
Case Report
Austin J Gastroenterol. 2024; 11(1): 1128.
Lack of Reactivation of Latent Tuberculosis despite PD-1 Immunotherapy and Chemotherapy: A Case Report and Systematic Review
Xiaojuan Yang¹; XingHong Xian²; Qing Zhu¹; Ke Wang2,3*
1Abdominal Oncology Ward, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
2Department of Pulmonary and Critical care Medicine, West China Hospital, Sichuan University, Chengdu, China
3Lung Cancer Center, West China Hospital, Sichuan University, Chengdu, China
*Corresponding author: Ke Wang Division of Respiratory Medicine, Lung Cancer Center, West China Hospital, Sichuan University, No. 37 Guo Xue Xiang, 610041 Chengdu, China. Tel: +18980602252; Fax: 00862155666857 Email: wang2ke@126.com
Received: December 01, 2023 Accepted: January 04, 2024 Published: January 11, 2024
Abstract
Background: Programmed Death 1 (PD-1) has a controversial role in the treatment of Tuberculosis (TB). Several clinical cases of TB development have been reported by administration of anti–PD-1 antibodies for patients with advanced unresectable cancers.
Case presentation: In this article, we report an interesting case about a male patient with history of cured pulmonary tuberculosis and a 4-month history of repeated hemoptysis. Imaging showed one mass in the lower lobe of left lung and the other in the left upper lobe. The diagnosis of Non-Small-Ccell Lung Cancer (NSCLC) was determined by biopsy specimens in the lower lobe nodule and cured pulmonary tuberculosis determined by past medical history. He was given neoadjuvant treatment with an anti–PD-1 inhibitor and chemotherapy. He underwent surgery. Pathological examination revealed Mtb in left upper lobe nodule but previous imaging showed without reactivation and slight reduction in tubercular infection inflammation. The left lower lobe nodule was poorly differentiated carcinoma.
Conclusions: Overall, the role of immune checkpoint inhibitors in patients with cancers and latent tuberculosis infection is inconclusive. Although we cannot establish whether patients with stage IIIA NSCLC and concurrent latent pulmonary tuberculosis after surgical evaluation can receive a PD-1 and chemotherapy as neoadjuvant therapy without the activation of pulmonary tuberculosis, our case endorses the need of further evaluation.
Keywords: NSCLC; PD-1 inhibitors; reactivation; Mtb
Abbreviations: PD-1: Programmed Death 1; Mtb: Mycobacterium Tuberculosis; NSCLC: Non-Small-Cell Lung Cancer; irAEs: Immune-related Adverse Events; pPR: Part Pathological Rresponse; 18F-FDG PET-CT: 18F-Fluorodeoxyglucose Positron Emission Tomography Combined with Computed Tomography
Background
Approximately 35% of Non-Small Cell Lung Cancer (NSCLC) patients are considered locally advanced non-metastatic disease at the time of diagnosis [1]. After surgical evaluation, patients may be treated with induction chemotherapy as a standard-of-care treatment for cT4N0M0, clinical stage IIIA. A recent report evidenced that neoadjuvant administration of two doses of Programmed Death 1 (PD-1) inhibitors in surgically resectable NSCLC (stage I, II, or IIIA) led to a major pathological response in about half of tumor (45%) without delays in planned surgery [2].
On the other hand, checkpoint inhibitors including PD-1 inhibitors can induce immune-related adverse events (irAEs) which can affect normal tissues [3]. Multiple cases of tuberculosis after immunotherapy have been observed [4-8], but all types of cancer are unresectable, including relapsed and metastatic diseases. Here, we report a stage IIIA NSCLC patient with concurrent pulmonary tuberculosis having a Pathological Part Response (pPR), but without the activation of pulmonary tuberculosis after the neoadjuvant treatment with pembrolizumab and chemotherapy.
Case Presentation
A 60-year old male patient with history of cured pulmonary TB was admitted to West China Hospital of Sichuan University in October 2019. He was a never smoker and with a 4-month history of repeated hemoptysis, but without other relevant clinical details such as fever, chest pain, anorexia, and weight loss. For previous pulmonary TB, he suffered from this disease in 2016 and was cured with a three-drug regimen for one year at an outside institution. The 18F-fluorodeoxyglucose (18F-FDG) Positron Emission Tomography (PET) combined with computed tomography PET-(CT) images showed intense uptake at the mass in the dorsal segment of lower lobe of left lung whose maximum cross section was approximately 6.4×5.3cm (Figure 1A and B] and which invades the apical and posterior segment of the left upper lobe and adjacent pleural (SUVmax 16.21) (Figure 1C). In the remaining lobes of bilateral lungs, there are many solid nodules, streak shadows, patch shadows, consolidation shadows and light transmission areas without lung texture. The nodules are mainly located in the bilateral upper to middle lobes, and most of them are distributed in clusters, with calcification. The largest nodule is located in another part of the apical and posterior segment of the left upper lobe which was measured 1.3×0.8cm, with faint uptake (SUVmax 2.13) (Figure 2A and 2B). 18F-FDG PET/CT scanning demonstrated without lymph node involvement. Unfortunately, the initial investigations for pulmonary TB such as sputum examination for acid-fast bacilli and GeneXpert were not performed. CT-guided, trans-cutaneous fine-needle aspiration was taken on the dorsal segment of lower lobe of left lung nodule. Immunohistochemistry staining revealed that he had a poorly differentiated lung adenocarcinoma, with pan-cytokeratin (PCK) (+) (Figure 3A), cytokeratin (CK) 7 (+) (Figure 3B), Epithelial Membrane Antigen (EMA) (+) (Figure 3C), thyroid transcription factor-1 (TTF-1) (+) (Figure 3D), cytokeratin (CK) 5/6 (-), Desmin (-), S-100 (-), Synaptophysin (Syn) (-), P63 (-), Chromogranin A (CgA) (-), ALK-V (-), ROS-1 (-). After systemic evaluation, he was diagnosed with poorly differentiated adenocarcinoma, cT4N0M0, clinical stage IIIA lung cancer based on the AJCC Cancer Staging Manual, Eighth Edition (2018). Thoracic surgeon suggested neoadjuvant treatment instead of upfront surgery. The patient received two cycles of neoadjuvant treatment with a PD-1 inhibitor pembrolizumab 200 mg (2 mg/Kg) plus carboplatin area under curve 5 mg/mL per min and pemetrexed 500 mg/m2 in cycles of once every 3 weeks (Figure 2E). Subsequently, the PET-CT scans revealed marked reduction of FDG uptake in the previous involved fine-needle aspiration nodule (SUVmax 2.35 compared with 16.21 at the outset) (Figure 1D, E and F) and slight reduction in another nodule (SUVmax 1.66 compared with 2.13 at the outset) (Figure 2C and D). Then he underwent left lower lobectomy and left upper wedge resection with mediastinal lymph node dissection for lung cancer. Pathological examination revealed Mycobacterium TB (Mtb) in left upper lobe nodule and TB A polymerase chain reaction (TB-qPCR) detection Mtb DNA fragments. In addition, positive acid-fast stain was observed in that surgical specimens (Figure 4A). The pathologic specimens in the left lower lobectomy revealed that poorly differentiated carcinoma with massive necrosis areas where there was only remaining 10% tumor tissues (Figure 4B). Circulating tumor cells prior to operation also did not be found (CTCs) in this patient.
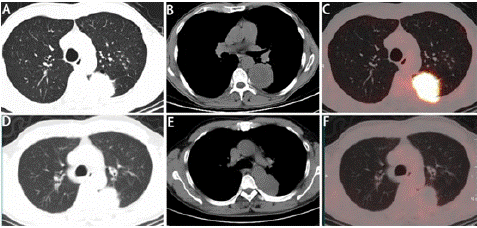
Figure 1: 18F-FDG PET-CT images and response evaluation of the tumor lesion after neoadjuvant treatment with pembrolizumab and chemotherapy. (A and B) CT scan at diagnosis. (C) 18FFDG-PET shows abnormal FDG uptake of the dorsal segment of lower lobe of left lung before neoadjuvant treatment. (D and E) CT scan after two cycles of neoadjuvant treatment. (F) 18FFDG-PET shows marked reduction of FDG uptake in the previous tumor lesion after neoadjuvant treatment. A and D: lung window; B and E mediastinal window.
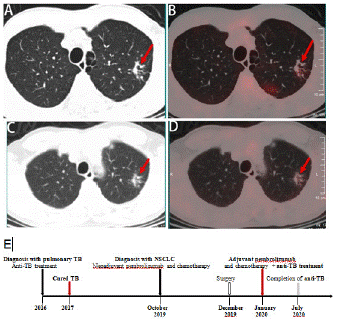
Figure 2: 18F-FDG PET-CT images and response evaluation of the tuberculosis lesion after neoadjuvant treatment with pembrolizumab and chemotherapy (arrow). (A) CT scan at diagnosis (arrow). (B) 18FFDG-PET shows slightly abnormal FDG uptake of the apical and posterior segment of the left upper lobe before neoadjuvant treatment (arrow). (C) CT scan after neoadjuvant treatment (arrow). (D) 18FFDG-PET shows slightly reduction of FDG uptake in the tuberculosis lesion after neoadjuvant treatment (arrow). A and C: lung window. (E) Timeline of therapy and disease status.
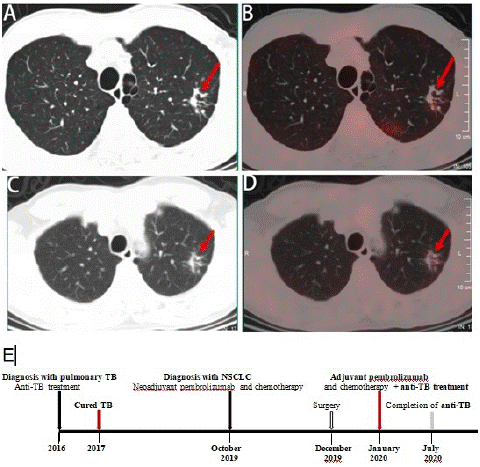
Figure 3: Immunohistopathological analysis of fine-needle aspiration biopsy specimens. (A) pan-cytokeratin (PCK) (+), (B) cytokeratin (CK) 7 (+), (C) epithelial membrane antigen (EMA)(+), (D) thyroid transcription factor-1 (TTF-1) (+). (HE staining, ×200).
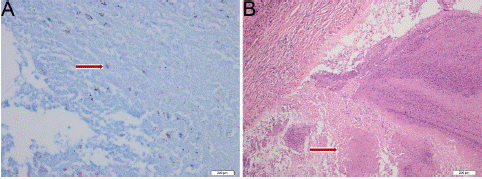
Figure 4: Histopathological findings of examination of the surgical specimens in left upper lobe nodule and left lower lobe nodule. (A) Positive acid-fast stain was observed in left upper lobe specimens (acid-fast stain, ×400). (B) massive necrosis areas in left lower lobe nodule specimens (HE staining, ×100).
After surgery, the patient not only continue receiving adjuvant treatment with the PD-1 blockade pembrolizumab and chemotherapy but also begin receiving antituberculosis treatFigure ments of combined of isoniazid 300mg once daily (QD) and rifampicin 450mg QD and ethambutol 750mg QD for six months. In the last follow-up imaging assessment, there were no signs of recurrence or metastases and no activation of tuberculosis.
Discussion and Conclusions
To the best of our knowledge, the present case report of a patient without the development of active pulmonary TB after the neoadjuvant treatment with anti–PD-1 antibody therapy combined with chemotherapy, is unprecedented in previous clinical reports. Additionally, We assessed the probability of the Adverse Drug Reaction (ADR) being related to pembrolizumab using Naranjo algorithm. A score of -2 indicates a impossible ADR.
It has been reported that seven cases of acute pulmonary tuberculosis during immune checkpoint blockade therapies have been observed. The first reported patient was a 72-year-old man with IV lung cancer and received an anti–PD-1 therapy, nivolumab, as third-line therapy. After eight cycles, active pulmonary tuberculosis developed.4 The second case, which was an 87-year-old Chinese man with repeatedly-relapsed Hodgkin’s lymphoma, was treated with an anti–PD-1 therapy, pembrolizumab (2 mg/kg). After five cycles of pembrolizumab, patients developed fever and unintentional weight loss, and then the culture of sputum yielded Mtb.5 Chu et al. reported a 59-year-old male patient with IV adenocarcinoma of the lung who received nivolumab (3 mg/Kg). After third cycle of nivolumab, rapid accumulation of pericardial effusion was found and then Mtb grew on the pericardial effusion culture.6 Picchi et al. described two cases, one was a 50-year-old male patient who was treated with pembrolizumab for metastatic melanoma. After 4 cycles of treatment, he developed asymptomatic pleurisy and was diagnosed with tuberculosis infection by histology and tuberculin skin test conversion. The other male patient was 64 years old and he was diagnosed with metastatic NSCLC. After 2 cycles of nivolumab, he suffered from spinal cord compression and Mtb grew on bone culture.7 Barber et al. proposed additional two cases, the first patient is a metastatic nasopharyngeal carcinoma patient treated with nivolumab. After three cycles of an anti–PD-1 therapy, he suffered from disseminated pulmonary tuberculosis. The second patient was treated with pembrolizumab for metastatic Merkel cell carcinoma. The culture from his lung specimen grew Mtb after 12th cycles of pembrolizumab [8]. The mechanism of checkpoint blockade–associated tuberculosis is unknown. Reungwetwattana et al. stated two possible reasons [9]. The first hypothesis is immune reconstitution inflammatory syndrome, and that means the rapid restoration of CD4 cells in patients with human immunodeficiency virus-infected after antiretroviral therapy.10 The second hypothesis is lymphopenia caused by drugs, thus may leading to opportunistic infections such as tuberculosis.9 Barber et al. examined Mtb-specific immune responses and found that the increase of interferon-γ-producing Mtb-specific CD4 T cells is related to a tuberculoma developed [8]. As we can see, previous seven patients suffer from recurrent or metastatic cancers, which means higher tumor burden than the present case achieving neoadjuvant treatment. It needs further studies.
However, a recent report demonstrated that immune checkpoint blockade therapies may be a strategy for cure for treating chronic persistent viral infections, including HIV and hepatitis B virus (HBV) [11]. Fujita K et al. suggested that PD-1 inhibitors reactivate cellular immunity (the cytotoxic T cells) to unleash anti-tumor immunity [4]. Jasenosky et al. and Puronen CE et al. also evidenced that T-cell immunity also has a critical role in the control of Mtb infection as well as HIV infection [12,13]. In a previous clinical study, there were seven patients with metastatic NSCLC and HIV infection who were treated with PD-1 inhibitors and the results showed that nivolumab and pembrolizumab can be safe and effective among these patients.14 Given the potential effects of PD-1 inhibitors in patients with NSCLC and HIV infection, presumably, we may have the hypothesis that activating the cellular immunity may have the same potential effects on Mtb infection. Some cell and animal models demonstrated that immunotherapeutic regimens could have positive effects on Mtb infection [15-18]. In one vitro cells of patients model, abrogation of the PD-1 pathway enhances M. tuberculosis–specific in vitro T-Cell proliferation, which was correlated with restoration of protective immunity [18]. Another cell model indicated that PD-L1 blocking had effect on in vitro cytotoxicity against IFN-γ-activated macrophages loaded with Mtb.17 A review also concluded that PD-1 inhibitors might be an attractive strategy to improve host immunity against Mtb.16 Moreover, the combination of the second- and third-line anti-TB drugs with checkpoint inhibition in patients with multidrug-resistant TB (i.e., the resistance in vitro to at least isoniazid and rifampicin) may allow immunity to develop with the reduction of the bacterial load [15]. Additionally, Sakai S et al. indicated that PD-1 deficiency may not exacerbate all mycobacterial infections, as PD-1-/- mice more readily clear Bacillus Calmette-Guérin (BCG). In that study, PD-1-PD-L1 pathway impairs CD4+T-cell response in the late stage of BCG infection [19]. Here, the present case treated with neoadjuvant pembrolizumab did not suffer from active pulmonary tuberculosis, and that means using a PD-1 treatment at an early stage and for short-term use. Further clinical researches are required to elucidate this issue.
Overall, the role of immune checkpoint inhibitors in patients with cancers and latent tuberculosis infection is inconclusive. Although we cannot establish whether patients with stage IIIA NSCLC and concurrent latent pulmonary tuberculosis after surgical evaluation can receive a PD-1 and chemotherapy as neoadjuvant therapy without the activation of pulmonary tuberculosis, our case endorses the need of further evaluation.
Author Statements
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of Sichuan university (Chengdu, China). Written informed consent was obtained from the participant.
Consent for Publication
Written consent was obtained from the patient for publication of this case report and the accompanying images.
Availability of data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
The work was supported by the National Science two Technology Major Projects (grant numbers 81870034 and 82070019). The role of study sponsor was in the interpretation of data and decision to submit the manuscript for publication.
Authors’ Contributions
XJY and KW were the main contributors to drafting the manuscript. KW and QZ contributed in diagnosing the disease, data collection and data analysis. XJY and XHX contributed to literature search, figure preparation. XJY and QZ contributed to study design and performed the final manuscript review. All authors have read and approved the manuscript.
References
- Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010; 28: 2181-2190.
- Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. 2018; 378: 1976-1986.
- Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Cancer. 2016; 99: 148-150.
- Fujita K, Terashima T, Mio T. Anti-PD1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J Thorac Oncol. 2016; 11: 2238-2240.
- Lee JJ, Chan A, Tang T. Tuberculosis reactivation in a patient receiving anti-programmed death-1 (PD-1) inhibitor for relapsed Hodgkin’s lymphoma. Acta Oncol. 2016; 55: 519-520.
- Chu YC, Fang KC, Chen HC, Yeh YC, Tseng CE, Chou TY, et al. Pericardial Tamponade Caused by a Hypersensitivity Response to Tuberculosis Reactivation after Anti-PD-1 Treatment in a Patient with Advanced Pulmonary Adenocarcinoma. J Thorac Oncol. 2017; 12: e111-e114.
- Picchi H, Mateus C, Chouaid C, Besse B, Marabelle A, Michot JM, et al. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clin Microbiol Infect. 2018; 24: 216-218.
- Barber DL, Sakai S, Kudchadkar RR, Fling SP, Day TA, Vergara JA, et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med. 2019; 11: eaat2702.
- Reungwetwattana T, Adjei AA. Anti-PD-1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J Thorac Oncol. 2016; 11: 2048-2050.
- Lapadula G, Soria A, Bandera A, Squillace N, Sabbatini F, Franzetti F, et al. Unmasking tuberculosis in the era of antiretroviral treatment. Eur Respir J. 2012; 39: 1064-1075.
- Shen L, Shi H, Gao Y, Ou Q, Liu Q, Liu Y, et al. The characteristic profiles of PD-1 and PD-L1 expressions and dynamic changes during treatment in active tuberculosis. Tuberculosis. 2016; 101: 146-150.
- Torrelles JB, Sieling PA, Arcos J, Knaup R, Bartling C, Rajaram MVS, et al. Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses. J Biol Chem. 2011; 286: 35438-35446.
- Puronen CE, Ford ES, Uldrick TS. Immunotherapy in People With HIV and Cancer. Front Immunol. 2019; 10: 2060.
- Ostios-Garcia L, Faig J, Leonardi GC, Adeni AE, Subegdjo SJ, Lydon CA, et al. Safety and Efficacy of PD-1 Inhibitors Among HIV-Positive Patients With Non-Small Cell Lung Cancer. J Thorac Oncol. 2018; 13: 1037-1042.
- Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2018; 18: 91-104.
- Ramos-Espinosa O, Islas-Weinstein L, Peralta-Álvarez MP, López-Torres MO, Hernández-Pando R. The use of immunotherapy for the treatment of tuberculosis. Expert Rev Respir Med. 2018; 12: 427-440.
- Jurado JO, Alvarez IB, Pasquinelli V, Martínez GJ, Quiroga MF, Abbate E, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 2008; 181: 116-125.
- Singh A, Mohan A, Dey AB, Mitra DK. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon ?-producing T cells from apoptosis in patients with pulmonary tuberculosis. J Infect Dis. 2013; 208: 603-615.
- Sakai S, Kawamura I, Okazaki T, Tsuchiya K, Uchiyama R, Mitsuyama M. PD-1-PD-L1 pathway impairs T(h)1 immune response in the late stage of infection with Mycobacterium bovis bacillus Calmette-Guérin. Int Immunol. 2010; 22: 915-925.