
Case Report
Ann Hematol Onco. 2023; 10(6): 1440.
Myelodysplastic Syndrome with Excess Blasts and Erythroid Predominance (MDS-EB-E) With NPM1 Mutation and P53 Expression
Satarupa Mohapatra, DM; Somanath Padhi, MD*; Prabodha Kumar Das, MD, FRCPA; Gaurav Chhabra, MD
Department of Pathology and Laboratory Medicine; and Medical Oncology/Hematology, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India
*Corresponding author: Somanath Padhi, MD Additional Professor, Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. Email: somanath.padhi@gmail.com
Received: August 01, 2023 Accepted: September 13, 2023 Published: September 20, 2023
Abstract
Nucleophosmin 1 (NPM1) mutated Myelodysplastic Syndrome (MDS) is a distinctly rare sub-group of myeloid neoplasm with limited available data describing its biological behavior and outcome using different therapeutic strategies. We describe a case of NPM1 mutated MDS in a middle-aged male with excess blasts and excess erythroid precursors (MDS-EB-E) morphologically mimicking Acute Erythroleukemia (AEL) with an unfavorable outcome following Hypomethylating Agent (HMA) therapy. We briefly reviewed the clinicopathological and genetic landscape data of NPM1 mutated MDS along with the role of p53 immunohistochemistry in differentiating MDS-EB-E from AEL.
Keywords: Myelodysplastic syndrome; TP53; NPM1; Next generation sequencing
Introduction
Myelodysplastic Syndrome (MDS) is a distinct group of clonal hematopoietic stem cell neoplasm characterised by recurrent cytogenetic abnormalities, peripheral blood cytopenia (s), normo to hypercellular marrow with dyspoietic changes, and an increased risk of transformation to acute myeloid leukemia. The 2017 modification of WHO classification of hematopoietic neoplasms describes MDS with excess blast and erythroid predominance (MDS-EB-E) as a distinct entity representing the so called erythroid-myeloid type Acute Erythroleukemia (AEL) in previous classification [1]. Although the genomic landscape data of such entity is not fully known, but presence of high-risk karyotype, and TP53, RUNX1, ASXL1 mutations are reported to be associated with adverse prognosis [2,3]. We aim to describe a case of MDS-EB-E with NPM1 mutation and highlight the biological behaviour of such rare entity published in the literature.
Case Presentation
A middle-aged male without any prior co-morbidity underwent hematological evaluation at our center for six-month history of exertional dyspnea secondary to transfusion dependent anemia (average of two packed red cell per month) associated with fatigue and weight loss. On examination, he had significant conjunctival pallor, no icterus, cyanosis, edema, lymphadenopathy and/or hepatosplenomegaly. His clinical presentation mimicked aplastic anemia, MDS, or underlying hematological malignancy such as leukemia/lymphoma. His complete blood count and peripheral blood smear examination revealed dimorphic anemia (normocytic normochromic admixed with macrocytic normochromic red cells) (secondary to transfusion) with a Hb; 65g/L (ref.; 120-140 g/L), mean cell volume; 99fL (ref.; 80-98 fL), corrected reticulocyte count; 1% (ref.; 0.5-2%), nucleated RBCs; 5/100 leukocytes]; leukopenia [leukocyte count; 2.7x109/L (ref.; 4-11x109/L) with 2% myeloid blasts, and scattered pseudo-Pelger Heut neutrophils (5% of differential); and thrombocytopenia [platelet count; 33x109/L (ref.; 150-450x109/L] with giant platelets; thus, suggestive of MDS.
Bone marrow aspirate examination revealed florid erythroid hyperplasia (70% of marrow nucleated cells) (myeloid to erythroid ratio=1:3) with dysplastic erythroid morphology in the form of megaloblastic nuclear chromatin, diffuse weak cytoplasmic positivity for Per-iodic Acid Schiff (PAS) stain, and nuclear membrane irregularity in the form of budding (noted in >30% erythroid cells); 15% non-erythroid myeloid blasts; and adequate but dysplastic (micro) megakaryocytes (Figure 1a).
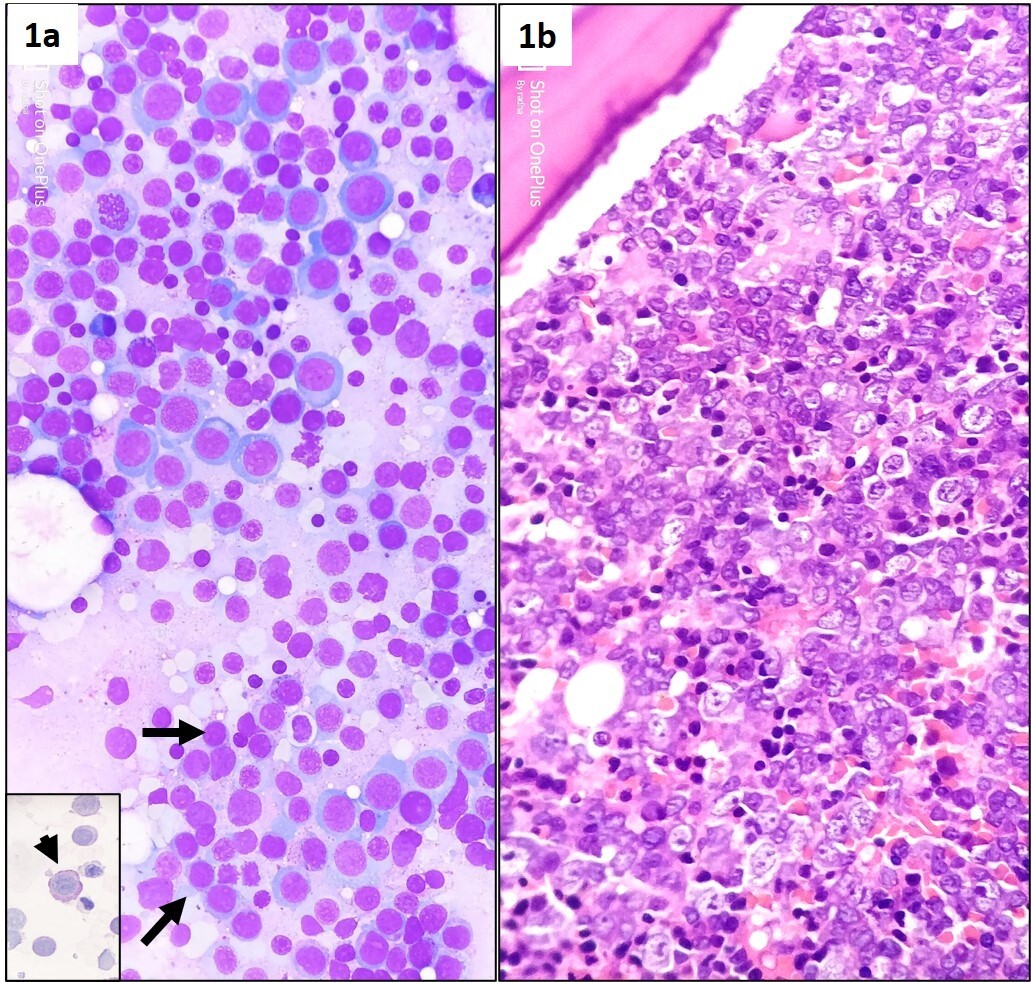
Figure 1: Stained bone marrow aspirate smears (1a) from right posterior superior iliac spine showing hypercellularity, erythroid hyperplasia (>50% of nucleated marrow differential) with megaloblastic nuclear chromatin, suppressed myelopoiesis with increased number of intermediate size myeloid blasts (black arrow). Also note the presence of diffuse cytoplasmic positivity for per-iodic Schiff stain in dysplastic erythroid precursors (inset, 1a, and arrow head) (May Grunewald Giemsa, x400). Trephine biopsy (1b) demonstrating hypercellular marrow with megaloblastic dysplastic erythroid hyperplasia (Hematoxylin eosin, x400).
Bone marrow trephine biopsy sections showed revealed hypercellularity for age (90%) with erythroid hyperplasia showing dysplastic (megaloblast like) islands (Figure 1b), multifocal interstitial clustering of myeloid blasts forming ALIP (CD 34, CD 117, and MPO positive); and presence of micromegakaryocytes showing loose clustering. Reticulin stain showed MF grade 1 fibrosis (WHO). In addition, dysplastic erythroid precursors showed E-cadherin positivity in <25% of cells and a heterogeneous nuclear positivity for P53 in >25% of cells (Figure 2a-2e). Flowcytometric immunophenotyping from BMA sample revealed 10% non-erythroid myeloid blast expressing CD 34 (strong), CD 117 (strong), HLA-DR (strong), CD13 (heterogenous), and CD33 (strong); but were negative for cytoplasmic MPO and other myeloid, B, or T lineage markers. The erythroid lineage cells were negative for CD34, CD117, but expressed CD71 (Figure 3).
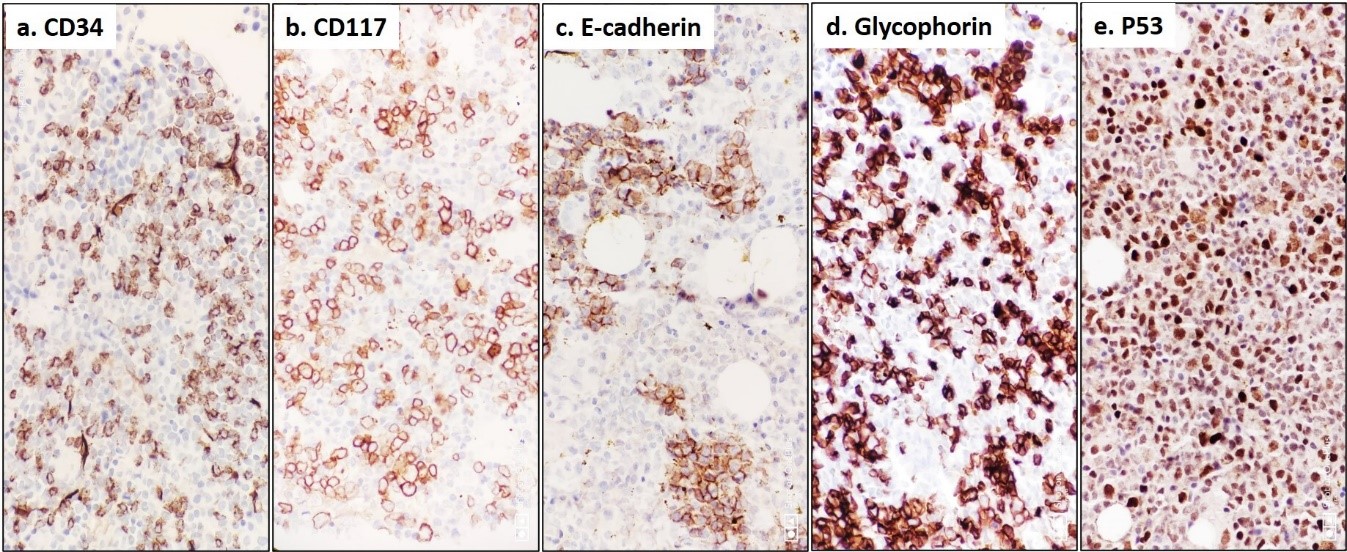
Figure 2: Immunohistochemistry on trephine biopsy demonstrating increased interstitial infiltrate and clusters of immature myeloid precursors (blasts) highlighted by CD 34 (a) and CD 117 (b) antibodies. Note the dysplastic early erythroid precursors and increased maturing erythroid lineages highlighted by E-cadherin (c) and Glycophorin (d). Also note the heterogeneous nuclear positivity for P53 among hyperplastic erythroid islands (e) (Peroxidase-antiperoxidase, x400).
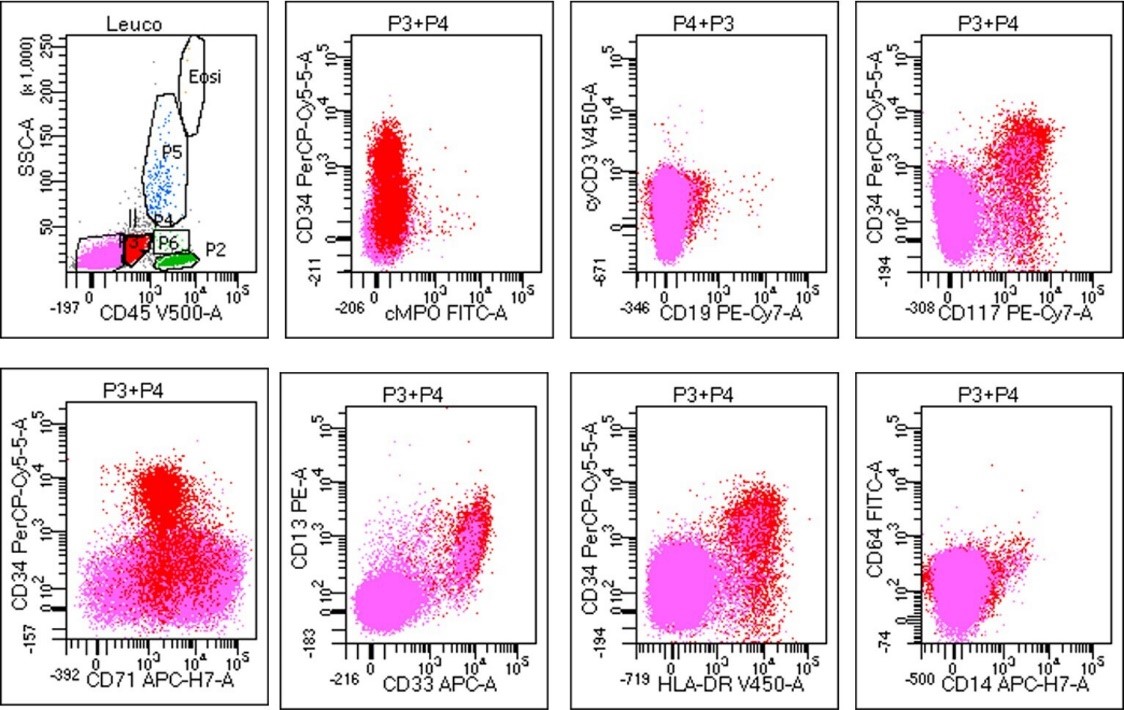
Figure 3: Flow cytometric immunophenotyping analysis from bone marrow aspirate in the index case with population of interest (blasts) gated in red (P3) and erythroid cells gated in pink (P4) on CD45 versus SSC. The blasts were positive for CD 34 (heterogeneous), CD 117, CD13, CD33, and HLA-DR; but negative for cyMPO, cyCD3, CD19, CD79a CD71, CD14, and CD64. These gated population of blasts constituted 12% of the cell population.
Conventional G-banding cytogenetics from BMA sample did not reveal any abnormality whereas targeted Next Generation Sequencing (NGS) assay revealed presence of NPM1 W288Cfs*12 mutation. His serum biochemistry profile was within normal reference limit; and PNH testing by flowcytometry was negative. The patient was therefore classified as MDS with excess blast 2 and erythroid predominance (MDS-EB2-E) as per WHO-2017 classification with revised international prognostic scoring system-5 (high risk category) [1,2]. His routine biochemical and serological tests were negative for antinuclear antibodies, anti-dsDNA, Hepatitis, HIV, EBV, CMV, Dengue, Leptospira, Brucella and Malaria. He was started on azacytidine at a dose of 75mg/m2 for seven days in every 28 days along with transfusion support and oral antifungal therapy with tablet voriconazole (200 mg twice daily) and was closely monitored with follow up CBC and biochemical parameters. Patient tolerated the therapy well. However, after three cycles of treatment, the patient developed febrile neutropenia with pneumonia with onset of ARDS ultimately succumbing to the same at the intensive care unit three months post initial diagnosis. A bone marrow biopsy could not be performed as the patient was in a very critical condition; hence, the cause of the persistent cytopenia remains undecided.
Discussion
MDS-EB-E is the revised phenotypic entity in the 2017 revised WHO classification which represents the erythroid/myeloid variant of AEL with =50% erythroid precursors in previous (2008) classification [1]. As suggested by previous authors, we used a combination of IHC markers such as CD 34, CD 117; and E-cadherin which reliably delineated the interstitial clusters of myeloid blasts; and dysplastic erythroid islands, respectively, in our case [5]. Recent data suggest that uniform and stronger nuclear expression of p53 in erythroid precursors (on trephine biopsy) was specific for leukemic erythroid blasts; and discriminated this entity from closer erythroid mimics as was evident in our case [6]. Moreover, immunohistochemical expression of p53 on trephine core biopsies have been reported to be an indicator of TP53 mutation burden and adverse clinical outcome in subjects with high risk MDS as well as in those with AML with Myelodysplasia Related Changes (AML-MRC), although TP53 mutation was not evident by NGS in our case [7-9].
Mutations in NPM1 is reported in up to 30% cases of Acute Myeloid Leukemia (AML), and in up to 60% of cases of AML with a normal karyotype [10]. NPM1 mutated AMLs are a distinct subgroup of myeloid neoplasms with a distinct clinico-morphological features and a favorable outcome following intensive chemotherapy; especially in the absence of coexistent DNTM3A and FLT3-ITD mutations [1]. In contrast, NPM1 mutated MDS/ MDS-MPN (myeloproliferative neoplasm) are distinctly rare (0 to 9%); and these are reported to have an unpredictable clinical course with a higher risk of AML transformation. The largest series published until date from MD Anderson Cancer Centre, Texas, USA reported an incidence of 1.6% (31/1900 newly diagnosed MDS/MDS-MPN) with a median age of 62 years (range; 19 to 86 years) [10]. As per IPSS-R, 13 (42%), 12 (39%), and 6 (19%) belonged to intermediate risk, high risk, and very high-risk category, respectively; and 19/31 (61%) were of MDS-EB phenotype [16 (52%); MDS-EB-2] (median blast percentage; 10%, range; 1-19%); and 24 (77%) had a normal karyotype. Younger age at presentation, female gender, anemia, higher blast count, and a normal karyotype were more commonly associated with NPM1 mutated subjects when compared with NPM1 wild type subjects (P<0.05). In contrast to NPM1 mutated AML and as noted in our case, the myeloid blasts expressed CD 117, HLA-DR, CD 13, CD 33, and CD 38; were negative for CD 14; whereas CD 34 positivity was noted in 21/31 (67%) of cases. On NGS study, codon 288 (W288fs) in exon 11 was found to be the most commonly mutated (20/31, 64.5%), followed by codon 290 (W290fs) in exon 11 and codon 269 (I269fs) in exon 11 (one case each). Mutations in genes other than NPM1 such as NRAS (N=7), DNMT3A (N=6), TET2 (N=4), WT1 (N=4), PTPN11 (N=3), and FLT3 (N=3) were also noted. Patients treated with chemotherapy (10/31, 32%) had higher complete response rates (90% vs 28%, P=0.004), longer median progression-free survival (not reached vs 7.5 months, P=0.023), and overall survival (not reached vs 16 months, P = 0.047) compared to those treated with HMA [10].
Conclusion
NPM1 mutated MDS is rare, potentially aggressive group of myeloid neoplasms with a distinctive molecular signature that may have a favorable response to intensive chemotherapy. A combination of immunohistochemical markers incorporating CD 34, CD 117, and E-cadherin should be performed in all cases of MDS to highlight the Abnormal Localization of Immature Cells (ALIP), and the dysplastic erythroid islands, respectively for accurate diagnosis and further categorization. Conventional cytogenetics along with next generation sequencing can confirm the underlying molecular abnormalities and help in accurate risk stratification.
Author Statements
Disclosure
This is to acknowledge that no financial interest or benefit
Author Contribution
The authors SM, SP did conceptual design, data collection, data interpretation, diagnosis, literature review, writing and editing of manuscript. GC did flowcytometric evaluation and review of manuscript. SM and PKD provided patient details, management and follow up data. All authors approved the final version of the manuscript to be published.
References
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127: 2391-405.
- Almeida AM, Prebet T, Itzykson R, Ramos F, Al-Ali H, Shammo J, et al. Clinical Outcomes of 217 patients with acute erythroleukemia according to treatment type and line: a retrospective multinational study. Int J Mol Sci. 2017; 18: 837.
- Zuo Z, Medeiros LJ, Chen Z, Liu D, Bueso-Ramos CE, Luthra R, et al. Acute myeloid leukemia (AML) with erythroid predominance exhibits clinical and molecular characteristics that differ from other types of AML. PLOS ONE. 2012; 7: e41485.
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012; 120: 2454-65.
- Ohgami RS, Chisholm KM, Ma L, Arber DA. E-cadherin is a specific marker for erythroid differentiation and has utility, in combination with CD117 and CD34, for enumerating myeloblasts in hematopoietic neoplasms. Am J Clin Pathol. 2014; 141: 656-64.
- Alexandres C, Basha B, King RL, Howard MT, Reichard KK. p53 immunohistochemistry discriminates between pure erythroid leukemia and reactive erythroid hyperplasia. J Hematopathol. 2021; 14: 15-22.
- Fernandez-Pol S, Ma L, Ohgami RS, Arber DA. Immunohistochemistry for p53 is a useful tool to identify cases of acute myeloid leukemia with myelodysplasia-related changes that are TP53 mutated, have complex karyotype, and have poor prognosis. Mod Pathol. 2017; 30: 382-92.
- McGraw KL, Nguyen J, Komrokji RS, Sallman D, Al Ali NH, Padron E, et al. Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse clinical outcome in myelodysplastic syndromes and secondary acute myeloid leukemia. Haematologica. 2016; 101: e320-3.
- Molteni A, Ravano E, Bandiera L, Riva M, Nichelatti M, Cantoni S, et al. The immunohistochemical expression of p53 in bone marrow biopsy has unfavorable prognostic impact in higher risk myelodysplastic syndromes if it is at least 10%. Blood. 2016; 128: 5546.
- Montalban-Bravo G, Kanagal-Shamanna R, Sasaki K, Patel K, Ganan-Gomez I, Jabbour E, et al. NPM1 mutations define a specific subgroup of MDS and MDS/MPN patients with favorable outcomes with intensive chemotherapy. Blood Adv. 2019; 3: 922-33.