
Case Report
Austin J Endocrinol Diabetes. 2025; 12(1): 1109.
Severe Hyperglycemia Induced by Alpelisib in Advanced Breast Cancer: Two Case Reports and A Call for Early Glycemic Monitoring
Peixe C1,2*, Alexandre MI1,2, Rocha JV1,2, de Griné Severino M1, Lopes M1, Barbosa AP1,2, Gomes AR1,2 and Nobre EL1,2
¹Endocrinology Department, Hospital de Santa Maria, Unidade Local de Saúde de Santa Maria, Lisbon, Portugal
²Faculty of Medicine, University of Lisbon, Lisbon, Portugal
*Corresponding author: Carolina Peixe, Endocrinology Department, Hospital de Santa Maria, Unidade Local de Saúde de Santa Maria, Lisbon, Portugal; Faculty of Medicine, University of Lisbon, Lisbon, Portugal Email: carolribeiropeixe@gmail.com
Received: June 03, 2025 Accepted: June 17, 2025 Published: June 20, 2025
Abstract
Introduction: Alpelisib is an emerging treatment for advanced breast cancer. It inhibits alpha-selective phosphatidylinositol 3-kinase (PI3K), impairing insulin action and promoting hepatic glycogenolysis, leading to hyperglycemia and compensatory insulin release. Alpelisib-induced hyperglycemia has been reported in 59% of patients, typically with early onset (median of 2 weeks), and 28% of patients experience moderate (grade 3, fasting plasma glucose >250-500 mg/dL) or severe (grade 4, fasting plasma glucose >500 mg/dL) hyperglycemia. Alpelisib’s short half-life (8–9 hours) allows glucose normalization within 24– 72 hours after interruption. We describe two cases of severe hyperglycemia associated with alpelisib in metastatic breast cancer patients.
Clinical Cases: Two women without previous hyperglycemia and with treatment-resistant HR-positive, HER2-negative breast cancer, both harboring PIK3CA mutations, initiated alpelisib. In both patients, severe asymptomatic hyperglycemia occurred within one month of treatment initiation. In both cases, metformin was started and alpelisib was suspended, leading to fasting glucose normalization within 72 hours. Alpelisib was subsequently resumed, but both patients experienced recurrent hyperglycemia with the reintroduction of the drug, requiring insulin treatment.
Conclusions: The expanding use of alpelisib will likely result in a significant rise in secondary hyperglycemia cases. Delayed diagnosis may compromise metabolic control, potentially leading to alpelisib dose reduction or discontinuation and compromising efficacy and patient survival. The rapid onset of severe, asymptomatic hyperglycemia warrants regular glucose monitoring in patients initiating this therapy.
Keywords: PI3K inhibition; Alpelisib; Hyperglycemia; Glucose monitoring; Breast cancer treatment
Introduction
Breast cancer is the most common cancer worldwide, with over 2.2 million cases in 2020. Approximately 1 in 12 women will develop breast cancer during their lifetime [1].
Breast cancer is classified into three major biological subgroups influencing treatment decision: hormone receptor-positive (HR+), human epidermal growth factor receptor 2-positive (HER2+), and triple negative (HR-/HER2-) [2]. HR+ and HER2- subtypes account for over 70% of cases, typically treated with hormone therapy (HT), often combined with cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in advanced disease [1,2]. Approximately 40% of patients with HR+/ HER2- advanced breast cancer harbor activating mutations in the phosphatidylinositol 3-kinase (PI3K) gene, associated with resistance to HT and poorer prognosis [3,4].
Alpelisib is a small-molecule alpha selective PI3K inhibitor that can help overcome resistance to hormone-based treatment [4] and is also the only PI3K inhibitor approved for HR+, HER2-, PIK3CAmuted advanced breast cancer progressing on or after HT [3,4]. Regulatory approval of alpelisib, and its inclusion in breast cancer treatment guidelines, was based on the phase 3 SOLAR-1 study of alpelisib (300 mg/day) which targeted patients with HR+, HER2- breast cancer that had progressed on or after HT [5].
PI3K is part of an intracellular signaling pathway that plays an important role in regulating glucose metabolism and cellular proliferation [5]. Dysregulation of this pathway in numerous cancers facilitates oncogenesis and undermines treatment efficacy. Additionally, the role of PI3K in glucose homeostasis results in newonset hyperglycemia, which is observed with PI3K inhibition [3]. Hyperglycemia typically manifests early during alpelisib’s treatment (the median time of onset is about 2 weeks from initiation) due to PI3K inhibition what impends insulin's metabolic actions, resulting in impaired glucose uptake in skeletal muscle and adipose tissue, alongside with increased hepatic glycogenolysis, culminating in elevated blood glucose levels and compensatory insulin release [4,5].
Hyperglycemia is observed in 59% of patients undergoing treatment with alpelisib, being moderate (grade 3, fasting plasma glucose (FPG) > 250 mg/dl) or severe (grade 4, FPG > 500 mg/ dl) in 28% of them. Despite the severity of hyperglycemia, these patients rarely experience acute diabetes complications or spoliative symptoms [6]. The hyperinsulinemia that occurs in these patients may provide breast cancer cells with a survival mechanism and reduce the efficacy of alpelisib [4]. Furthermore, if not successfully managed, hyperglycemia can cause alpelisib dose reductions, treatment interruption or treatment discontinuation. Optimal hyperglycemia management is therefore required to maximize treatment benefit [3,4].
Case Presentations
Case Report 1
71-year-old woman, with history of infiltrating ductal carcinoma breast cancer, HR+, HER2-, diagnosed at 40 years-old and treated with radical left mastectomy. Recurrence in the contralateral breast occurred with 65 years-old and was treated with radical mastectomy and HT. Bone metastases appeared in 3 years later, without response to multiple adjuvant treatments, including HT and chemotherapy. Genomic studies revealed a PIK3CA mutation, leading to the initiation of alpelisib at a dose of 300 mg/day in association with tamoxifen. Her metabolic background included obesity grade 2 (body mass index (BMI) 35.2 kg/m²) and no prior diabetes diagnosis (plasma glycated hemoglobin (HbA1c) 5.6%).
Two weeks after starting alpelisib treatment, the fasting capillary glucose (FCG) was 527 mg/dL, without acute complications or spoliative symptoms. Treatment with metformin 850 mg three times a day was initiated and alpelisib was suspended. FCG values returned to normal within 72 hours. After 10 days without any treatment, the Oncologist restarted alpelisib at a lower dose (200 mg/day). The next day the patient presented an FCG of 257 mg/dL and pioglitazone 30 mg daily was initiated. One week later, due to uncontrolled hyperglycemia (FCG 200-250 mg/dL), basal insulin (10 IU/day) was started.
After 3 months of treatment with alpelisib, pioglitazone and insulin, the HbA1c was 7.6%. Alpelisib treatment was maintained for 4 months with no need for additional treatment or increase in insulin doses. However, due to oncologic disease progression, alpelisib was stopped and glycemia normalized within 24 hours without antidiabetic drugs.
Case Report 2
44-year-old woman, with history of infiltrating ductal carcinoma of the breast, HR+, HER2- diagnosed at 37 years-old. The patient underwent neoadjuvant chemotherapy, and radical left mastectomy, and was subsequently treated with HT. Multimetastases (bone, lung, pleura, and pericardium) appeared 4 years after the initial diagnosis. Six lines of adjuvant treatments were given, including multiple lines of HT and chemotherapy, without response. Genomic studies revealed a PIK3CA mutation, leading to the initiation of alpelisib (300 mg/day) alongside tamoxifen. The patient did not have a previous diagnosis of diabetes mellitus or glucose intolerance (HbA1c 5.3%) or obesity (BMI 23.1 kg/m²).
Indications were given for daily assessment of FCG levels to monitor the risk of hyperglycemia. One month after starting alpelisib treatment, the patient had an FCG of 552 mg/dL, without acute complications or spoliative symptoms. Metformin (850 mg twice a day) was initiated, and alpelisib was suspended. After suspension, the patient reported normal FCG values within 48 hours (Table 1).
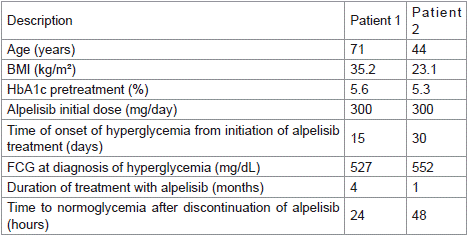
Table 1: Patients information summary. BMI - body mass index;
HbA1c - plasma glycated hemoglobin; FCG - fasting capillary glucose.
After 20 days without treatment, the Oncologist restarted alpelisib at a dose of 200 mg/day. The very next day, the patient presented an FCG of 380 mg/dL. Basal insulin (initial dose 10 IU/day) was associated with metformin treatment. Over the next month, there was the need to increase basal insulin dose (until 18 IU/day) and add prandial insulin. Alpelisib was definitively interrupted after 1 month of treatment due to disease progression, and glycemia normalized in 48 hours with no need for anti-diabetic treatment.
Discussion
The role of PI3K in regulating glucose metabolism and cellular proliferation is crucial for understanding the effects of alpelisib in patients with advanced or metastatic breast cancer. The PI3K/AKT signalling pathway plays a central role in insulin actions, promoting glucose uptake by skeletal muscle and adipose tissue and glycogen storage in the liver. Activation of this pathway is triggered by insulin binding to its receptor, resulting in receptor phosphorylation and subsequent activation of PI3K and AKT, leading to GLUT4 glucose transporter translocation to the plasma membrane in insulin target organs. Any alteration in the PI3K/AKT pathway can result in insulin resistance and hyperglycemia [3,7]. Alpelisib, a PI3K inhibitor, disrupts this signalling pathway, blocking glucose uptake by skeletal muscle and adipose tissue, and activating hepatic gluconeogenesis [3- 5].
Hyperglycemia is the main adverse effect of alpelisib, being a factor for dose reduction in 20% of patients and a cause of permanent treatment discontinuation in 6.3% of patients [3].
Based on our clinical experience, we observed that patient 1 developed hyperglycemia within two weeks of starting the treatment, while patient 2 developed it after one month. This finding is consistent with the results reported in SOLAR-1 study, where hyperglycemia occurred early during treatment, with a median onset time of 15 days after initiating the treatment, and onset time ranged from 5 to 395 days [5,8].
Obesity (BMI =30kg/m2), age =70 years, HbA1c between 5.7 and 6.4%, and gestational diabetes are all risk factors for alpelisib induced hyperglycemia because it implies a baseline propensity to insulin resistance [4,9]. These risk factors were present in 74.7% of patients with any grade of hyperglycemia and 86.2% of patients with grade 3 or 4 hyperglycemia in SOLAR-1 [3-5].
The presence of two of these risk factors (age and obesity) may have contributed to the early development of hyperglycemia observed in patient 1. However, Patient 2 did not exhibit any of these risk factors yet developed a severe hyperglycemic condition requiring more intensive treatment.
After the initiation of alpelisib, blood glucose should be monitored at least once every week in the first 2 weeks, once every 2 weeks during the next 8 weeks and at least once a month thereafter with the assessment of FCG in fingerprick samples or by assessing fasting plasma glucose (FPG) in a peripheral blood sample; HbA1c should be monitored 4 weeks after initiating treatment and every 3 months thereafter [4,5]. Compared to self-monitoring of FCG, a continuous glucose monitoring system provides a close monitoring of glycemic behavior in a non-invasive way, allowing the early detection and treatment of hyperglycemia; therefore, if available, continuous glucose monitoring devices should be considered, especially in patients with one or more risk factors [3,4]. Given that hyperglycemia occurred in >50% of patients with previous normal glycemic status and normal BMI who received alpelisib in the SOLAR-1 study [5,8] all patients require lifestyle management advice on how to minimize the risk of hyperglycemia [4]. For all patients with type 2 diabetes mellitus, it is inappropriate to start alpelisib treatment without a previous endocrinological evaluation [9]. The safety of alpelisib in patients with type 1 and uncontrolled type 2 diabetes has not been established as these patients were excluded from clinical trials [5].
The hyperinsulinemia that occurs in patients with alpelisibinduced hyperglycemia may provide breast cancer cells with a survival mechanism and reduce its efficacy [3,4]. Furthermore, if not successfully managed, hyperglycemia can lead to alpelisib dose reductions, treatment interruption or discontinuation. As seen in our patients, grade 3/4 (FPG 250-500 mg/dL) hyperglycemia require alpelisib interruption [4,5]. Grade 1 (FPG < 160 mg/dl) and grade 2 (FPG 160-250 mg/dl) hyperglycemia are usually asymptomatic and manageable without alpelisib dose adjustment [4,5]. Optimal hyperglycemia management is therefore required to maintain alpelisib dose and maximize treatment benefit [3,4].
Based on the mechanism of action of alpelisib and to attenuate the pronounced insulin resistance and hyperglycemia associated with it, it is pertinent to note that insulin sensitizers may be preferable over insulin secretagogues for treatment [4]. Metformin is widely recognized as a standard treatment in these cases (4,10). Despite the potential for diarrhea as an adverse effect of both alpelisib and metformin [8,10], the incidence and severity of diarrhea were comparable in patients who received concomitant metformin and those who did not in the SOLAR-1 trial [5,8]. To mitigate gastrointestinal adverse effects, metformin dosage should be titrated gradually [4]. The initiation of metformin at a dose of 850 mg, three times a day in patient 1 may have accounted for its poor tolerability.
Currently there is no secondary agent that is widely accepted when hyperglycemia remains uncontrolled with metformin [10]. Other non-insulin antihyperglycemic medications that can be used in conjunction with metformin or as alternatives to it include the insulin sensitizer pioglitazone and the sodium-glucose co-transporter 2 (SGLT2) inhibitors [4]. Based on their mechanism of action, SGLT2 inhibitors have been proposed as the second line, and potentially first line, treatment options in cases of metformin intolerance [4,10]. Pioglitazone cannot be used as a first-line agent due to its delayed onset of action (6–8 weeks) [4]. GLP-1 receptor agonists are potential therapeutic agents; however, there is limited experience regarding their use in settings PI3K inhibitions. Additionally, the common gastrointestinal side effects of GLP-1 receptor agonists overlap with those seen with the administration of alpelisib (10). Dipeptidyl Peptidase-4 (DPP-4) inhibitors could be an option since they are well-tolerated drugs with few adverse effects. However, the limited available evidence suggests they have low efficacy in treating alpelisib-induced hyperglycemia. Sulfonylureas should be avoided as primary treatment for this type of hyperglycemia because they function as insulin secretagogues and elevate insulin levels, potentially diminishing the primary antitumor effect of alpelisib [4]. Insulin, and/or sulfonylureas can be utilized as rescue medication for hyperglycemia >250 mg/dL, not adequately controlled by non-insulin antihyperglycemic agents alone [4], as illustrated in our cases. Given the short half-life of alpelisib, 8 to 9 hours, renders the hyperglycemic effect negligible for at least 24-72 hours after treatment is interrupted [3,4]. Insulin administration requires careful consideration due to the risk of hypoglycemia after the half-life effect of alpelisib.
Although our two patients needed to interrupt alpelisib treatment in the moment of hyperglycemia diagnosis, both achieved adequate glycemic control and were able to continue alpelisib treatment after the use of anti-hyperglycemic agents. Insulin use was necessary in both cases, underscoring the safety of its usage when coupled with close monitoring of glycemic profiles, particularly in instances of alpelisib discontinuation.
Conclusion
Alpelisib is a selective alpha inhibitor of PI3K, whose use has experienced a notable increase in recent years, particularly in cases of HR+ breast cancer [4]. Hyperglycemia stands out as its most frequent adverse effect, manifesting in approximately 59% of patients [6]. Consequently, the expansion of alpelisib utilization is expected to correlate with a significant uptick in secondary hyperglycemia cases.
The hyperglycemia induced by alpelisib typically emerges around 15 days following the initiation of therapy, primarily stemming from a state of insulin resistance [5,8]. Therefore, treatment should involve insulin sensitizer medication, being metformin the preferred choice. Insulin and/or other insulin secretagogue drugs should be reserved for situations where hyperglycemia remains uncontrolled despite initial treatment [4]. Blood glucose levels are expected to normalize within one week of treatment cessation, requiring special care for patients taking insulin due to the associated risk of hypoglycemia.
A delayed diagnosis can significantly impact metabolic control, potentially leading to alpelisib dose adjustments or discontinuation, and compromising treatment efficacy and patient survival.
With these 2 case reports, we want to highlight the rapid onset of severe asymptomatic but still benign course of the hyperglycemia in this setting, and therefore to emphasize the need for close monitoring of the patients to maintain the benefit of the oncological treatment, which necessarily requires a multidisciplinary approach.
References
- DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiology Biomarkers and Prevention. 2015; 24: 1495–1506.
- Waks AG, Winer EP. Breast Cancer Treatment. JAMA. 2019; 321: 288.
- Pla Peris B, Arranz Martin A, Ballesteros García A, Sebastián-Valles F, Marazuela Azpiroz M. Alpelisib-Induced Diabetes Mellitus: Case Report, Pharmacodynamics and Management Considerations. Front Endocrinol (Lausanne). 2022; 13.
- Tankova T, Senkus E, Beloyartseva M, Borštnar S, Catrinoiu D, Frolova M, et al. Management Strategies for Hyperglycemia Associated with the a-Selective PI3K Inhibitor Alpelisib for the Treatment of Breast Cancer. Cancers. MDPI; 2022; 14.
- Rugo HS, André F, Yamashita T, Cerda H, Toledano I, Stemmer SM, et al. Time course and management of key adverse events during the randomized phase III SOLAR-1 study of PI3K inhibitor alpelisib plus fulvestrant in patients with HR-positive advanced breast cancer. Annals of Oncology. 2020; 31: 1001–1010.
- André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA -Mutated, Hormone Receptor–Positive Advanced Breast Cancer. New England Journal of Medicine. 2019; 380: 1929–1940.
- Carrillo M, Rodriguez RM, Walsh CL, Mcgarvey M. Alpelisib-Induced Diabetic Ketoacidosis: A Case Report and Review of Literature. AACE Clin Case Rep. 2021; 7: 127–131.
- Shields M, Mo Q, Armitage M, Sharpe SC, Costa RLB. A systematic review and meta-analysis of selected toxicity endpoints of alpelisib [Internet]. Oncotarget. 2020; 11: 3793.
- Gallagher EJ, Moore H, Lacouture ME, Dent SF, Farooki A, Goncalves MD, et al. Managing hyperglycemia and rash associated with alpelisib: expert consensus recommendations using the Delphi technique. NPJ Breast Cancer. 2024; 10.
- Liu D, Weintraub MA, Garcia C, Goncalves MD, Sisk AE, Casas A, et al. Characterization, management, and risk factors of hyperglycemia during PI3K or AKT inhibitor treatment. Cancer Med. 2022; 11: 1796–1804.