
Research Article
Gerontol Geriatr Res. 2025; 11(1): 1110.
Cognitive Impairment Assessment in Older Adults: A Narrative Review of Available Tools
Rhalimi M1,2, Tran A1,3 and Valade A1,3
1Centre hospitalier Bertinot Juel, Chaumont-en-Vexin, France
2INSERM U1088, Université de Picardie, Amiens, France
2Faculté de pharmacie, Université de Montréal, Canada
*Corresponding author: Mounir Rhalimi, Centre hospitalier Bertinot Juel, Chaumont-en-Vexin, INSERM U1088, Université de Picardie, Amiens, 34 Bis Rue Pierre Budin, Chaumont-en-Vexin, 60240, Hauts-de-France, France Tel: +333.44.49.54.51; Email: m.rhalimi@ch-chaumontenvexin.fr
Received: March 18, 2025 Accepted: April 03, 2025 Published: April 07, 2025
Abstract
Purposes: First to define and to differentiate the concepts of delirium, dementia, confusion and disorientation. Second to review and compare current relevant published cognitive impairment assessment. This review will assess how well these tools as can be integrated in a clinician’s routine to estimate the reliability of the information given by a patient. Third, to discuss the potential positioning of the STOT (Spatial-Temporal Orientation Test) among currently available tools.
Purposes: First to define and to differentiate the concepts of delirium, dementia, confusion and disorientation. Second to review and compare current relevant published cognitive impairment assessment. This review will assess how well these tools as can be integrated in a clinician’s routine to estimate the reliability of the information given by a patient. Third, to discuss the potential positioning of the STOT (Spatial-Temporal Orientation Test) among currently available tools.
The authors reviewed existing cognitive impairment assessment tools and compared the evaluated neurocognitive domains, the duration of these tests, the sensitivity, the specificity and the predictive values.
Results: We identified 132 tests through PubMed search, and we included 30 of them in our analysis. Twenty-five tools tested for orientation and 23 for memory. Seventeen tools evaluated dementia, while 10 of them tested for mild cognitive impairment. Eleven of them evaluated delirium. Thirteen tests in our list take up to 5 minutes to complete, and three of these tests take 3 minutes or less to administer.
Conclusions: Some tests have the potential to be integrated in clinical pharmacists’ routine, since they take less than 3 minutes to administer. The STOT would probably be one of the easiest tools to use systematically considering the simplicity of the questions, but data is needed to validate its use.
Keywords: Delirium; Dementia; Mild Cognitive Impairment; Neurocognitive disorders; Neuropsychological Tests; Older people
Key Summary Points
Aim: This review allows clinical pharmacists and other clinicians to compare cognitive tools that evaluate cognitive functions and to choose among them the more adapted to their services.
Findings: The need for quick cognitive assessment tools to evaluate the reliability of information given by patients is not well addressed in the literature. While there are a few reviews of cognitive tools that have been published, we have found none that evaluates them in this particular context. However, in our daily work as clinical pharmacists, we have found this type of evaluation to be necessary, especially in the context of polymedicated patients in the geriatric ward. Thus, our review analyses the current cognitive tools with a novel approach, evaluating their possible use to test for the reliability of patient-given information.
Message: Multiple existing tools have the potential to measure quickly and accurately a patient’s cognitive function, from which we can now study the reliability of patient-given information.
Introduction
As geriatric clinical pharmacists, we perform acts which allow us to identify pharmacologic problems, prevent idiopathic events and optimize pharmacotherapy. High prevalence of cognitive impairments in geriatric populations is an obstacle to gathering precise information directly from the patient. Indeed, when clinicians question patients on their health and lifestyle, there is a possibility that the answer is erroneous. This inaccuracy could be harmful to the patient. Using a brief and effective test to evaluate the patient's cognitive function every time information was needed from them would give clinicians a better idea of the reliability of those answers and the necessity to look for other information sources, (eg, family members and nursing homes). The Spatial-Temporal Orientation Test (STOT) was devised to address this problem and has been used for many years at the Bertinot Juël Hospital in Chaumont-en-Vexin, France [1].
It consists of 4 questions regarding the actual year, the current geographical location of the patient, the patient’s address and the time of the day. For now, we consider the patient disorientated if doesn’t score 4 correct answers out of 4. However, this test has yet to be validated, and numerous other tools currently exist to evaluate cognitive function.
Furthermore, in the context of possible neurocognitive disorders, it is important to distinguish between the different concepts such as dementia, delirium, confusion and disorientation.
• Thus, there are three main objectives to this review: first to define and to differentiate the concepts of delirium, dementia, confusion and disorientation;
• second to review and compare current relevant published tools;
• third to discuss the potential positioning of the STOT among currently available tools.
Definitions and Concepts
Causes of cognitive impairment sometimes overlap, sometimes are very distinct. It is necessary to differentiate symptoms from illnesses. Regarding medical definitions, the World Health Organization's (WHO) International Classification of Diseases (ICD) is recognized as an international standard, but the Diagnostic and Statistical Manual of Mental Disorders (DSM) prevails as the main reference in the psychiatric department. The classification of dementia by the DSM has recently been updated. In the fourth edition of the DSM, published in the nineties, dementia was classified in a category of its own, while the fifth edition published in 2013 included it in the new “Major neurocognitive disorders” (MCD) category along with other possible causes of impairment such as HIV or brain trauma [2-4]. Major neurocognitive disorders diagnosis is defined as a significant cognitive decline of one or more cognitive domains over time. According to DSM-V, there are six neurocognitive domains: learning and memory, language, executive function, complex attention, perceptual-motor function and social cognition [2]. Supplementary Figure S1 presents DSM-V classifications of the six neurocognitive domains and their components [5]. Mild neurocognitive disorders or impairment (MCI) are also mentioned for the first time in this latest edition and require basically the same criteria as MCD with distinction being assessed upon severity [5]. Delirium is an acute alteration of cognitive domains and it is excluded from MCI and MCD according to ICD-10 and DSM-V definitions.
A major obstacle to evaluating validity of a patient’s affirmations is underdiagnosis of cognitive impairment, especially delirium [6]. Delirium was shown to be a predictor of increased mortality, complications rate, length of hospital stay, relocation at discharge, cost for society, permanent cognitive and functional decline and delirium history was even associated to a higher risk of developing dementia [7-9]. It is essential to recognize it to prevent and treat it whenever possible. The DSM-V criteria as well as the ICD-10 definitions offer a reference in spotting delirium cases but the difficulty to recognize the mentioned symptoms among healthcare providers and its low diagnosis rate are already well documented [4,10]. Even through published literature, reported delirium prevalence is subject to large variations. These variations seem to be influenced by clinical context, but methods also seem to have an important impact, as there are variations between publications for similar contexts [10]. These elements strengthen the need to implement tools to systematically screen for cognitive impairment in healthcare settings, especially in clinical contexts with high prevalence of geriatric patients.
Dementia prevalence across the world was estimated to 24 million cases in 2010 and it was predicted this number would double every 20 years [11]. This disease is usually presented as a chronic, irreversible and associated to organic damages in which symptoms can only worsen gradually over time at a variable rhythm. The conversion rate from MCI to dementia is estimated to be around 10% yearly [12,13]. The principal causes of dementia are Alzheimer’s disease, vascular (postcerebrovascular event permanent damages) and Lewy body dementia. Other causes or aggravating factors include substance abuse, vitamin B deficiencies, hypothyroidism, etc [4]. Even if MCI and dementia usually present stable symptoms, the subtlety of symptoms and slow progression can delay diagnosis until a more advanced stage.
Therefore, an absence of documented MCI, MCD or even delirium does not guarantee normal cognitive function, and thus does not warrant reliable information.
Differentiation between delirium and other neurocognitive disorders is also complicated, even for caregivers working on their bedside daily [6]. Summarily, delirium can develop suddenly, it is usually not associated to any organic damages, and it is, most notably, reversible. Other notable differences between dementia and delirium are mentioned in Table 1.
Delirium
MCI and MCD
Apparition
Fast and sudden
Progressive
Evolution
Fluctuations
Steady
Resolution
Reversible in days to weeks
Irreversible
Cognitiv
e domains
Executive
Possible rapid decrease in executive functions
Progressive degradation of executive functions
Attention
Hardly sustained, short concentration
Harder to concentrate but attention sustainable
Memory
Short term memory importantly impaired; important recognition memory loss
Short, then long term memory loss;
semantic memory loss more probable as disease progresses
Language
Wrong word choice, incoherent sentences, etc.; naming and comprehension possibly decreased; probable impact of visual
Mainly name and words forgetting as disease progresses (impacted by semantic memory loss and other developed
misperceptions
extralinguistic deficiencies)
Perceptual-motor
Frequent hallucinations, misinterpretation, significant loss of awareness (altered gnosis)
Hallucinations are uncommon; agnosia possible with progression of the disease
Social cognition
Probable loss of emotions recognition
Usually not affected until advanced stages
Emotions and behavior
Possible fear, aggressiveness
Possible anxiety, fear, irritability, apathy
Sleep
Altered sleeping-waking cycle
Usually, normal
Table 1: Clinical presentation mapping for delirium and dementia [2,14,19–24].
Many predisposing factors as well as triggering factors for delirium have been mentioned in literature (Supplementary Table S2). Dementia is a predisposing factor for delirium; both often coexist and can be hard to differentiate. The presence of short- and long-term related questions for both time and space components of orientation evaluated by the STOT aims to assess whether short- or long-term memory is the most affected. The results could help rapidly distinguish between dementia and delirium. Terms “confusion’’ and “disorientation’’ are closely related and WHO presents them as synonyms. Indeed, ICD-10 definition for “disorientation” simply states “Confusion not otherwise specified”. Confusion generally describes an alteration of one or more cognitive domains. Neurocognitive disorders are only one possible cause of confusion amongst many. Other possible causes include various medical situations, iatrogenic effects, exposition to harmful substances, etc. However; “disorientation” seems frequently employed in the meaning of “spatiotemporal disorientation” which is a symptom of confusion mainly related to the cognitive domain of memory (more specifically to dysfunction of the recognition memory) and perceptual-motor domain [14,15]. These domains are frequently impaired in both neurocognitive disorders and delirium. Also, spatiotemporal disorientation is one of the most easily detectable symptoms of confusion, which is why the STOT concentrates on this aspect to evaluate cognitive function.
Methods
The first step was to identify the existing tests concerning cognitive function impairment: the search was done on PubMed and using the keywords “neuropsychological tests’’, “cognitive dysfunction”, “evaluation’’, “screening’’, and “diagnosis’’ to find relevant publications. Since we aren’t looking to diagnose delirium or dementia, or to differentiate one from the other, we included tests to evaluate both conditions. We excluded articles that weren’t in published English and those concerning mental impairment in a specific population other that patients over 65 (eg, stroke patients). We looked at reviews of tools, from which we extracted additional tests that weren’t directly found through this search. After gathering a list of all the tests that are mentioned through this search, the second step was to look for the original articles where these tests were first mentioned and validated. We only included tests that have been published in a journal and those that have been validated. We excluded tests that were done via telephone and those that could not be completed in one meeting. We also excluded tests that needed a support other than pen and paper, such as a screen.
We extracted the main objective of the test, sensitivity, specificity, positive and negative predictive values from the original validation study as available, and then compared with subsequent studies when a lot of data was available, favoring reviews and meta-analysis. We also looked for the duration of each test and if they included an evaluation of the spatial temporal orientation. For some tools, we had to dig for more information if we didn’t find all the data we needed within the articles we had already gathered. For instance, we sometimes had to research for specific data like sensibility, specificity or predictive values individually. Considerable reputation, clinical experience and more recent publications were also weighted in our selection. Some tools required a modified approach, such as MMSE, for which the original publication didn’t present sensitivity, specificity or accuracy data. We thought this could be explained by the evolution in standards over several decades. In this case, we chose to include meta-analyses, which constituted a reasonable approach considering the important literature. These meta-analyses were chosen to optimize sample size and diversity while minimizing duplicates.
Results
As illustrated in Figure 1, we identified 132 tools with our PubMed search, including articles that were referenced in other publications. We retained 30 tools which matched our inclusion and exclusion criteria and we separated them into three categories: those evaluating and screening for chronic neurocognitive disorders from MCI to dementia, those targeting delirium, and those developed with time-saving in mind and that don’t differentiate between dementia and delirium (STOT-like tools). Tables 2 to 4 present the basic characteristics of the different tools and specifies each domain that they test for. Supplementary tables S3 to S5 present the performance of the different tools and include sensitivities, specificities and predictive values of the different tests. We also included other practical information concerning each publication such as the main author’s name, size of the studied population, purpose or object of the study, comparators (if applicable) and reference cognitive assessment method.
Name
Abbreviation
Year of publication
Evaluated
conditionCognitive domains
Emotional disturbances
Test duration (minutes)
Number of items or subtests
Mild cognitive impairment (MCI)
Dementia
Perceptual-motor
functionLearning/
memoryComplex attention
Language
Social cognition
Executive
functionGnosis (awareness)
Psychomotor disturbance
Visual perception
Visuoconstructional
Spatiotemporal orientation
Learning
Memory
Working memory
Thought process
Other executive functions
Mini Mental State Examination and
Standardized MMSE [27.28]MMSE/ SMMSE
1975
and 1997Adjusted cutpoint
X
X
X
X
X
10-15
11
Mini-Cog [29]
2000
X
X
X
3
2
Demenz Detektion (German) [30]
DemTect
2004
X
X
X
X
X
8-10
5
Memory and Executive
Screening [31]MES
2012
X
X
X
X
7
7
Memory orientation
screening test [32]MOST
2010
X
X
X
X
< 5
4
Montreal Cognitive
Assessment [33]MoCA
2005
X
+/-
X
X
X
X
X
X
10
30
Short-MoCA [34]
s-MoCA
2005
X
+/-
X
X
X
X
X
X
< 10
8
AB Cognitive Screen [35]
ABCS
2003
X
+/-
X
X
X
X
3-5
5
Quick Mild
Cognitive Impairment [16,36]Qmci
2012
X
+/-
X
X
X
X
5
6
Hasegawa's
Dementia Scale- Revised [37,38]HDS-R
1994
X
X
X
X
X
10
9
7 Minutes
Neurocognitive Screening [39]7MS
1998
X
X
X
X
X
7
4
Ascertain
Dementia 8 [40]AD8
2005
X
X
X
X
X
<3
8
Addenbrooke's Cognitive
Examination III [41]ACE- III
2017
X
X
X
X
X
X
15
5
Memory Alteration Test [42]
M@T
2007
X
X
X
X
5-7
40 to 50
Memory Impairment Scale [43]
MIS
1999
X
X
X
X
4
4
Table 2: Basic characteristics and domains of MCI and dementia tools.
Name
Abbreviation
Year of publication
Cognitive domains
Emotional disturbances
Delirium elements
Duration (minutes)
Number of items or subtests
Perceptual-motor function
Learning/ memory
Complex attention
Language
Social cognition
Executive function
Acute onset
Fluctuations
Sleep-wake cycle
Gnosis (awareness)
Psychomotor disturbance
Visual perception
Visuoconstructional
Spatiotemporal orientation
Learning
Memory
Working memory
Thought process
Other executive functions
Cognitive Assessment Method [44]
CAM
1990
X
X
X
X
X
X
X
X
5-10
9
CAM adapted for intensive care units [45]
CAM-ICU
2001
X
X
X
X
X
X
< 5
4
Intensive Care Delirium Screening Checklist [46]
ICDSC
2001
X
X
X
X
X
X
X
X
X
X
7-10
8
Recognizing Acute Delirium As part of your Routine [47]
RADAR
2015
X
X
X
X
2-3
3
Delirium Observation Screening Scale (25 items) [48]
DOS
2003
X
X
X
X
X
X
X
X
< 5
25
Delirium Observation Screening Scale (13 items) [49]
DOS
2003
X
X
X
X
X
X
X
X
< 5
13
Neecham Confusion Scale [50,51]
NEECHAM
1996
X
X
X
X
X
X
X
10
9
Memorial Delirium Assessment Scale [52]
MDAS
1997
X
X
X
X
X
X
X
X
X
10
10
Delirium-O-Meter [53]
DOM
2005
X
X
X
X
X
X
X
X
3-5
12
Delirium Symptom Interview [54,55]
DSI
1992
X
X
X
X
X
X
10-15
109
Delirium Rating Scale Revised-98 [56,57]
DRS-R-98
2001
X
X
X
X
X
X
X
X
X
X
X
X
15-20
16
Table 3: Basic characteristics and domains of delirium tools.
Name
Abbreviation
Year of publication
Evaluated condition
Cognitive domains
Emotional disturbances
Duration (minutes)
Number of items or subtests
Cognitive dysfunction
MCI
Dementia
Delirium
Perceptual-motor function
Learning/ memory
Complex attention
Language
Social cognition
Executive function
Gnosis (awareness)
Psychomotor disturbance
Visual perception
Visuoconstructional
Spatiotemporal orientation
Learning
Memory
Working memory
Thought process
Other executive functions
Short Portable Mental Status Questionnaire [58]
SPMSQ
1975
X
X
X
X
X
5-10
10
Abbreviated Mental Test [59]
AMT
1972
X
X
X
X
X
5-7
10
4-items AMT [60]
AMT-4
1997
X
X
X
X
3-5
4
6-items Cognitive Impairment
Test [61]
6CIT
1983
X
X
X
X
X
X
2-3
6
Table 4: Basic characteristics and domains of STOT-like tools.
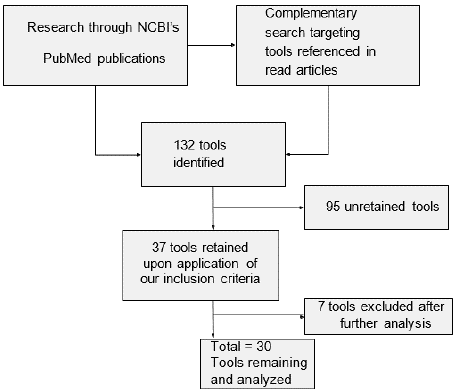
Figure 1: Flow diagram of the literature search.
Orientation and memory are the elements evaluated in the most tests: 25 of them tested for orientation and 23 for memory. Seventeen tools evaluated dementia, while ten of them tested for mild cognitive impairment. Eleven of them evaluated delirium. Study sizes are very variable between tests: some studies have less than 50 subjects, others have more than 500 subjects, and in meta-analyses such as those of the MMSE, pooled data can come from more than ten thousand subjects. Sensitivity varies between 20 and 100%, and 21 tests have at least one study suggesting a sensitivity of more than 90%. Specificity varies between 25 and 100%. Thirteen tests in our list take up to 5 minutes to complete, and three of these tests take 3 minutes or less to administer. Most tools, especially the shorter ones, test for a specific condition rather than cognitive function as a whole. None of the tests screens specifically for spatial temporal disorientation, but this element is often included in the evaluation process regardless of whether the system tests for dementia or delirium.
Discussion
While the sensitivity, specificity and predictive values of numerous tools included in our review are similar, the size of published data give us a good idea of what tests are frequently used and allows a more global interpretation of the validity of each tool. Unsurprisingly, the MMSE is amongst the most commonly used in clinical practice, but is also a common reference in dementia diagnosis and follow-ups. However, it has limitations such as suboptimal sensibility and specificity, especially in patients with MCI where the Montreal Confusion Assessment (MoCA) appears superior. For delirium screening, the Confusion Assessment Method (CAM) has an important amount of relatively strong data supporting its effectiveness. The optimal duration of a tool depends on the context in which it will be used. Since we want to evaluate reliability of data extracted from patients, it should logically be quicker than the ones generally used for diagnostic. Most tools that we examined are not very time-consuming, requiring less than 10 minutes to complete. However, even this amount of time can become a burden should it be used repeatedly as part of a routine. For example, if a clinician sees 20 patients per day, doing these tests before seeing each patient would take them at least an hour and a half per day. Therefore, the required time is an important factor if we want to validate information acquired from patients every time we interact with them. In this context, time required to go through a formulary like the MMSE, the MoCA or even the CAM, which all take around 10 minutes to complete, explains the interest for shorter, quicker tools derived from them. For example, the short-MoCA (s-MoCA) has less evaluation elements than the MoCA while assessing the exact same domains, and it presents similar results compared to the original MoCA. In contrast, Mini-Cog, a derivative of the MMSE, hasn’t been able to come up with consistent results and seems to magnify some of MMSE’s weakness such as questionable specificity.
Focusing on time-saving alternatives, the Short Portable Mental Status Questionnaire (SPMSQ), Abbreviated Mental Test (AMT) and 6-items Cognitive Impairment Test (6CIT) all have a similar structure to the STOT with a few questions that can be answered relatively fast. Despite these similarities, the use of SPMSQ and 6CIT is backed by stronger evidence. The 6CIT, while having only six predefined questions, might still be longer to administer than the STOT, since answering some of its questions can be complicated and take more time (eg, counting backward).
However, the number of items does not often reflect the duration of the test. For example, the Memory Alteration Test (M@T) contains between 40 and 50 items, but takes less time to complete than Hasegawa's Dementia Scale-Revised (HDS-R) composed of only nine items. The longest test in our list is the Delirium Rating Scale Revised-98 (DRS-R-98).
Delegating cognitive function assessment to different caregivers could be a plausible approach to bypass the time constraint. However, it has been thoroughly demonstrated that results obtained may vary between caregiver categories, at least with some tools like MMSE. There is a possibility that this discrepancy is the result of varying moments of administration, especially when considering the possibility of the daily fluctuations of delirium. Specialized caregivers seem to obtain better results in these situations, but these results could be influenced by their basic knowledge. Interestingly, some tools were designed specifically for nurses, such as the RADAR test and the Delirium-OMeter, and are adapted and evaluated for nurse-patient interactions. The same working process could be used in developing and evaluating tools for other caregivers, such as clinical pharmacists.
Clinical settings can also motivate the modification of existing tools. For instance, a couple of variations of the CAM currently exists, and some are supported by good evidence and used relatively often in clinical settings. One of such variation is the ICU adapted version (CAM-ICU), meant to be used on intubated patients.
Other tools with less supporting evidence also present interesting characteristics. The Quick MCI (Qmci) test presents interesting results in differentiation of MCI from normal cognition and was compared to MoCA on that matter. Qmci was created by modifications to AB Cognitive Screen’s (ABCS) subsections and takes only 3 to 5 minutes to complete, but results are presented in the form of a score from 0 to 100 that seems to bring cutoff issues. In fact, the authors originally suggested a cutoff of less than 62 for differentiating MCI from normal cognition, but a more recent study [16] seem to demonstrate that a lower cutoff would be more accurate. The same problems occur with MoCA as different authors suggest different cutoffs - at least four different values have been proposed. It seems to us that this is a serious obstacle in evaluating, comparing and optimizing results of these tools in everyday caregiving and it could be an interesting matter to address in a further literature review.
Overall, the DSM-V criteria to delirium diagnosis when applied by a clinician remains the cornerstone of neurocognitive disorders and delirium recognition and it seems to us that these criteria should be the gold standard when validating a new tool to obtain reliable accuracy data. However, MMSE is also used in many cases as the comparative in validation studies. Other tests have also been used as references, such as the CAM and the DSR-R-98. Clinical context and destined users should to be considered for choosing the right comparator. An optimal cutoff should also always be determined.
While classifying the different domains that each test assesses, we noticed that for certain types of questions, there isn’t a consensus on the targeted domain. For example, questions requiring naming days of the week or months of the year backwards, is classified under the section ‘disturbance of consciousness’ in the Delirium Symptom Index (DSI) while the 6-CIT considers it a calculation test. Establishing the neurocognitive domains, we want to evaluate prior to building a new evaluation tool based upon its objective (eg, screening delirium, evaluating dementia).
Bernabeu-Wittel et al. [17] analyzed each of the SPMSQ’s questions separately. As previously mentioned, some of these questions are very similar to those of the STOT and evaluate the same cognitive domains. Therefore, the relatively conclusive results of this analysis are interesting and are part of what encourages us to pursue the STOT’s validation process.
Limitations
Since there is an important number of tests that currently exists, there was more than two thirds of them that could not be included in our review. Some existing reviews of cognitive tests are more thorough, such as the systematic review for the U.S. Preventive Services Task Force [18], although we have not found one that included every test that we have come across in our search.
Furthermore, for some of the tests, we extracted the validity data from one publication, which was often the original validation study. This can be a source of bias, particularly publication bias, since the author is the creator of the tool. Additionally, having data from multiple studies would have given a better estimate of the sensitivity and specificity of the different tools through our review. Effectively, there has been some variations in data with almost every tool. Since delirium is known to be difficult condition to diagnose. In fact, even between experts, there isn’t always a consensus, and clinical context also seems to be a significant source of variation. These discrepancies between publications on a same tool make it harder to draw conclusions and hinders comparison of different tools, especially those with similar statistics. Thus, these variations and their possible causes must be kept in mind when comparing tests to one another to minimize the impact on our analysis as much as possible.
Some other variable would have been interesting to compare between tools, such as details of the populations in which the tools were tested and inter-rater variability, as we have sometimes seen these two elements having an impact on a tool’s performance.
Conclusion
The notions of delirium, dementia, confusion and disorientation are closely intertwined. While dementia and delirium are two separate conditions that affect one another, confusion is a manifestation of these disorders and disorientation is one face of confusion. Cognitive dysfunction can affect the reliability of patients’ answers to clinicians’ questions, but the numerous currently available tests have limited applicability as a systematic pre-interview screen. While some tests are quite short (less than 3 minutes to administer) the STOT would probably be even shorter considering the simplicity of the questions, which makes it easier to be used for this function. Validation studies including sensitivity, specificity and ROC calculations would be needed to support its systematic use the healthcare system. Furthermore, it would be relevant to consider the relationship between disorientation and the reliability of the patient’s answers: does a disoriented patient’s answers are necessarily erroneous? Further studies are needed on this subject to help guide clinicians’ approach to potentially confused patients.
Acknowledgements
We would like to thank Valérie Le Chatton, occupational therapist, for her help in developing the STOT, and Joanne May Ramil, B.Sc. candidate in behavioural neuroscience for reviewing the text.
Author Contributions
Every contributing author has been named and all named author have contributed significantly to this article.
References
- Rhalimi F, Rhalimi M, Rauss A. Pharmacist’s Comprehensive Geriatric Assessment: Introduction and Evaluation at Elderly Patient Admission. Drugs - Real World Outcomes. 2017; 4: 43-51.
- American Psychiatric Association, ed. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association, ed. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4. ed., 7. print. Washington, DC; 1998.
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992.
- Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. 2014; 10: 634-642.
- Brooke J. Differentiation of delirium, dementia and delirium superimposed on dementia in the older person. Br J Nurs. 2018; 27: 5.
- McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium Predicts 12-Month Mortality. Arch Intern Med. 2002; 162: 457-463.
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does Delirium Contribute to Poor Hospital Outcomes? J Gen Intern Med. 1998; 13: 234-242.
- O’Keeffe S, Lavan J. The Prognostic Significance of Delirium in Older Hospital Patients. J Am Geriatr Soc. 1997; 45: 174-178.
- Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does This Patient Have Delirium?: Value of Bedside Instruments. JAMA. 2010; 304: 779.
- Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. The Lancet. 2005; 366: 2112-2117.
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of Mild Cognitive Impairment to Dementia in Clinic- vs Community-Based Cohorts. Arch Neurol. 2009; 66: 1151-1157.
- Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009; 119: 252-265.
- Lippmann S, Perugula ML. Delirium or Dementia? Innov Clin Neurosci. 2016; 13: 56-57.
- Schnider A, von Däniken C, Gutbrod K. Disorientation in amnesia: A confusion of memory traces. Brain. 1996; 119: 1627-1632.
- O’Caoimh R, Molloy D. The Quick Mild Cognitive Impairment Screen (Qmci). In: Cognitive Screening Instruments: A Practical Approach. 2017: 255-272.
- Bernabeu-Wittel M, Nieto Martín D, Moreno-Gaviño L, Ollero-Baturone M, en representación de los investigadores del Proyecto Profund. Diagnostic value of a simplified Pfeiffer questionnaire for polypathological patients. Rev Clin Esp. 2017; 217: 320-324.
- Lin JS, O’Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013; 159: 601-612.
- Martins S, Fernandes L. Delirium in Elderly People: A Review. Front Neurol. 2012; 3.
- Massimo L, Kales HC, Kolanowski A. State of the Science: Apathy As a Model for Investigating Behavioral and Psychological Symptoms in Dementia. J Am Geriatr Soc. 2018; 66: S4-S12.
- Kempler D, Goral M. Language and Dementia: Neuropsychological Aspects. Annu Rev Appl Linguist. 2008; 28: 73-90.
- Beglinger LJ, Mills JA, Vik SM, et al. The Neuropsychological Course of Acute Delirium in Adult Hematopoietic Stem Cell Transplantation Patients. Arch Clin Neuropsychol. 2011; 26: 98-109.
- Wallesch CW, Hundsalz A. Language Function in Delirium: A Comparison of Single Word Processing in Acute Confusional States and Probable Alzheimer's Disease. Brain Lang. 1994; 46: 592-606.
- Goyal AR, Bergh S, Engedal K, Kirkevold M, Kirkevold ø. The course of anxiety in persons with dementia in Norwegian nursing homes: A 12-month follow-up study. J Affect Disord. 2018; 235: 117-123.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014; 383: 911-922.
- Josephson SA, Miller BL. Confusion and Delirium. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill Education; 2015.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189–198.
- Molloy DW, Standish TI. A guide to the standardized Mini-Mental State Examination. Int Psychogeriatr. 1997; 9: 87-94; discussion 143-150.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The Mini-Cog: a cognitive‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000; 15: 1021–1027.
- Kalbe E, Kessler J, Calabrese P, et al. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry. 2004; 19: 136-143.
- Guo Q, Zhou B, Zhao Q, Wang B, Hong Z. Memory and Executive Screening (MES): a brief cognitive test for detecting mild cognitive impairment. BMC Neurol. 2012; 12: 119.
- Clionsky MI, Clionsky E. Development and Validation of the Memory Orientation Screening Test (MOSTTM): A Better Screening Test for Dementia. Am J Alzheimers Dis Dementiasr. 2010; 25: 650-656.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc. 2005; 53: 695-699.
- Larner AJ. Short Montreal Cognitive Assessment: Validation and Reproducibility. J Geriatr Psychiatry Neurol. 2017; 30: 104-108.
- Molloy DW, Standish TI, Lewis DL. Screening for Mild Cognitive Impairment: Comparing the SMMSE and the ABCS. Can J Psychiatry. 2005; 50: 52-58.
- O’Caoimh R, Gao Y, McGlade C, et al. Comparison of the quick mild cognitive impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing. 2012; 41: 624-629.
- Imai Y, Hasegawa K. The Revised Hasegawa’s Dementia Scale (HDS-R)- Evaluation of Its Usefulness as a Screening Test for Dementia. Hong Kong J Psychiatry. 1994; 4: 20.
- Tsai N, Gao ZX. Validity of Hasegawa’s Dementia Scale for screening dementia among aged Chinese. Int Psychogeriatr. 1989; 1: 145-152.
- Solomon PR, Hirschoff A, Kelly B, et al. A 7 Minute Neurocognitive Screening Battery Highly Sensitive to Alzheimer’s Disease. Arch Neurol. 1998; 55: 349- 355.
- Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005; 65: 559-564.
- Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke’s Cognitive Examination III in Frontotemporal Dementia and Alzheimer’s Disease. Dement Geriatr Cogn Disord. 2013; 36: 242-250.
- Rami L, Molinuevo JL, Sanchez-Valle R, Bosch B, Villar A. Screening for amnestic mild cognitive impairment and early Alzheimer’s disease with M@T (Memory Alteration Test) in the primary care population. Int J Geriatr Psychiatry. 2007; 22: 294-304.
- Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the memory impairment screen. Neurology. 1999; 52: 231-238.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990; 113: 941-948.
- van Eijk MM, van den Boogaard M, van Marum RJ, et al. Routine Use of the Confusion Assessment Method for the Intensive Care Unit. Am J Respir Crit Care Med. 2011; 184: 340-344.
- Gusmao-Flores D, Salluh JIF, Chalhub RÁ, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012; 16: R115.
- Voyer P, Champoux N, Desrosiers J, et al. Recognizing acute delirium as part of your routine [RADAR]: a validation study. BMC Nurs. 2015; 14: 19.
- Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003; 17: 31-50.
- Jorgensen SM, Carnahan RM, Weckmann MT. Validity of the Delirium Observation Screening Scale in Identifying Delirium in Home Hospice Patients. Am J Hosp Palliat Med. 2017; 34: 744-747.
- Neelon VJ, Champagne MT, Carlson JR, Funk SG. The NEECHAM Confusion Scale: Construction, Validation, And Clinical Testing. Nurs Res. 1996; 45: 324-330.
- Gemert van LA, Schuurmans MJ. The Neecham Confusion Scale and the Delirium Observation Screening Scale: Capacity to discriminate and ease of use in clinical practice. BMC Nurs. 2007; 6: 3.
- Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The memorial delirium assessment scale. J Pain Symptom Manage. 1997; 13: 128-137.
- de Jonghe JFM, Kalisvaart KJ, Timmers JFM, Kat MG, Jackson JC. Delirium- O-Meter: a nurses’ rating scale for monitoring delirium severity in geriatric patients. Int J Geriatr Psychiatry. 2005; 20: 1158-1166.
- Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992; 5: 14-21.
- Schuurmans MJ, Deschamps PI, Markham SW, Shortridge-Baggett LM, Duursma SA. The measurement of delirium: review of scales. Res Theory Nurs Pract. 2003; 17: 207-224.
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001; 13: 229–242.
- O’Sullivan R, Meagher D, Leonard M, et al. A comparison of the revised Delirium Rating Scale (DRS-R98) and the Memorial Delirium Assessment Scale (MDAS) in a palliative care cohort with DSM-IV delirium. Palliat Support Care. 2015; 13: 937-944.
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975; 23: 433-441.
- Jitapunkul S, Pillay I, Ebrahim S. The abbreviated mental test: its use and validity. Age Ageing. 1991; 20: 332-336.
- Swain DG, Nightingale PG. Evaluation of a shortened version of the Abbreviated Mental Test in a series of elderly patients. Clin Rehabil. 1997; 11: 243-248.
- Abdel-Aziz K, Larner AJ. Six-item cognitive impairment test (6CIT): pragmatic diagnostic accuracy study for dementia and MCI. Int Psychogeriatr. 2015; 27: 991-997.
- Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009; 43: 411-431.
- Seitz DP, Chan CC, Newton HT, et al. Mini-Cog for the diagnosis of Alzheimer’s disease dementia and other dementias within a primary care setting. Cochrane Database Syst Rev. 2018; 2: CD011415.
- Larner AJ. DemTect: 1-year experience of a neuropsychological screening test for dementia. Age Ageing. 2007; 36: 326-327.
- McLennan SN, Mathias JL, Brennan LC, Stewart S. Validity of the montreal cognitive assessment (MoCA) as a screening test for mild cognitive impairment (MCI) in a cardiovascular population. J Geriatr Psychiatry Neurol. 2011; 24: 33-38.
- O’Caoimh R, Timmons S, Molloy DW. Screening for Mild Cognitive Impairment: Comparison of “MCI Specific” Screening Instruments. J Alzheimers Dis JAD. 2016; 51: 619-629.
- Kim KW, Lee DY, Jhoo JH, et al. Diagnostic Accuracy of Mini-Mental Status Examination and Revised Hasegawa Dementia Scale for Alzheimer’s Disease. Dement Geriatr Cogn Disord. 2005; 19: 324-330.
- Ozer S, Noonan K, Burke M, et al. The validity of the Memory Alteration Test and the Test Your Memory test for community-based identification of amnestic mild cognitive impairment. Alzheimers Dement. 2016; 12: 987-995.
- Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001; 29: 1370-1379.
- Ely EW, Inouye SK, Bernard GR, et al. Delirium in Mechanically Ventilated Patients: Validity and Reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA. 2001; 286: 2703-2710.
- van Eijk MMJ, van Marum RJ, Klijn IAM, de Wit N, Kesecioglu J, Slooter AJC. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009; 37: 1881-1885.
- Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001; 27: 859-864.
- Teale EA, Munyombwe T, Schuurmans M, Siddiqi N, Young J. A prospective observational study to investigate utility of the Delirium Observational Screening Scale (DOSS) to detect delirium in care home residents. Age Ageing. 2018; 47: 56-61.
- Shyamsundar G, Raghuthaman G, Rajkumar AP, Jacob KS. Validation of memorial delirium assessment scale. J Crit Care. 2009; 24: 530-534.
- Erkinjuntti T, Sulkava R, Wikström J, Autio L. Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987; 35: 412-416.
- Malhotra C, Chan A, Matchar D, Seow D, Chuo A, Do YK. Diagnostic performance of short portable mental status questionnaire for screening dementia among patients attending cognitive assessment clinics in Singapore. Ann Acad Med Singapore. 2013; 42: 315-319.
- Schofield I, Stott DJ, Tolson D, McFadyen A, Monaghan J, Nelson D. Screening for cognitive impairment in older people attending accident and emergency using the 4-item Abbreviated Mental Test: Eur J Emerg Med. 2010; 17: 340-342.
- Hessler JB, Schäufele M, Hendlmeier I, et al. The 6-Item Cognitive Impairment Test as a bedside screening for dementia in general hospital patients: results of the General Hospital Study (GHoSt): 6CIT and dementia in the general hospital. Int J Geriatr Psychiatry. 2017; 32: 726-733.