
Case Report
Austin Gynecol Case Rep. 2022; 7(2): 1034.
Monochorionic – Monoamniotic Twins’ Placenta
Trojner Bregar A1,2*, Paljk Likar I1, Tul N1,2 and Cvetko E3
¹Division of Obstetrics and Gynecology, Department of Perinatology, University Medical Center, Ljubljana, Slovenia
²Division for gynecology and obstetrics, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
³Institute of Anatomy, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
*Corresponding author: Andreja Trojner Bregar Division of Obstetrics and Gynecology, Department of Perinatology, University Medical Center, Ljubljana, Slovenia
Received: November 10, 2022; Accepted: December 21, 2022; Published: December 26, 2022
Case report
A 33-year-old healthy primigravida with a body mass indexof 21 kg/m², unexplained infertility, pregnancy after IVF, and single embryo transfer, was referred to our department at 12 weeks. We diagnosed monochorionic – monoamniotic twin pregnancy with normal growth, anatomy, and dopplers in both fetuses, anterior normal appearing placenta with eccentric insertion of both umbilical cords, and cervical length of 40 mm. After extensive counseling on the risks and complications of monochorionic – monoamniotic twins, the parents decided to continue with the pregnancy. The course of the pregnancy was uneventful for the mother and fetuses with normal results on all biweekly performed ultrasound examinations. At 30 weeks she received corticosteroids for fetal lung maturation.
A planned Cesarean delivery was performed at 32 weeks +1 day because the fetuses were in a single amniotic sac.
Two healthy female neonates were delivered (Table 1). Both girls were transferred to the neonatal intensive care unit and discharged after 16 days of uneventful courses without any medical intervention. Three days later were discharged from the hospital, healthy.
Postpartum examination of the placenta was a huge surprise. A monochorionic placenta had massive interlacing of umbilical cords with a true knot in each umbilical cord, and an unusual partially velamentous course of umbilical vessels (Figure 1, Figure 2).
We prepared the placenta for conservation.
After delivery, excess blood was manually milked out of the placenta to minimize blood clots interfering with the casting process. The placenta was then bathed in saline solution with heparin to reduce thrombosis.
All arteries and veins of the umbilical cords were injected with acrylate monomers (acrylate powder and liquid (Poli Repair S, PoliDent, Volcja Draga, Slovenia) mixed with different dyes to identify fetal connections (Figure 1). We typically used 40 ml monomer for arteries and 80 ml for venous casting because of the more compliant circulation. The resin was injected into the system until back-pressure prevented further injection. Following injection of the umbilical cords, the placenta was placed in a bath at room temperature, in which the injected material polymerized completely in approximately 60 min. Subsequently, the placenta was placed in a 30% hydrogen chloride solution. After two days, it was carefully rinsed with water jets to remove necrotic tissue (Figure 3, Figure 4).
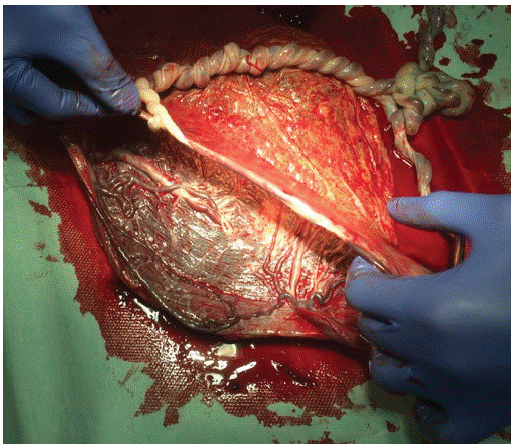
Figure 1: Ultrasound finding of the right ovarian tumor
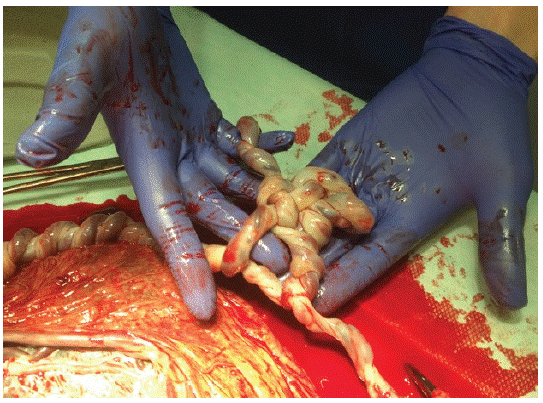
Figure 2: Tangeled umbilical cords with true notch.
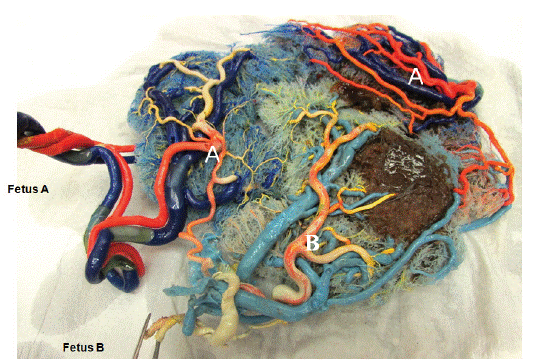
Figure 3: Anatomical preparation of the monochorionic placenta.

Figure 4: Anatomical preparation of the monochorionic placenta.
Fromthe obstetricians’ point of view is interesting that the pregnancy and development of fetuses were uneventful, although the presence of a completely irregular course of umbilical cords. What are the reasons and causes for the irregular development of vessels are unknown? But it is well known that vessel anomalies are common in all monochorionic pregnancies and that in monochorionic – monoamniotic twins umbilical cord entanglement has almost 100% prevalence [7].
It is estimated that pregnancies resulting from ART procedures account for 0.2–3.9 % of births in Europe [1]. The rate of twin pregnancies after ART (IVF-ET) in Europe is 17%, and for Slovenia specifically 8.4% [2]. It is known that the principal determinant of the perinatal outcome of twins is chorionicity, moreover, monoamnionicity carries an increased risk of adverse pregnancy outcomes compared to monochorionic diamniotic twins [3].
Pregnancies with monochorionic monoamniotic twins are rare and have a high risk for premature delivery, low birth weight, and congenital anomalies. Twin-to-Twin Transfusion Syndrome (TTTS), Twin Anemia Polycythemia Sequence (TAPS), and Selective Intrauterine Growth Restriction (sIUGR) may also develop owing to placental vascular anastomoses and unequal placental sharing [4,5].
There are some unique complications in monoamniotic pregnancies such as conjoined twins [6] and umbilical cord entanglement - with an almost 100% prevalence in monochorionic monoamniotic twins pregnancies [7]. An umbilical artery notch has been associated with cord entanglement and adverse perinatal outcomes in monochorionic monoamniotic twins [8]. However new findings suggest, that the true notch and entanglement of the umbilical cord with the absence of signs of fetal deterioration do not necessarily lead to an abnormal perinatal outcome [5]. Fortunately in our reported case, the monochorionic monoamniotic pregnancy had a normal outcome and a happy ending and beginning.
References
- IVF/ICSI twin pregnancies: risks and prevention. Pinborg A Hum Reprod Update. 2005; 11: 575-93.
- Calhaz-Jorge C, De Geyter C, Kupka MS, Erb K, Mocanu E, et al. European IVF-Monitoring (EIM); Consortium for the European Society on Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2014: results generated from European registries by ESHRE. Hum Reprod. 2017; 32: 1957-1973.
- D’Antonio F, Odibo A, Berghella V, Khalil A, Hack K, et al. Perinatal mortality, timing of delivery and prenatal management of monoamniotic twin pregnancies: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2018.
- Lewi L, Deprest L, Hecher K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. Am J Obstet Gynecol. 2013; 208: 19–30.
- Aurioles-Garibay A, Hernandez-Andrade E, Romero R, Garcia M, Qureshi F, et al. Presence of an umbilical artery notch in monochorionic/ monoamniotic twins. Fetal Diagn Ther. 2014; 36: 305- 11.
- Mathew RP, Francis S, Basti RS, Suresh HB, Rajarathnam A, et al. Conjoined twins – role of imaging and recent advances. J Ultrason. 2017; 17: 259-266.
- Dias T, Mahsud-Dornan S, Bhide A, Papageorghiou AT, Thilaganathan B. Cord entanglement and perinatal outcome in monoamniotic twin pregnancies. Ultrasound Obstet Gynecol. 2010; 35: 201–4.
- Hugon-Rodin J, Guilbert JB, Baron X, Camus E. Notching of the umbilical artery waveform associated with cord entanglement in a monoamniotic twin pregnancy. J Matern Fetal Neonatal Med. 2013; 26: 1559-61.