
Research Article
Austin J Forensic Sci Criminol. 2023; 10(1):
Estimating the Presence of Blood with Reference to Anti-Forensic-A Comparative Study
Gautam K; Soumya G; Joshi R; Rawal R*
Department of Biochemistry and Forensic Sciences, School of Sciences, Gujarat University, India
*Corresponding author: Rakesh Rawal Department of Biochemistry and Forensic Sciences, School of Sciences, Gujarat University, Ahemdabad, India. Email: rakeshrawal.gu@gmail.com
Received: March 30, 2023 Accepted: May 09, 2023 Published: May 16, 2023
Abstract
Number of presumptive tests is there for detection of blood in forensic science. But problems arise in choosing the presumptive test that gives the least false positive result. Difficulties arise due to the small quantity of blood found, so to prevent damage of the sample appropriate reagent is required for the detection of blood. Blood, artificial blood, fake blood, and some false positive-giving substances (including commercial products such as Bleach, Enamel paint, Sauce, and Betadine, chemicals such as copper sulphate, ferrous oxide, and potassium permanganate, vegetables/cereals/fruits such as cauliflower, strawberries, red kidney beans, and rust) were used in these studies. Presumptive tests like Kastle-Meyer, Tetra methyl benzidine, leucomalachite green, BLUESTAR® FORENSIC and Fluorescein test were used. To overcome this problems, comparison of these test reagents are done. In comparison to all other test reagents, Lecomalachite green has the highest accuracy, followed by BLUESTAR® FORENSIC, Fluorescein, Kastle-Meyer, and Tetramethylbenzidine test, which has the lowest accuracy.
Keywords: Presumptive test; Kastle-Meyer; Tetra methyl benzidine; Leucomalachite green; Bluestar® forensic; Fluorescein; Artificial blood
Introduction
Blood is a fluid that transports oxygen and nutrients to the cells while removing carbon dioxide and other waste products from the body. Blood is both a fluid and a tissue. It is a tissue since it is made up of a collection of cells that have been specially developed and have a similar function. These cells are contained in a liquid matrix called plasma, which gives blood its fluid form. If blood flow is interrupted, death will occur in a matter of minutes because highly vulnerable cells are influenced by an unfavourable environment (Pokupcic, 2017) [11]. Blood is the most important trace evidence in forensic analysis [11]. Several colourimetry or presumptive tests, including the Kastle-Meyer test (phenolphthalein), the TMB (tetramethyl benzidine) test, and the LMG (leucomalachite Green) test [1], are accessible because significant volumes of blood may be seen with the naked eye. Little amounts of blood or probable spots where blood has been wiped away as evidence, however, are challenging to spot with the naked eye, hence chemiluminescent or fluorescent reagents are utilised, such as Presumptive tests, which emit colour when a compound reacts chemically and identifies the substance, are a part of qualitative analysis. These presumptive tests operate on the oxidation-reduction reaction theory, which is catalyzed by the haemoglobin heme group [1,9].
RH2 + H2O2R+ 2H2O
The chemistry underlying this reaction is that haemoglobin will catalyze the reaction with the enzyme peroxidase and that all the reagents are in a reductant form, denoted as RH2. H2O2 is an oxidant. Presumptive test reaction happens because hemoglobin speeds up the reaction; it produces colour and is denoted as R, but reductant RH2 is colorless. There have been numerous studies of presumptive tests for blood detection using a variety of test reagents and methods, including on pure cotton, fibers, glasses, tiles, floors, or wood, which is then heated or chemically treated in the case of wood [4,9]. The transport of oxygen and carbon dioxide through blood is the most important function of blood, and artificial blood is a theoretical replacement for blood. Artificial blood might be able to save people's lives, especially in big disasters where there is a lot of blood loss; however, the products that are currently in development are unable to perform blood's secondary functions, such as fighting infections. Contamination, in which organisms that are not actually present in a blood sample are grown in culture, is the cause of false positives. Cases, where false positive tests are considered, are the presumptive tests which are performed for the identification of blood which include the tetramethyl benzidine test (commonly knowns as TMB test). This test utilises a similar methodology to the benzidine test. The underlying concept depends on the peroxidase activity that exists in blood. This activity is seen in the blood's haemoglobin, which degrades the peroxidase and releases an oxygen molecule that reacts with the test regulator, such as TMB, and causes colour changes. It is significant to highlight that other substances can produce the same effects as blood and that "peroxidase-like activity" is a class trait. TMB produces false-positive results for recycled paper, horseradish, garlic, green grapes, and red grapes [7]. The TMB and phenolphthalein both operate according to the same principles. The reagent reacts in this test, also known as the Kastle Meyer test, and the peroxidase activity causes a pink colour to appear [5]. The presence of horseradish, potatoes, turmeric, malt extract, cobalt, manganese, iron, lead, and saliva might lead to false positive test findings. [3] The last component is luminol, a spray that is applied to the suspected region and then examined under an ultraviolet light source. The substance produces a strong blue fluorescence that can only be observed under UV light when combined with the bloodstaining substance haematin. Luminol yields falsely positive findings for copper salts, brass, bronze, and other alloys containing copper. The purpose of the study is to overcome the problem of selecting the appropriate test reagent when a small amount of blood is found as evidence at the scene of a crime that gives the least false positive reaction, So test reagents of the presumptive test for detection of blood, artificial blood, and fake blood, and some falsely positive substances (including commercial products such as bleach, enamel paint, sauce, and betadine; chemicals such as copper sulfate, ferrous oxide, and potassium permanganate; and vegetables, cereals, and fruits such as cauliflower, strawberries, red kidney beans, and rust) were used in the study to compare the test reagents [3].
As an outcome, it was thought that this study would achieve the following aim:
1. To establish the difference between artificial blood and fake blood.
2. In order to determine whether this has an equal impact on all of the presumptive tests.
3. To differentiate between false positive and false negative scenarios in blood examination.
Shouldn't ever anything be compromised with apparent blood stains in every component?
Materials and Methods
Materials
The BLUESTAR® FORENSIC [6] Latent bloodstain reagent (Blue star forensics A-2581), Phenolphthalein solution (John Glaister, n.d.), Tetramethyl benzidine solution [2], LMG (Leucomalachite green), fluorescin solution were use [1].
BLUESTAR® FORENSIC [6] reagent was prepared by adding 1 tablet H2O2 (catalyse) + 1 tablet beige NaOH-Urea (reagent) to 125ml distilled water aand llow it to dissolve for 1-2 minutes by stirring gently (Note: Do not shake the contained) [8].
Kastle-Meyer (Phenolphthalein) [5] reagent was prepared by Stock solution adding Phenolphthalein powder, Potassium hydroxide (KOH), Zinc dust, Distilled water. And Boil the solution till color disappears. (pink to colourless or pale yellow) [1].
TMB (tetramethyl benzidine) [2] reagent was prepared by Stock solution adding TMB reagent and Glacial acetic acid mix. Use warm water or a water bath to warm the solution to dissolve and store in a cool place [2].
Leucomalachite Green [10] (LMG) reagent was prepared by Stock solution adding, Glacial acetic acid, Distilled water, LMG reagent, Zinc dust. Boil the solution until the green color disappears and becomes a pale yellow or white color solution [7].
Fluorescein reagents [14] was prepared by Stock solution adding Fluorescein, Ethanol, Glacial acetic acid, Zinc powder.Immediately after stirring, the solution will become colourless. Wait at least 30 minutes for reduction reaction takes place. The procedure can be sped up by putting the vial in hot water. Losing the cap allows hydrogen to escape that has been created. Although this fluorescein stock solution can be stored over fresh zinc, freshly prepared fluorescein yields the best results. Amber bottles are used to hold these solutions [12].
1. Blood: The blood was collected in EDTA tubes from the same subject (with written consent) and stored at 4 degree Celsius.
2. Artificial blood was prepared by extracting haemoglobin form hen blood. (with ethical Consent was taken).
3. Fake blood was prepared from two chemicals (Potassium thiocyanate and Iron (III) chloride).
4. False positive substances are collected including commercial products (Bleach, Enamel paint, Sause and Betadine) chemicals (copper sulphate, ferrous oxide and potassium permanganate) vegetables/cereals/fruits (cauli flower, Strawberry and red kidney beans) and Rust.
Kastle-Meyer reagent, TMB reagent, and LMG reagent were prepared. Tests were performed by adding stock solutions. In addition, colour formation indicates positive results within 10–20 seconds, whereas no colour change within 10–20 seconds indicates negative results. Spraying the BLUESTAR® FORENSIC reagent on the suspected area produces a blue colour chemiluminescent reaction, indicating a positive reaction. And a stock solution of fluorescein reagents was prepared and take 10ml of stock solution in 20ml of ethanol in a spray bottle, and add 3% H2O2. 10ml H2O2 and 100ml Ethanol to the spray bottle [8]. Spray the working solution to the suspected area, and then make 3% H2O2 visible through an alternative light source at the 425–485nm wave length. The oxidised fluorescein will produce green fluorescence, showing a positive reaction [12]. Artificial blood was prepared from hen’s blood by extracting haemoglobin. The procedure for the formation of artificial blood involves treating blood with carbon monoxide (CO) to convert the oxyhemoglobin (HbO2) to carboxyhemoglobin (HbCO). This process is called a carbonylation reaction. Then, to separate the cells and fluid, centrifuge them at room temperature at 4000rpm for 3 minutes. The supernatant was washed with an equal weight of isotonic saline (0.9% NaCl w/w) and centrifuged at 3000rpm for 10 minutes. The supernatant was discarded, and the supernatant was added to an isotonic saline solution (0.9% NaCl w/w), mixed, and centrifuged at 3000rpm for 10 minutes. The supernatant was discarded, and for hemolysis, 0.01M NaCl and 5mm Tris-HCl buffer at pH 7.4 were used at a ratio of 1:2 (w/w) of RBC-hypotonic solution at 3°C for 24 hours. After hemolysis, the suspension of lysed cells was submitted to heating at 60°C for 30 minutes in a water bath in the dark and then centrifuged at 8000rpm for 15 minutes. The bottom layer was discarded. Dilute the solution in a ratio of 1:1 with RBC-saline solution, water, or buffer, and centrifuge at 8000rpm for 15 minutes. For converting oxyhemoglobin to carboxyhemoglobin or for carbonylation, one needs carbon monoxide. So, in these experiments, Carbon monoxide (CO) was formed by storing carbon dioxide (CO2) in a closed container, which converted carbon dioxide (CO2) to carbon monoxide (CO), as there was not enough oxygen in the container to produce carbon dioxide (CO2) [5].
Results and Discussion
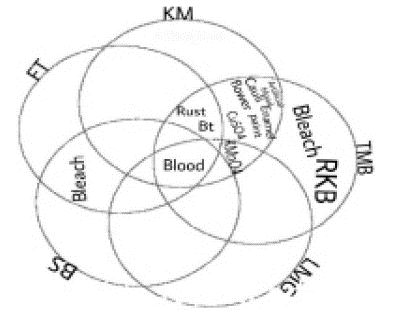
Figure 1: This Venn diagram shows test and substance reactions.
(KM: Kastle-Meyer; TMB: Tetramethylbenzidine; LMG: Leucomalachite Green; BS: BlueStar® Forensic; FT: Fluorescein Tests; Bt: Betadine).
1. Result of human blood with Kastle Meyer, TMB, LMG reagent and fluorescein test.:
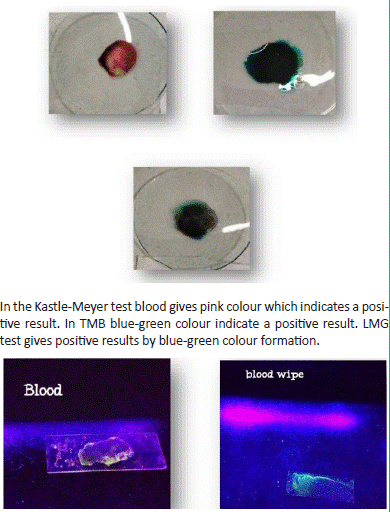
Figure 2: Sample on glass plate and under UV light.
2. Result of enamel paint with Kastle Meyer, TMB, LMG reagent and fluorescein test.

Figure 3: Sample on glass plate and under UV light.
3. Result of cauliflower with Kastle Meyer, TMB, LMG reagent and fluorescein test.
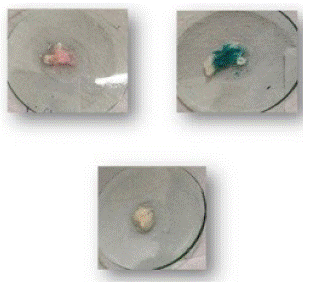
Figure 4: Sample on glass plate.
4. Result of strawberry with Kastle Meyer, TMB and LMG reagent.
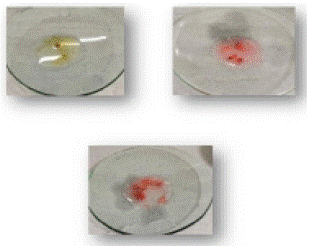
Figure 5: Sample on glass plate.
5. Result of bleach with Kastle Meyer, TMB, LMG reagent and fluorescein test.
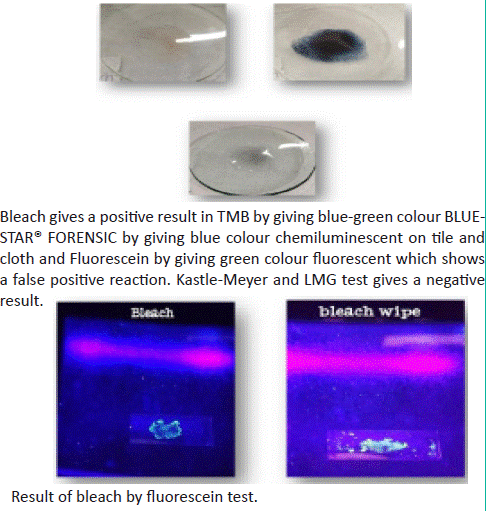
Figure 6: Sample on glass plate and under UV light.
6. Result of enamel paint with Kastle Meyer, TMB, LMG reagent and fluorescein test.
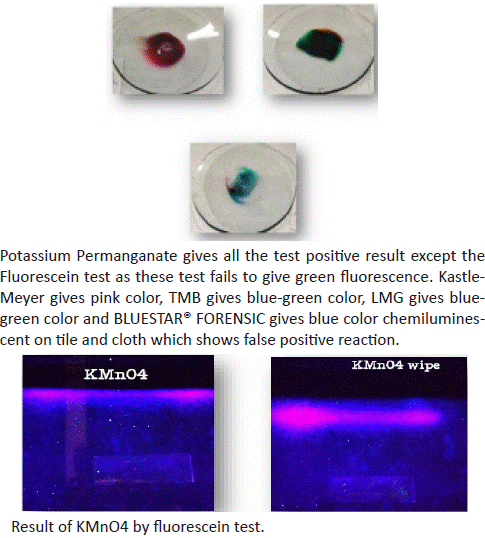
Figure 7: Sample on glass plate and under UV light.
7. In the BLUESTAR@ FORENSIC (luminal) test, the blue colour chemiluminescent indicates a positive result one the cloth and tile.
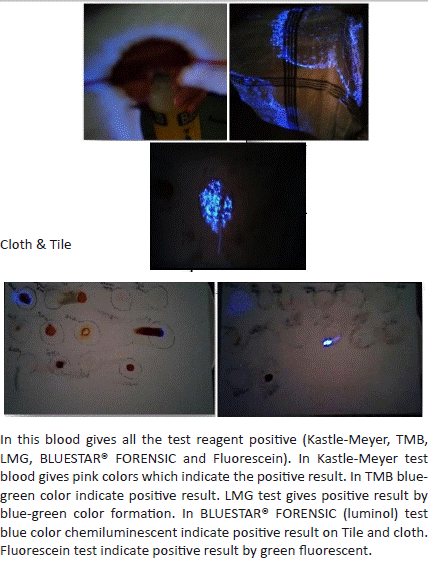
Figure 8: Sample under UV light.
8. Result of fake blood with fluorescein test.
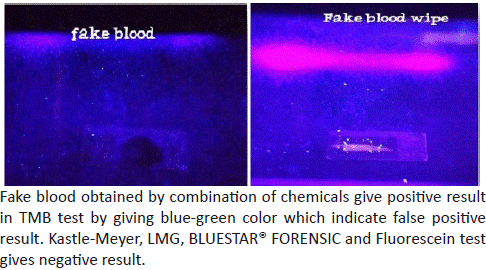
Figure 9: Sample under UV light.
9. Result of artificial blood with fluorescein test.
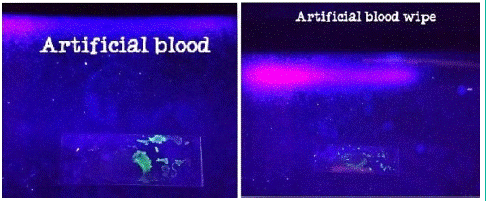
Figure 10: Sample under UV light.
Artificial blood in which hemoglobin extracted from hen’s blood gives positive reaction in Kastle-Meyer test by indication of pink color, TMB test with blue-green color and Fluorescein test by green fluorescent which can be considered as a false positive reaction. LMG test and BLUESTAR® FORENSIC test gives negative as there is no positive indication.
10. Result of copper sulphate with Kastle Meyer, TMB, LMG reagent and fluorescein test.

Figure 11: Sample on glass plate and under UV light.
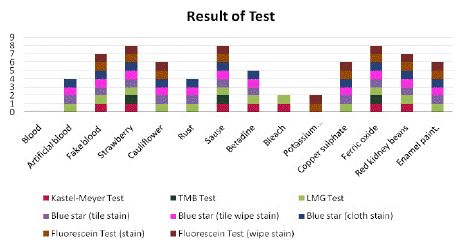
Graph:
Conclusions
In comparison to these five test reagents—Kastle-Meyer, TMB (tetramethylbenzidine), LMG (leucomalachite green), BLUESTAR® Forensic, and Fluorescein—we found that these tests give positive results in blood. Further comparison of these test reagents is done using false-positive-giving substances, and it is found that LMG (leucomalachite green) only produces false-positives for one substance when compared to the other reagents. In comparison to other reagents, BlueStar® Forensic produces just two false positive reactions. The fluorescein test produces four false positives. Six substances are falsely positive in the Kastle-Meyer test. The TMB (tetramethylbenzidine) test results in ten false positives. From the following, we can conclude that the LMG (leucomalachite green) test yields accurate findings, whereas the Kastle-Meyer test reagent yields most of the chemicals positive, indicating a lack of accuracy. In comparison to Kastle-Meyer and TMB, LMG produces accurate results for scrab blood. In comparison to the fluorescein test, the BLUESTAR® forensic test can be used to look for blood in wiped spots that are suspected. But there are some limitations to BLUESTAR® Forensic, including that it can only be visual in dark places.
Author Statements
Author Contribution
Conceptualization: Khushboo Gautam, writing—original draft preparation, Khushboo Gautam and Gv sai soumya.; writing—review and editing Dr. Rakesh Rawal, Khushboo Gautam, Gv sai soumya, Dr. Rushikesh Joshi.; visualization, Dr. Rakesh Rawal. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standards
Ethical consent was obtained from the ethics committee of Gujarat University and statements on informed consent from the volunteers before the collection of blood samples.
Acknowledgement
We Acknowledges and support from the department of biochemistry and forensic Science, Gujarat University
References
- Cox M. A Study of the Sensitivity and Specificity of Four Presumptive Tests for Blood. Journal of Forensic Sciences. 1991; 36: 1503-11.
- Garner DD, Cano KM, Peimer RS, Yeshion TE. An evaluation of tetramethylbenzidine as a presumptive test for blood. Journal of Forensic Sciences. 1976; 21: 816-21.
- Foley MM, Brown CO, Westring CG, Danielson PB, Mc Kiernan HE. Effects of organic acids and common household products on the occurrence of false positive test results using immunochromatographic assays. Forensic Science International. 2020; 308: 110165.
- Jillian Vaughan. Effects of Bluestar on the Kastle-Meyer Presumptive Test for Blood. Office of Justice Programs. 2023; 61: 38-49.
- John Glaister. The Kastle-Meyer Test for the Detection of Blood—PMC. 2023.
- Katherine Martin. Ada County Sheriff’s KM, Ada County Sheriff’s. North west association of forensic scientists. 2019; 44.
- Lee H, Park MJ, Sun SH, Choi DH, Lee YH, et al. Ascorbic acid and vitamin C-containing beverages delay the leucomalachite green reaction to detect latent bloodstains. Legal Medicine (Tokyo, Japan). 2016; 23: 79–85.
- Luedeke M, Miller E, Sprague JE. Technical note: The effects of Bluestar(®) and luminol when used in conjunction with tetramethylbenzidine or phenolphthalein. Forensic Science International. 2016; 262: 156–159.
- Marielle Vennemann, Georgina Scott, Lynn Curran, Felix Bittner, Shanan S Tobe. Sensitivity and specificity of presumptive tests for blood, saliva and semen—PubMed. 2023.
- Noor S Alenazy, Ahmed M Refaat, Saranya R Babu. Comparison of the effects of two presumptive test reagents on the ability to obtain STR profiles from minute bloodstains. Science Direct. 2015; 5: 103-108.
- Raymond MA, Smith ER, Liesegang J. The physical properties of blood—Forensic considerations. Science & Justice: Journal of the Forensic Science Society. 1996; 36: 153–160.
- Ricardo Tomboc. The Fluorescein Method of Latent Blood Detection. 2023.
- Seashols SJ, Cross HD, Shrader DL, Rief A. A comparison of chemical enhancements for the detection of latent blood. Journal of Forensic Sciences. 2013; 58: 130–133.
- Thorogate R, Moreira JCS, Jickells S, Miele MMP, Daniel B. A novel fluorescence-based method in forensic science for the detection of blood in situ. Forensic Science International: Genetics. 2008; 2: 363–371.