Abstract
Background: Disparities exist in both health and health care. Differences in the quality of care received by individuals may result in differential outcomes, including increased prevalence of disease or decreased life expectancy.
Purpose: The purpose of this paper is to describe successful quality collaborative efforts in cardiovascular medicine that demonstrate the impact of creating high quality systems to standardize care, reduce disparities, and improve patient outcomes.
Methods: Six cardiovascular collaboratives within Michigan were reviewed based on their success, innovation, and contributions to knowledge and practice.
Conclusion: Equitably improving care can help to eliminate disparities. Providers, researchers, thought leaders, and institutions must work together. Monitoring outcomes and benchmarking practice while embedding evidencebased guidelines into systems, is one way to improve care quality for all.
Keywords: Access to care, Disparities; Evidence-based medicine; Minority health; Quality of care; Health care Management; Quality management
Abbreviations
CVD: Cardiovascular Disease; CABG: Coronary Artery Bypasses Graft; PCI: Percutaneous Coronary Intervention; BMC2: Blue Cross Blue Shield of Michigan Cardiovascular Consortium; AMI: Acute Myocardial Infarction; MRCD: Maximum Radiographic Contrast Dose; MSTCVS: Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative; STS: National Society of Thoracic Surgeons; ACS: Acute Coronary Syndrome; GRACE: Global Registry of Acute Coronary Events; AAA: Abdominal Aortic Aneurysm; ICD9- CM: International Classification of Diseases, Ninth Revision, Clinical Modification; GAP-MI: American College of Cardiology Guidelines Applied in Practice Acute Myocardial Infarction Initiative; GAPHF: Mid-Michigan Guidelines Applied in Practice Heart Failure Initiative; MEDPAR: Medicare Provider Analysis and Review
Introduction
Cardiovascular Disease (CVD) is the leading cause of death in the United States with disproportionately high rates among minorities [1]. Heightened awareness and technological advances are leading the war toward eliminating such disparities. Equitably improving care quality may further close this gap, but to do so at a lower cost is the challenge for this generation. This review describes the collaborative efforts of six cardiovascular consortiums. These collaboratives translated their research on access, quality, and biology to the bedside through standardizing quality improvements. This research ultimately reduced both health (outcomes) and health care (management) disparities.
Background
Defining disparities
Disparities are unequal differences between groups and are often perceived on a continuum from unintentional to discriminatory [2]. In particular, the term “racial disparity” is highly debated among both researchers and policy makers. For example, interpretations of significant racial differences may range from a phenomenon of interest [3] to being considered representative of unfair and unjust availability and access to care [4] or simply as a result of other differences not yet defined [5]. Within this review a health care disparity is defined as a difference in the quality of care received by individuals, and the associated health outcomes.
Disparities in cardiovascular disease
It is well known that striking disparities exist in CVD, its management, and outcomes; where hypertension, diabetes, and obesity disproportionately affect minorities [6]. CVD accounts for one third of the difference in life expectancy between blacks and whites [7]. The age-adjusted death rate for blacks is 22% greater than whites [8]. Whites tend to use more healthcare services and receive more inappropriate procedures, while blacks are more likely to refuse treatment [9]. Specific to CVD, white Medicare patients are four times more likely to receive coronary artery bypass graft (CABG) surgery than blacks [10]. Blacks are less likely to receive thrombolytic therapy or PCI when they present with chest pain [11]. More myocardial infarctions occur in American Indians/Alaskan Natives under 65 years of age (36%)than in the general population [8,12]. Even when adjustments are made for socioeconomic status and education these differences persist [13]. Clearly standardized evidence-based that are racially competent are needed. The purpose of this paper is to describe successful quality collaboratives in cardiovascular medicine that demonstrate the impact of creating high quality systems to standardize care, reduce disparities, and improve patient outcomes.
Methods
Two types of collaborative efforts are presented in this review. The first set engaged regional thought leaders to develop, monitor, and benchmark local practice against national and international registries. The second are interventional projects that demonstrate the power of regional consortiums translating evidence-based practice to the bedside.
Regional collaborative registries
Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2): BMC2 is a multicenter registry for tracking outcomes of patients who underwent percutaneous coronary intervention (PCI). BMC2 began in 1996 with the goal of improving care for PCI patients in Michigan [14]. This registry underwent rigorous auditing and contains data on consecutive patients [15]. The design and goals of the BMC2 consortium began with eight hospitals (academic and nonacademic centers). Detailed descriptions of the structure and function of BMC2 have been previously published [14,15]. Briefly, the structure includes an executive committee, a working group, and a writing committee each made of physicians, administrators, and nurse coordinators from individual sites. Together these groups provided oversight, top down buy-in, quality assurance, and promoted scholarly efforts [14, 15]. Significant contributions from BMC2 include the creation of a prediction tool for estimating in-hospital mortality post-PCI, an algorithm for determining the appropriate administration of blood products post-PCI, and a calculation tool for the maximum radiographic contrast dose for patients undergoing PCI. The simple additive bedside prediction tool for estimating inhospital mortality post-PCI allows patients, families, and providers to make more informed decisions [16]. The algorithm for judicious use of blood products promotes standardized transfusion practices among providers treating patients with post-AMI/PCI anemia [17,18]. The Maximum Radiographic Contrast Dose (MRCD) is the first tool available to help providers avoid contrast-induced nephropathy requiring dialysis post-PCI [19]. The contrast dye used during PCI can cause kidney damage that necessitates dialysis. Though this is a rare complication it is serious problem and affects an unacceptably high percentage of patients [19].
Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS): The MSTCVS is an interdisciplinary state society of Cardiac and Thoracic Surgeons perfusionists, cardiac care teams, and data managers. Over 10 years, the MSTCVS has evolved into a robust quality collaborative whose purpose is to enhance the quality, care, and outcomes of all cardiac surgery patients in Michigan. This collaborative is partially funded by Blue Cross Blue Shield of Michigan and active participation is required to receive funding. Presently, all 33 Michigan hospitals that perform adult cardiac surgery in Michigan are represented [20].
The MSTCVS uses validated outcomes data from their regional database and the National Society of Thoracic Surgeons (STS) database [21], to design and implement changes throughout the state [20]. Two of the recent successes of the MSTCVS were in increasing the utilization of the internal mammary artery for coronary artery bypass grafting (CABG) and the reduction of prolonged ventilation post CABG. On average, use of the internal mammary artery during CABG is associated with improved survival. The MSTCVS designed an educational series, and implemented an ownership environment to improve utilization of the internal mammary artery. All sites, with an emphasis on low-utilization sites, received seminars on the rational for utilization and surgeons were required to submit a justification for cases where the internal mammary artery was not used. Similarly, the MSTCVS identified variations in ventilator dependency following cardiac surgery. Prolonged ventilation is associated with poorer outcomes and increases cost by approximately $30,000 per case [21]. Hospitals with higher than average ventilator times were identified with unblended data at quarterly meetings where candid discussions on the issue took place. The group acknowledged system and resource barriers that contributed to these variations and worked with outlier hospitals to improve their performance.
Acute Coronary Syndrome Registry (ACS), University of Michigan: The University of Michigan maintains a consecutive registry of all ACS patients. Similar to the goals of the Global Registry of Acute Coronary Events (GRACE) [22], the goal of the ACS registry is to improve the quality of care received by ACS patients at the University of Michigan. The registry contains demographic, co-morbidity, clinical presentation, and treatment data similar to the GRACE registry. The ACS registry has been used to describe differences and relationships between patient characteristics, treatment practices, and outcomes [23].
Vascular surgeons collaborative research: Not formally a consortium, this group represents a more traditional type of collaborative effort among an exceptional team of vascular surgeons. This group came together to investigate how access to care influences outcomes for patients with abdominal aortic aneurysms (AAAs). It is well known that blacks have higher mortality than whites from AAA [24]. Using the National Inpatient Sample from 1995 to 2000, data was abstracted on patients with ICD9-CM codes for AAA who were less than 65 years of age (to exclude access to Medicare) [25]. A follow-up study addressing factors predicting racial variation in mortality was undertaken using the Medicare Provider Analysis and Review from 2001 to 2006 [24].
Regional collaborative interventional projects
American College of Cardiology Guidelines Applied in Practice Acute Myocardial Infarction Initiative (GAP-MI): The GAP-MI program was a prospective interventional study, funded by the American College of Cardiology, to evaluate the change in quality of care and outcomes when national guidelines were systematically applied in practice. The first guidelines for the management of acute myocardial infarction (AMI) were published in 1996 [26]. Despite evidence supporting improved quality and outcomes from the use of these guidelines, application remains low and inconsistent; especially among Medicare and Medicaid patients, women, and elderly [27, 28]. The GAP-MI project together with the Michigan Peer Review Organization sought to integrate and measure the utilization of AMI guidelines in practice [26, 29]. The infrastructure of this program included members from the American College of Cardiology, Michigan Peer Review Organization, and the Southeast Michigan Quality Forum for Cardiovascular Care. This consortium was a unique collaborative that carried considerable clinical expertise and credibility. A project oversight team, responsible for the intervention, was selected from the above groups and a core group of physician and nurse champions were appointed to facilitate the intervention [26, 29].
Within the Southeast Michigan consortium, 32 hospitals participated in three separate studies. Ten hospitals were initially selected as intervention sites. Cases were identified using ICD-9-CM code for AMI [26, 29]. Baseline and study data was collected from control and intervention sites between July 1, 1998 and December 15, 2000. In accordance with ethical practice, control sites continued to make quality improvements as part of their usual operations [26, 29].
The core of the GAP-MI program consisted of customized tool kits designed to enhance utilization of the National guidelines. The kit included preprinted admission and discharge order sets (including a physician-patient discharge contract), pocket guides for physicians, clinical pathways for nurses, and educational materials for patients [26, 29]. Intervention sites received chart stickers and performance charts.
Mid-Michigan Guidelines Applied in Practice Heart Failure Initiative (GAP-HF): The main objective of GAP-HF was to determine the effect of heart failure guideline adherence on quality of care, mortality, and rehospitalization [30]. The GAP-HF initiative was an expansion of the original multifaceted GAP-MI project redesigned for heart failure patients. The collaboration included members from the American College of Cardiology, Michigan Peer Review Organization, the Greater Flint Health Coalition, Michigan Cardiovascular Outcomes Research and Reporting Program, and local physician leaders. Fourteen mid-Michigan hospitals participated in this program (8 interventional, 6 control) [23]. The sampling procedure for this intervention mirrored the GAP-MI project using ICD9-CM codes for heart failure in place of AMI. The intervention was also based on the GAP-MI program employing the American College of Cardiology guidelines for heart failure instead.
Results
Blue cross blue shield of michigan cardiovascular consortium (BMC2)
Today the BMC2 registry has data on more than 260,000 PCIs from more than 44 hospitals throughout Michigan (A. Jensen, personal communication, March 2, 2011). In 2001, the Simple Bedside Additive Tool for Prediction of In-hospital Mortality post- PCI was published from a sample of 10,796 consecutive procedures [16]. AMI, shock, renal insufficiency, cardiac arrest, number of diseased vessels, greater than 70 years of age, low ejection fraction, thrombus, peripheral vascular disease, and gender were identified as independent predictors of mortality in this population. The model demonstrated excellent discrimination with an area under the ROC curve of 0.90. The model was further validated in a sample of 5,863 consecutive cases with similar discrimination (area under the ROC = 0.92). In 1998 BMC2 established a sample of 4,623 patients with post-PCI anemia from a review of 67,051 consecutive PCIs [17]. Blood transfusions studied at one institution were associated with a higher risk of in-hospital mortality (OR = 2.02, 95% CI 1.47-2.79, p <0.001). After cardiology adopted the transfusion algorithm for the appropriate usage of blood products the transfusion rate at the targeted hospital decreased by nearly 66%, and was lower than the mean of the consortium by 2000 [18].
Intervention to reduce the incidence of renal nephropathy requiring dialysis post-PCI was conducted on 10,592 consecutive PCI patients. A follow-up validation study was later conducted on a sample of 5,863 patients. In the primary study in-hospital mortality for patients with nephropathy-requiring dialysis was higher than for those without this complication (39.0% vs. 1.4%, P <0.001). Multivariate analysis revealed five independent risk factors for nephropathy requiring dialysis: peripheral vascular disease, diabetes, pre-procedure renal insufficiency, heart failure, and cardiogenic shock (c-statistic = .89). However, exceeding the MCRD was the strongest predictor of nephropathy requiring dialysis (OR=6.2, 95% CI =3.0-12.8). The risks of nephropathy-requiring dialysis and in-hospital mortality were higher for patients whose contrast load exceeded that recommended by the MCRD (nephropathy 2.4% vs. 0.2%, p <0.001; in-hospital mortality 4.8% vs. 1.1%, p < 0.001) [19]. Following a statewide adoption of the MCRD there was a 57% reduction in nephropathy-requiring dialysis in post-PCI patients (Figure 1). Cumulative evaluation of BMC2 demonstrates global success in reducing procedural complications and negative outcomes (Figure 2) [31].
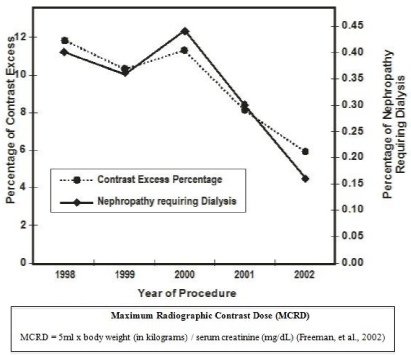
Figure 1: From BMC2, the percentage of patients exceeding the maxium
weight and creatinine-adjusted contrast dose, and percentage of patients
developing nephropathy requiring dialysis, pre and post introduction of the
MCRD (1997-2000). Adapted from “Nephropathy requiring dialysis after
percutaneous coronary intervention and the critical role of an adjusted
contrast dose” [19].
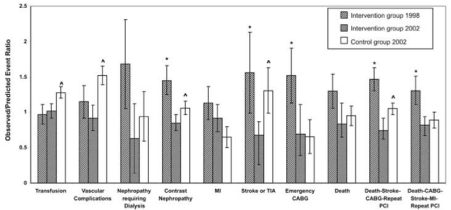
Figure 2: From BMC2, Standardized events ratios (SERs) in the
preintevention period (diagonal bars: 1998) and in the postintervention
period (grid bars; 2002) in the intervention group. The open bars represent
standardized event ratios in 2002 in the control group.*Significant difference
in preintervention and postintervention SERs. Significants SER differences
in postintervention 2002 patients vs. Control 2002 potions. Adapted from”
Association of a continuous quality improvement initiative with practice and
out variations of contemporary percutaneous coronary interventions [31].
Michigan society of thoracic and cardiovascular surgeons quality collaborative (MSTCVS)
The MSTCVS has access to more than 125,000 cardiac surgery patients from their own database and the STS (Prager, personal communication, March 3, 2011). In 2005 usage of the internal mammary artery for CABG varied 60% to 90% across Michigan programs [20]. Following MSTCVS interventions the percentage of outlier hospitals using the internal mammary artery increased from 82% to 93% between 2005 and 2008 [20]. Since 2007, Michigan has exceeded the national average for this procedure (Figure 3).
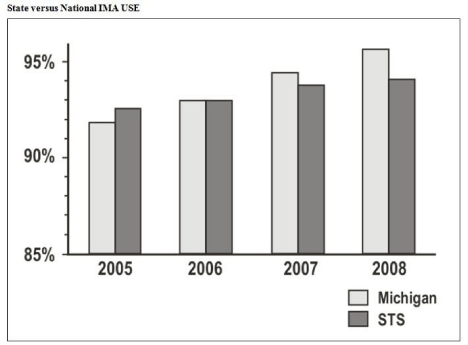
Figure 3: From MSTCVS, Michigan compared to the STS on rates of internal
mammary artery usage. Adapted from “Cardiac surgeons and the quality
movement. The Michigan Experience [20].
The reduction in ventilator dependency after CABG at outlier sites is another accomplishment for the MSTCVS. Following intervention, the range of prolonged ventilation rates at outlier hospitals decreased from 13.7% -36% in 2005 to 11.2%-15.2% in 2008. Thus dropping the state average from 19% in 2005 to 13.8% (p = 0.0004) in 2007 [20].
Acute coronary syndrome registry (ACS), University of Michigan
The ACS registry has more than 4100 consecutive admissions with two-year follow-up [23]. Analyses from this registry have contributed to the identification of quantifiable differences between men and women with ACS. Men present with AMIs at younger ages than women (62.0 years vs. 67.0 years, p < 0.001), and are more likely to be smokers (67.7% vs. 49.4%, p < 0.001). Women have more hypertension (78.4% vs. 65.0%, p< 0.001), higher GRACE risk scores for in-hospital mortality (112.0 vs. 99.4, p <0.001), and higher postdischarge mortality at 6 months (133.3 vs. 122.9, p <0.001) than men. Women are less likely to receive PCIs (44% vs. 60%, p <0.001) are 1.7 times more likely to have an adverse event, and 1.9 times more likely to die in-hospital than men. However, after adjusting for the severity of the event (GRACE risk score) and treatment disparities (PCI) these differences were eliminated. Moreover, an analysis of coronary angiograms comparing men and women showed that most of the apparent disparity in PCI utilization is explained by differing biology. Twice as many women (12%) had minor or no coronary blockages at cardiac catheterization than men (6.1%) [32]. The key to this observation is that PCI is not indicated (or helpful) when the degree of residual coronary narrowing after an ACS event is minor or nil.
Vascular surgeons collaborative research
A sample of 5,363 patients was used to evaluate the relationship between payer status and outcomes of AAA repair. This study revealed that AAA rupture was more likely for uninsured individuals and Medicaid recipients than for those with private insurance (36% vs. 18% vs. 13%, p < 0.001; (Figure 4). Patients without insurance were 2.3 times more likely to rupture than those with private insurance. Operative mortality for the uninsured and Medicaid populations were higher than for those with private insurance (45.3% vs. 31.3% vs. 26.2%, p < 0.001) [25].
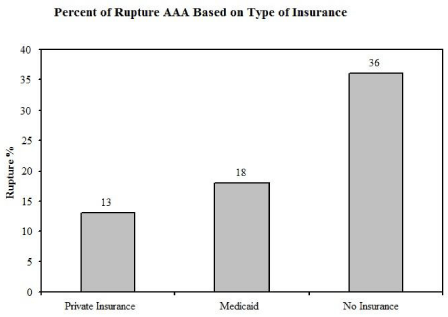
Figure 4: Patients without insurance present more often with ruptured AAA
than those with insurance. Adapted from payer status is related to differences
in access and outcomes of abdominal aortic aneurysm repair in the United
States [25].
In the MEDPAR follow-up study, data from 160,785 patients were used to examine the relationship between race and mortality. Black patients were found to have a 36% higher adjusted mortality rate after surgical repair than non-black patients. Mortality rates were greater in hospitals that treated more black patients (OR=1.19, 95% CI 1.01-1.40). Nearly one third of this difference was explained by comorbidities (29%) and another third by socioeconomic status (26%). When these factors and the use of endovascular repair (6%) were taken into consideration, 25% of the mortality was attributed to receiving care in lower quality hospitals [24].
American College of Cardiology Guidelines Applied in Practice Acute Myocardial Infarction Initiative (GAP-MI)
In total, 2,857 patients were enrolled in the GAP-MI initiative (1,368 controls, 1,489 intervention) [26, 29]. Patients who received the GAP-MI intervention had lower in-hospital mortality (10.4 vs. 13.6, p <0.017), 30-day mortality (16.7 vs. 21.6, p <0.001) and mortality at 1-year (33.2 vs. 38.3, p <0.004; (Figure 5) [33]. Further studies confirmed that utilization of the GAP tools was responsible for these findings [29]. Evidence-based guideline adherence for in-hospital and discharge medical therapy was higher for GAP-MI participants. When the GAP-MI standardize discharge tools were used in practice, patients were less likely to die at 30 days (OR = 0.52, 95% CI 0.27-0.98, p <0.042) or 1 year post-discharge (OR = 0.53, 95% CI 0.36-0.76, p <0.0006) regardless of race or gender [34].
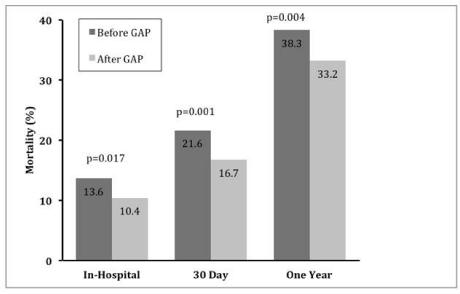
Figure 5: Reduction in mortality for Medicare patients enrolled in the GAPMI
study. Adapted from “An organizational frame work for the AMI ACC_
GAP(TM). Project” [33].
Mid-Michigan guidelines applied in practice heart failure initiative (GAP-HF)
The GAP-HF Initiative included 1,806 controls, and 1,822 participants; 23% were black. This study found that blacks had an overall lower mortality rate from heart failure than whites post discharge at 30 days (2.9 vs. 10.0, p < 0.001), 60 days (5.1 vs. 14.5, p < 0.001), 90 days (6.7 vs. 17.1, p < 0.001), and 180 days (11.0 vs. 23.8, p < 0.001; (Figure 6) [30, 35]. Furthermore, blacks were more often discharged on beta-blockers than whites (beta-blocker: 75.2% vs. 66.5%, p <0.005). There was a trend toward more use of ace-inhibitors (or angiotensin receptor blockers) and aldosterone inhibitors in blacks than whites (Ace-Inhibitors/Angiotensin receptor blockers: 80.2% vs. 77.8%, p <0.211; Aldosterone inhibitors: 55.6% vs. 47.6%, p <0.090). The only measure that white heart failure patients received more evidence-based care was preventative. Whites were more likely to have received a pneumococcal vaccine before discharge than blacks (53.4% vs. 48.2%, p <0.017). The adjusted odds ratios for mortality between black and white heart failure patients ranged from .36 to .48 at 30, 60, 90, and 180 days post-discharge; indicating that blacks have better survival than whites. Some of this difference may be attributable to baseline differences. Blacks admitted with heart failure were younger than whites (64.1 vs. 76.8 years), less likely to have coronary disease (54% vs. 70%), and were more likely to have a history of hypertension (88% vs. 79%) [35].
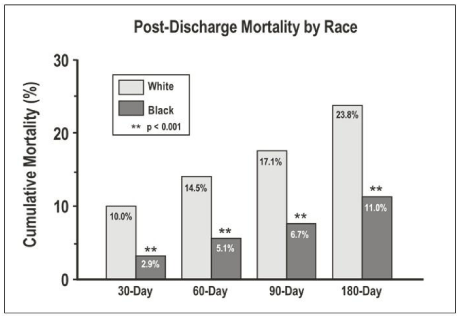
Figure 6: From the Mid-Michigan GAP-HF, post-discharge racial differences
in mortality at 30, 60, 90, and 180 days. Adapted from “African Americans
with heart failure are more likely to receive evidence based medications and
have lower risk of mortality in the Mid-Michigan Guidelines Applied in practice
for Heart Failure study [35].
Discussion
Disparities are multifactorial and often presume discrimination. This article challenges that concept, and proposes that disparities represent opportunities for improvement in hospital systems, access to quality care, and an ongoing pursuit to understand the pathophysiology of CVD. Unfortunately, social, political, and economic forces heavily regulate these focus areas. Michigan however, has repeatedly demonstrated that collaborative efforts to standardize care and quality for all does reduce disparities in both management and outcomes.
Access
The differential presentation of AAA between those with and without insurance is concerning and suggest that the current health care system fails to provide unbiased screening for asymptomatic aneurysm, or ready access for symptomatic aneurysms, or both. With regular access to care AAA’s can effectively be managed before rupture. However, those without insurance seemed to only present when their lives were threatened. Patients without insurance may choose to delay, physicians may delay testing or surgical referrals, and hospital systems may have additional obstacles for the uninsured [36]. When these variables were controlled for the single largest factor affecting outcomes was where you received care [37]. Both BMC2 and the MSTCVS confirmed that where you receive care impacts your outcome. Through continuous outcomes monitoring and measuring institutional variances in practice, outlier hospitals were identified in each collaborative and targeted for improvement. BMC2 effectively identified and implemented evidence-based practices to address high rates of blood transfusions and nephropathy requiring dialysis at targeted institutions. The MSTCVS was equally successful, within their consortium, at increasing the use of the internal mammary artery for CABG surgery and reducing the incidence of prolonged ventilation after surgery.
Systems
Health care systems are a primary domain for incubating disparities, and because where you go for care is paramount, care should be standardized across all systems. BMC2, MSTCVS, ACS, and the GAP programs enhanced quality of care for all patients, regardless of gender, race, or ethnicity, by affecting change within their systems. These exemplars of regional collaborative efforts standardized care and in doing so heralded in new standards of practice.
The MSTCVS approach and BMC2 share many similarities. One notable difference is the candid forum format of the MSTCVS, where unblinded data is presented to the consortium of peers for discussion and problem solving. This frank approach has been instrumental at identifying barriers to changing practice. MSTCVS has lead Michigan to exceed the nation in many quality measures for cardiac surgery through mandatory-reporting, development, and dissemination of best-practices [38].
The GAP-MI and GAP-HF projects are examples of successful regional quality intervention programs. The GAP-MI initiative demonstrated that embedding systems within institutions to promote and ensure utilization of evidence-based guidelines positively influences outcomes in AMI and heart failure [34]. The overall impact of these projects was demonstrated in the reduction of in-hospital and post-discharge mortality rates at institutions where the tools were used. Most importantly for AMI patients, these findings were regardless of race or gender and support the “racial competence” of the national guidelines [40]. Increasingly programs like GAP and “Get with the Guidelines” are reinforcing standardized of practice.
Etiology
Despite the overall success of the GAP programs, the GAP-HF initiative had the controversial, albeit not entirely novel, finding that blacks with heart failure survive better than whites. This “reverse disparity” is counterintuitive given the traditional disparities observed in cardiovascular care. The reason for blacks having lower mortality post-discharge with heart failure is not entirely clear. Multiple etiologic mechanisms for this variability in disease progression are plausible. Severity and underlying pathophysiology of heart failure may differ across racial groups. For example, degree of systolic impairment is one of the strongest predictors of death in heart failure patients. Preserved systolic function (i.e. patients with more diastolic disease) is associated with longer survival. Blacks tend to have more diastolic disease than systolic disease compared to whites; perhaps related to the greater incidence of long-standing hypertension [39- 44].
Further, epidemiological studies suggest a crossover in heart failure mortality risks between blacks and whites at about age 65. Blacks have a higher mortality risk than whites before age 65 but a lower mortality risk than white patients after 65 years [44]. Findings of racial differences in mortality may suggest a survivor effect, whereby black patients who survive to age 65 years with heart failure represent a healthier cohort than white patients of the same age. There may be other yet unmeasured biological differences that may account for the survival advantage of black patients. While GAP-HF has taught us to be mindful of our assumptions, the ACS registry reminds us to be conscious of confounding variables that can lead to incomplete conclusions. Consistent with other studies, initial findings from ACS confirmed that women fared worse and had fewer interventions than men after an AMI. This treatment difference (PCIs) was biologically justified because women more often presented with small vessel disease where PCI would not provide any additional benefit. After adjusting for treatment differences there were no differences in mortality between men and women.
Conclusion
All of the collaborative efforts presented in this paper demonstrate the value of providers, researchers, thought leaders, and institutions working together. Champions from each of these realms are essential to disseminate best practice models, with top-down buy-in to ensure the availability of resources. Institutions are being challenged to monitor outcomes and to benchmark their practice against national data. Regional quality care collaboratives take many forms and they are one solution to satisfy this demand. When the level of care is raised for all at an institutional level, it will no longer matter where you seek care.
Acknowledgement
The authors would like to thank Shireesha Bottu, MD, Saman Kandola, MD, Cydni Smith, MPH, and Rachel Sylvester, BS, for their contributions to this project.
References
- American Heart Association. Heart disease and stroke statistics-2009.
- Hebert P, Sisk J, Howell E. When does a difference become a disparity? Conceptualizing racial and ethnic disparities in health. Health Affairs. 2008; 27: 374-382.
- Rockville MD. Agency for Healthcare Research and Quality. Key Themes and Highlights from the National Healthcare Disparities Report; 2007.
- Whitehead M. The concepts and principles of equity and health. Health Promotion International.1991;6: 217-228.
- Betancourt JR. Eliminating racial and ethnic disparities in health care: what is the role of academic medicine? Academic medicine: journal of the Association of American Medical Colleges. 2006; 81: 788-792.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart Disease and Stroke Statistics-2014 Update: A Report From the American Heart Association. Circulation. 2013 ;18.
- Chang MH, Moonesinghe R, Athar HM, Truman BI. Trends in Disparity by Sex and Race/Ethnicity for the Leading Causes of Death in the United States-1999-2010. Journal of public health management and practice: JPHMP. 2015.
- American Heart Association. Health disparities fact sheet. 2009.
- Hayes SL, Riley P, Radley DC, McCarthy D. Closing the Gap: Past Performance of Health Insurance in Reducing Racial and Ethnic Disparities in Access to Care Could Be an Indication of Future Results. Issue brief (Commonwealth Fund). 2015; 5:1-11.
- Hravnak M, Ibrahim S, Kaufer A, Sonel A, Conigliaro J. Racial disparities in outcomes following coronary artery bypass grafting. The Journal of cardiovascular nursing. 2006; 21: 367-378.
- Nelson A. Unequal treatment: report of the Institute of Medicine on racial and ethnic disparities in healthcare. The Annals of Thoracic Surgery. 2003; 76: S1377-1381.
- Graham G. Population-based approaches to understanding disparities in cardiovascular disease risk in the United States. International journal of general medicine. 2014; 7: 393-400.
- Smedley BD, Stith AY, Nelson AR, editors. Unequal treatment: confronting racial and ethnic disparities in health care?. Washington: National Academies Press; 2003.
- Moscucci M, Share D, Kline-Rogers E, O'Donnell M, Maxwell-Eward A, Meengs WL, et al. The Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) collaborative quality improvement initiative in percutaneous coronary interventions. Journal of Interventional Cardiology. 2002;15: 381-386.
- Kline-Rogers E, Share D, Bondie D, Rogers B, Karavite D, Kanten S, et al. Development of a mulitcenter interventional cardiology database: the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) experience. Journal of Interventional Cardiology. 2002; 15: 387-392.
- Moscucci M, Kline-Rogers E, Share D, O'Donnell M, Maxwell-Eward A, Meengs WL, et al. Simple bedside additive tool for prediction of in-hospital mortality after percutaneous coronary interventions. Circulation. 2001; 104: 263-268.
- Jani SM, Smith DE, Share D, Kline-Rogers E, Khanal S, O'Donnell M, et al. Blood transfusion and in-hospital outcomes in anemic patients with myocardial infarction undergoing percutaneous coronary intervention [Supplemental Issue II]. Clinical Cardiology. 2007; 30: II-49-II56.
- Chetcuti SJ, Grossman PM, Kline-Rogers E, Montoye C, Smith DE, Moscucci M. Improving outcomes of percutaneous coronary intervention through the application of guidelines and benchmarking: reduction of major bleeding and blood transfusion as a model. Clinical Cardiology. 2007; 30: II-44-II-48.
- Freeman RV, O'Donnell M, Share D, Meengs WL, Kline-Rogers E, Clark VL, et al. Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. The American Journal of Cardiology. 2002; 90:1068-1073.
- Prager RL, Armenti FR, Bassett JS, Bell GF, Drake D, Hanson EC, et al. Cardiac surgeons and the quality movement: the Michigan experience. Seminars in Thoracic and Cardiovascular Surgery. 2009; 21: 21-27.
- Ferguson TB, Dziuban SW, Edwars FH, Eiken MC, Shroyer LW, Pailero PC, et al. The STS national database: current changes and challenges for the new Millennium. Annals of Thoracic Surgery. 2000; 69: 680-6891.
- The Grace Investigators. Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: A multinational registry of patients hospitalized with acute coronary syndromes. American Heart Journal. 2001; 141:190-199.
- MCORRP. Major Initiatives [homepage on the Internet]: University of Michigan; c2010 Ann Arbor, MI: University of Michigan.2010.
- Osborne NH, Upchurch Jr GR, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. Journal of Vascular Surgery. 2009; 50: 709-713.
- Boxer L, Dimick J, Wainess R, Cowan J, Henke P, Stanley J, et al. Payer status is related to differences in access and outcomes of abdominal aortic aneurysm repair in the United States. Surgery. 2003; 134:142-145.
- Eagle KA, Gallogly M, Mehta RH, Baker PL, Blount A, Freundl M, et al. Taking the national guideline for care of acute myocardial infarction to the bedside: developing the Guideline Applied in Practice (gap) initiative in southeast michigan. Joint Commission Journal on Quality and Patient Safety. 2002; 28: 5-19.
- McLaughlin TJ, Soumerai SB, Willison DJ, Gurwitz JH, Borbas C, Guadagnoli E, et al. Adherence to national guidelines for drug treatment of suspected acute myocardial infarction: evidence for undertreatment in women and the elderly. Archives of Internal Medicine. 1996; 1996;156:799-805.
- Ellerbeck EF, Jencks SF, Radford MJ, Timothy F, Craig AS, Gold JA, et al. Quality of care for medicare patients with acute myocardial infarction: a four-state pilot study from the cooperative cardiovascular project. Journal of the American Medical Association. 1995; 273: 1509-1514.
- Mehta RH, Montoye CK, Gallogly M, Baker P, Blount A, Faul J, et al. Improving Quality of Care for Acute Myocardial Infarction: The Guidelines Applied in Practice (GAP) Initiative. JAMA. 2002 March 13, 2002; 287:1269-1276.
- Greater Flint Health Coalition. Mid-Michigan guidelines applied in practice - heart failure (GAP_HF) project manuscript and abstracts. 2003-2007.
- Moscucci M, Rogers EK, Montoye C, Smith DE, Share D, O'Donnell M, et al. Association of a continuous quality improvement initiative with practice and outcome variations of contemporary percutaneous coronary interventions. Circulation. 2006; 113: 814-22.
- Dey S, Flather M, Devlin G, Brieger D, Gurfinkel E, Steg PG, et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009; 95:20-26.
- Montoye CK1, Eagle KA; Michigan ACC-GAP Investigators; ACC-GAP Steering Committee; American College of Cardiology . An organizational framework for the AMI ACC-GAP Project. J Am Coll Cardiol. 2005; 46: 1-29.
- Eagle K, Montoye C, Riba A, Defranco A, Parrish R, Skorcz S, et al. Guideline-Based Standardized Care Is Associated With Substantially Lower Mortality in Medicare Patients With Acute Myocardial Infarction:: The American College of Cardiology's Guidelines Applied in Practice (GAP) Projects in Michigan. Journal of the American College of Cardiology. 2005; 46:1242-1248.
- Olomu A, Montgomery D, Eagle KA, Koelling TM. African Americans with heart failure are more likely to receive evidence based medications and have lower risk of mortality in the Mid-Michigan Guidelines Applied in Practice for Heart Failure study. Circulation. 2009; 120: S516.
- Giacovelli JK, Egorova N, Nowygrod R, Gelijns A, Kent KC, Morrissey NJ. Insurance status predicts access to care and outcomes of vascular disease. J Vasc Surg. 2008; 48: 905-911.
- Arnaoutakis DJ, Propper BW, Black JH, 3rd, Schneider EB, Lum YW, Freischlag JA, et al. Racial and ethnic disparities in the treatment of unruptured thoracoabdominal aortic aneurysms in the United States. The Journal of surgical research. 2013; 184: 651-657.
- Bruckel JT, Gurm HS, Seth M, Prager RL, Jensen A, Nallamothu BK. Use of a Heart Team in Decision-Making for Patients with Complex Coronary Disease at Hospitals in Michigan Prior to Guideline Endorsement. Plos one. 2014; 9: e113241.
- Eagle KA, editor. Reforming cardiovascular health care in the United States - why, what, when, and how? Grand Rounds; University of Michigan, Ann Arbor. 2009.
- Alexander M, Grumbach K, Remy L, Rowell R, Massie BM. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. American Heart Journal. 1999; 137: 919-927.
- Dauterman K, Massie B, Gheorghiade M. Heart failure associated with preserved systolic function: a common and costly clinical entity. American Heart Journal. 1998;135: S310-S9.
- Ghali J, Kadakia S, Bhatt A, Cooper R, Liao Y. Survival of heart failure patients with preserved versus impaired systolic function: the prognostic implication of blood pressure. American Heart Journal. 1992; 123: 993-997.
- East MA, Peterson ED, Shaw LK, Gattis WA, O'Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004; 148:151-156.
- Yao L, Robert SA. Examining the Racial Crossover in Mortality between African American and White Older Adults: A Multilevel Survival Analysis of Race, Individual Socioeconomic Status, and Neighborhood Socioeconomic Context. Journal of Aging Research. 2011; 2011:8.
