut M, YAVUZ AS, Saydam G. Polycythemia vera: diagnosis, clinical course, and current management. Turkish journal of medical sciences. 2018; 48: 698-710.
- Iurlo A, Cattaneo D. Treatment of myelofibrosis: old and new strategies. Clinical Medicine Insights: Blood Disorders. 2017; 10: 1179545X17695233.
- Pimenta DB, Varela VA, Datoguia TS, Caraciolo VB, Lopes GH, Pereira WO. The bone marrow microenvironment mechanisms in acute myeloid leukemia. Frontiers in Cell and Developmental Biology. 2021; 9: 764698.
- Geyer HL, Andreasson B, Kosiorek HE, Dueck AC, Scherber RM, Martin KA, et al. The role of sexuality symptoms in myeloproliferative neoplasm symptom burden and quality of life: An analysis by the MPN QOL International Study Group. Cancer. 2016; 122: 1888-96.
- Bose P, Alfayez M, Verstovsek S. New concepts of treatment for patients with myelofibrosis. Current treatment options in oncology. 2019; 20: 1-25.
- Davydkin IL, Popelnyuk NS, Naumova KV, Mordvinova EV, Stepanova TY, Krivova SP, et al. QUALITY OF LIFE IN PATIENTS WITH MYELOPROLIFERATIVE NEOPLASMS.2019; 39: 58-65.
- Alshahrani AM, Bakheet OS, Makkawi MH, Alasmari SZ. Hematological Malignancies. Saudi Med J. 2024; 45: 295–306.
- Mohamed Elasbali A, Hussain Alharbi H, Al-Onzi Z, Saiem Al-dahr MH, Hamza A, Khalafalla E, et al. Epidemiology and Patterns of Leukemia in Northern Saudi Arabia. International Journal of Medical Research & Health Sciences. 2019; 8: 160–166.
- Bawazir A, Al-Zamel N, Amen A, Akiel MA, Alhawiti NM, Alshehri A. The Burden of Leukemia in the Kingdom of Saudi Arabia: 15 Years Period (1999– 2013). BMC Cancer. 2019; 19: 703.
- Alenzi FQ, Al-Amri AM, Alanazi FGB, Tamimi W, Alanazi A, Alenezy AK, et al. Alana Cellular and Molecular Responses of Saudi Chronic Myeloid Leukaemia Patients to Imatinib (STI-571): Ten Year Experience. J Ayub Med Coll Abbottabad. 2012; 24: 122–128.
- Hu Y, Li Q, Hou M, Peng J, Yang X, Xu S. Magnitude and Temporal Trend of the Chronic Myeloid Leukemia: On the Basis of the Global Burden of Disease Study 2019. JCO Glob Oncol. 2021; 7: 1429–1441.
- Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating Morphologic, Clinical, and Genomic Data. Blood. 2022; 140: 1200–1228.
- Yassin MA, Taher A, Mathews V, Hou H, Shamsi T, Tuglular TF, et al. MERGE: A Multinational, Multicenter Observational Registry for Myeloproliferative Neoplasms in Asia, Including Middle East, Turkey, and Algeria. Cancer Med. 2020; 9: 4512–4526.
- Alshemmari S, Almazyad M, Alwehaib A, Ameen R. Philadelphia-negative Myeloproliferative Neoplasms among Kuwaiti Nationals. Cancer Med. 2021; 10: 365–371.
- Sukrisman L. Clinical Characteristics and Prognostic Risks of Philadelphia- Negative Myeloproliferative Neoplasms at Cipto Mangunkusumo General Hospital. J Blood Med. 2022; 13: 495–503.
- Lusine S, Anahit TG, Maria B, Anahit S, Alla S, Samvel D. Incidence in Ph- Negative Myeloproliferative Neoplasms in Armenia from 2005 to 2019. Open J Epidemiol. 2020; 10: 355–368.
- Radivoyevitch T, Jankovic GM, Tiu RV, Saunthararajah Y, Jackson RC, Hlatky LR, et al. Sex Differences in the Incidence of Chronic Myeloid Leukemia. Radiat Environ Biophys. 2014; 53: 55–63.
- Karantanos T, Chaturvedi S, Braunstein EM, Spivak J, Resar L, Karanika S, et al. Sex Determines the Presentation and Outcomes in MPN and Is Related to Sex-Specific Differences in the Mutational Burden. Blood Adv. 2020; 4: 2567.
- Geyer HL, Kosiorek H, Dueck AC, Scherber R, Slot S, Zweegman S, et al. Associations between Gender, Disease Features and Symptom Burden in Patients with Myeloproliferative Neoplasms: An Analysis by the MPN QOL International Working Group. Haematologica. 2017; 102: 85.
- Algahtani FH, Alqahtany FS. Evaluation and Characterisation of Chronic Myeloid Leukemia and Various Treatments in Saudi Arabia: A Retrospective Study. J Infect Public Health. 2020; 13: 295–298.
- McMullin MF, Anderson LA. Aetiology of Myeloproliferative Neoplasms. Cancers (Basel). 2020; 12: 1810.
- Stivala S, Meyer SC. Recent Advances in Molecular Diagnostics and Targeted Therapy of Myeloproliferative Neoplasms. Cancers (Basel). 2021; 13: 5035.
- Heisterkamp N, Jenster G, Ten Hoeve J, Zovich D, Pattengale PK, Groffen J. Acute Leukaemia in Bcr/Abl Transgenic Mice. Nature. 1990; 344: 251–253.
- Chia YC, Siti Asmaa MJ, Ramli M, Woon PY, Johan MF, Hassan R, et al. Molecular Genetics of Thrombotic Myeloproliferative Neoplasms: Implications in Precision Oncology. Diagnostics. 2023; 13: 163.
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders. N Engl J Med. 2005; 352: 1779–1790.
- Tefferi A, Barbui T. Polycythemia Vera and Essential Thrombocythemia: 2017 Update on Diagnosis, Risk-Stratification, and Management. Am J Hematol. 2017; 92: 94–108.
- Chang YC, Lin HC, Chiang YH, Chen CGS, Huang L, Wang WT, et al. Targeted Next-Generation Sequencing Identified Novel Mutations in Triple- Negative Myeloproliferative Neoplasms. Medical Oncology. 2017; 34: 83.
- Feenstra JDM, Nivarthi H, Gisslinger H, Leroy E, Rumi E, Chachoua I, et al. Whole-Exome Sequencing Identifies Novel MPL and JAK2 Mutations in Triple-Negative Myeloproliferative Neoplasms. 2016; 127: 325-32.
- Cabagnols X, Favale F, Pasquier F, Messaoudi K, Defour JP, Ianotto JC, et al. Presence of Atypical Thrombopoietin Receptor (MPL) Mutations in Triple- Negative Essential Thrombocythemia Patients. Blood. 2016; 127: 333–342.
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. New England Journal of Medicine. 2013; 369: 2379–2390.
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. N Engl J Med. 2013; 369: 2391–2405.
- Vainchenker W, Kralovics R. Genetic Basis and Molecular Pathophysiology of Classical Myeloproliferative Neoplasms. Blood. 2017; 129: 667–679.