
Review Article
J Drug Discov Develop and Deliv. 2023; 9(2): 1048.
Schistosomiasis Drug Development: A Computational Approach
Khwesa Peredy*
Material and Computational Chemistry, College of Natural and Computational Sciences, Addis Ababa University, Ethiopia
*Corresponding author: Khwesa Peredy Material and Computational Chemistry, College of Natural and Computational Sciences, Addis Ababa University, P.O. BOX 1176, Addis Ababa, Ethiopia. Email: khwesaperedy@gmail.com
Received: October 17, 2023 Accepted: November 20, 2023 Published: November 27, 2023
Abstract
Schistosomiasis continues to be a significant global health concern, affecting millions of individuals worldwide. The limitations associated with the existing treatment, Praziquantel, highlight the urgent need for novel schistosomicides. The development of a structure-based drug with high efficacy offers a potential solution to the challenges posed by current treatments. Given the capital and time-intensive nature of traditional inhibitor identification methods, computational techniques have gained prominence as valuable tools in drug discovery. This study employed a comprehensive computational approach to identify potential drug candidates, laying the groundwork for future wet-lab experimentation.
In this research, in silico molecular docking analysis assessed the binding affinity of various compounds to the Schistosoma japonicum tegument protein 4 (SGTP4). To facilitate this analysis, homology modeling generated a reliable 3D structure of SGTP4. This rigorous approach, encompassing homology modeling and subsequent validation, ensured the reliability and high quality of the SGTP4 protein model, providing a solid foundation for subsequent molecular docking and drug discovery investigations. The ligands examined included Praziquantel , Licochalcone, P96, Licarin, and Harmonine. Ligand preparations were conducted using LigPrep within the Schrodinger Maestro suite, and pharmacokinetic parameters were evaluated using the QikProp tool within the Schrodinger-2019-4 software suite. Subsequently, AutoDockTools version 4 facilitated the docking analysis of the prepared ligands.
The docking results furnished valuable insights into the binding affinities and potential inhibitory activities of the assessed compounds against SGTP4. PZQ, Licochalcone, and P96 exhibited robust binding affinities and inhibitory potentials, characterized by their lower binding energies and Ki values. Licarin demonstrated moderate activity, whereas Harmonine exhibited comparatively weaker binding and inhibition.
The amalgamated results of docking analysis and ADMET simulations establish a foundation for the selection of lead compounds for further experimental evaluation in the quest for effective anti-schistosomal drugs. Among the evaluated compounds, Licochalcone and Licarin emerge as promising candidates, characterized by favorable molecular properties, drug-like attributes, and bioavailability. While PZQ retains its importance as a reference, its limitations underscore the necessity of exploring alternative compounds. Furthermore, P96 exhibits potential, particularly with structural modifications, warranting further investigation.
This study underscores the significance of computational methodologies in the initial stages of drug discovery. Nonetheless, it is essential to stress that wet-lab experimentation remains an indispensable step in drug development. The computational insights presented herein offer a compelling rationale for the wet-lab validation of the identified candidates. Such experiments can elucidate the mechanisms of action, evaluate safety profiles, and confirm efficacy against schistosomes. Therefore, we advocate for the integration of wet-lab experiments to complement computational approaches, presenting a synergistic strategy poised to address the deficiencies of PZQ and effectively combat schistosomiasis.
Keywords: Schistosomiasis, Drug discovery, Molecular docking, SGTP4 protein, Computational analysis, Neglected tropical disease
Introduction
Schistosomiasis, an NTD, is a parasite illness transmitted by water and is prevalent in economically disadvantaged regions of Sub-Saharan Africa. According to multiple sources it is predicted that out of the global total of around 240 million cases of the disease reported in 2020, the majority, specifically 90% of the cases, were documented in the Sub-Saharan Africa region [1-3]. The disease's prevalence is impacted by the low socioeconomic position of the area, which is characterized by inadequate sanitation, weak health policies, and overall substandard living conditions [4]. In Africa, there is a prevalent reliance on herbal medications, with a significant proportion of the population, particularly in rural regions, relying on such remedies [5]. Hence, it is imperative to comprehend the utilization of traditional herbal remedies within these populations, considering the high incidence of schistosomiasis.
According to Gryseels et al. , the species that are considered to be the most clinically significant are S. mansoni, S. japonicum, and S. haematobium [6]. On the other hand, S. mekongi, S. guineensis, and S. intercalatum exhibit lower prevalence rates. The World Health Organization (WHO) reports that there is a global infection rate of roughly 229 million individuals, resulting in an estimated yearly mortality rate of over 200,000 [7]. Nevertheless, it is likely that this assessment is conservative, as the current diagnostic technologies exhibit limited sensitivity in detecting parasite infections of low intensity [8]. According to Kassebaum et al., this particular condition holds the second position in terms of both prevalence and socio-economic impact, with malaria being the only disease ranking higher [9]. Furthermore, it is responsible for a significant loss of over 2.6 million disability-adjusted life years (DALYs).
Human infection occurs when cercariae larvae, which are discharged by intermediate snail hosts, penetrate the skin upon exposure to freshwater that has been contaminated [10]. Subsequently, cercariae gain entry into the host's circulatory system and undergo maturation into juvenile and adult worms [11] the adult schistosomes, both female and male, are found in the blood vessels, where they engage in reproductive activities resulting in the production of eggs. These eggs are then expelled either by feces in the case of S. mansoni, S. japonicum, S. intercalatum, S. guineenses, and S. mekongi, or through urine in the case of infections caused by S. haematobium [12]. The presence of eggs in human tissues leads to the initiation of inflammatory immunological responses, characterized by the formation of granulomata. These immune reactions contribute to the impairment of organ function, giving rise to various diseases such as intestinal, hepatosplenic, or urogenital disorders [13] The eggs that are deposited into the environment undergo hatching in aquatic habitats, subsequently releasing the larval form known as miracidia. The Miracidia are responsible for infecting the intermediate hosts, so facilitating the continuation of the parasite's life-cycle.
The development of a vaccine for schistosomiasis has presented significant challenges [14]. As a result, the treatment and management of schistosomiasis still rely heavily on praziquantel , a medicine that has been in use for nearly 50 years [15]. Schistosomiasis is primarily prevalent in the Sub-Saharan African region, where the prevalence of infection is on the rise. This increase can be attributed to various factors, including climate change and socio-economic influences. Currently, praziquantel stands as the sole pharmaceutical agent available for the management of this incapacitating ailment. Praziquantel exhibits several advantageous properties:The treatment exhibits efficacy against several species of Schistosomes; It is characterized by its affordability and easy accessibility; Furthermore, it demonstrates a favorable safety profile, being well-tolerated across all age groups of patients. Regrettably, the utilization of Praziquantel is constrained by the subsequent factors: There are several challenges associated with the use of praziquantel for the treatment of schistosomiasis. Firstly, drug resistance has been observed, which reduces the effectiveness of the medication. Secondly, there is a lack of patient compliance to treatment, particularly in certain populations. This non-adherence to the prescribed regimen can hinder the successful management of the disease. Additionally, praziquantel is ineffective against immature forms of the Schistosoma species, limiting its efficacy in treating infections at early stages. Lastly, it is important to note that praziquantel does not provide protection against re-infection of schistosomiasis, necessitating additional preventive measures. Moreover, there has been a rise in the phenomenon of parasite change and modification, leading to an escalation in the global parasite burden and the occurrence of co-infection with many strains of Schistosoma parasites [15-17]. In conjunction with instances of cerebral schistosomiasis in various regions worldwide, there exists a pressing necessity for an alternative pharmaceutical compound with anti-schistosomal properties or for enhancing the effectiveness of Praziquantel administration through methods such as nanotechnology, in order to attain targeted therapeutic outcomes against schistosomiasis, particularly within the Central Nervous System.
Praziquantel is widely recognized for its efficacy in targeting both adult and schistosomula stages across several types of schistosomes. In addition, it should be noted that PZQ is often supplied in a racemic mixture, meaning that only one of the two stereoisomers (namely, the R-PZQ stereoisomer) is pharmacologically active. In addition to its pharmacological inactivity, the S-PZQ compound is implicated in the bitter taste and the substantial dimensions of PQZ tablets. These characteristics have been seen to diminish patient adherence and render the medication unsuitable for pediatric use [18]. Furthermore, the utilization of PZQ in large-scale drug administration initiatives over an extended period of time has the potential to provide a selective pressure that could facilitate the emergence of resistance in parasites [19]. Indeed, studies have shown a decrease in the effectiveness of praziquantel in both laboratory and field isolates [20,21].
Since its initial clinical studies in the late 1970s, Praziquantel has demonstrated both safety and efficacy against all three primary manifestations of the disease. Furthermore, the ongoing reduction in costs has rendered this medication more accessible, with a current price range of approximately 7-19 US cents per 600 mg tablet [22]. According to [23], administering a solitary oral dosage ranging from 40 to 60 mg/kg is effective in attaining cure rates between 60% and 90% [23]. This approach is particularly advantageous in promoting adherence among patients, particularly children. Despite sporadic and isolated incidents reported by Botros et al., the occurrence of clinically significant and widespread resistance has not yet been observed [24]. The fortuitous circumstance described herein presents a notable juxtaposition to the circumstances surrounding certain other Neglected Tropical Diseases (NTDs) as documented by [25]. These NTDs necessitate the administration of outdated and frequently toxic medications via parenteral routes, requiring several days or weeks of treatment. Moreover, these diseases are progressively encountering challenges related to drug resistance, as indicated by Pink et al. [26].
However, the utilization of a solitary pharmaceutical agent for addressing the healthcare needs of a populace exceeding 200 million individuals who are infected, as well as an additional 700 million individuals who are at risk throughout three continents [1,27], appears notably precarious when contemplating the potential emergence of drug resistance. Moreover, it should be noted that PZQ is not exempt from certain challenges or drawbacks. One of the primary limitations of PZQ is its limited efficacy against migratory juvenile and sub-adult worms, as noted by [28]. Consequently, in order to achieve successful therapy and sustainable control, it is necessary to administer PZQ regularly. Recent scholarly discourse on the treatment landscape of human helminthiases has placed emphasis on the renewed exploration of alternate options to praziquantel , as highlighted by [29]. This includes the investigation of pharmacological combinations that involve the incorporation of PZQ, as discussed by [15].
The second choice offers the longer-term advantage of increasing PZQ availability while delaying the establishment of resistance to this most priceless of medicines, even if it is more challenging and expensive to create. In researching anti-schistosomal drugs (and anthelmintics in general), our knowledge of the molecular drug target or mechanism of action has been rather constrained. The suppression of redox and proteolytic enzymes [30] and heme aggregation [31] are two major breakthroughs in this field.
Contrasting sharply with the vast array of validated targets that serve as the cornerstone of drug development initiatives by Public-Private Partnerships (PPPs) addressing other globally significant infectious illnesses like malaria and the trypanosomiases is the paucity of established molecular therapeutic targets for this parasite. The recently published draft genomes of Schistosoma mansoni [32] and S. japonicum, as well as the initial efforts to prioritize these targets [15]; TDR Drug Targets Prioritization Database), may help to more effectively address the limited number of targets. Phenotypic screening, a method often used in the discovery of schistosome drugs, includes evaluating a compound's activity in vitro using whole organism experiments, usually using adult worms. Additionally, to evaluate the efficacy of these substances, animal models of illness are often used [33].
The majority of the time, these tactics are used without having a thorough understanding of the target or the action's process. It's also possible that they are methods whose bioactivity has already been confirmed in related parasitological or biomedical situations [34]. These organizations have shown their value. For instance, PZQ underwent testing in an animal model of schistosomiasis after being first developed as a veterinary cestocide [35]. Evidence about its mode of action was subsequently gathered. However, because these methodologies heavily rely on a small number of talented research teams with the necessary skills for managing the complex life cycle of schistosomes and dealing with only small numbers of the parasite, the rate of advancement using these approaches is relatively slow.
An Examination of Historical and Contemporary Therapeutic Approaches for Schistosomiasis
The proposed therapeutic option to eradicate schistosomiasis in 1984 was chemotherapy, as suggested by the WHO Expert Committee [35]. Chemotherapy remains the exclusive approach for the management of schistosomiasis, relying exclusively on a monotherapy regimen using a single dosage of Praziquantel.
Among the several pharmacological medicines that have been studied, praziquantel has emerged as the main anti-schistosomal drug. The pathogenic disease known as schistosomiasis is caused by the Schistosoma species, and praziquantel is effective against all known Schistosoma species. The treatments may lessen the severity of symptoms and reduce the burden that parasites create. of addition, this medication is well-liked for its simplicity of use, potency, and affordability.
Although the specific method for treating schistosomiasis is still not fully understood, one often proposed mechanism is the rapid remodeling of the muscles in the parasite worms [36,37]. Provided evidence of the phenomena mentioned above. These researchers saw that the worm's muscles changed and contracted as a consequence, which was probably caused by the quick input of calcium ions into the schistosome. A notable research by [37], which focused on the voltage-gated calcium channels present in schistosomes as a potential target for PZQ, provided evidence for the aforementioned assertion. The study's authors suggested that the observed effect of PZQ on the control of Ca2+ levels in schistosomes is consistent with the mechanism through which PZQ functions. It was discovered that the particular structural traits of -subunits found in schistosome channels vary from those of conventional -subunits seen in other situations. It has been shown that these distinct -subunits block the passage of current via the schistosome channels' 1 subunit, with which they are intricately linked.
A second hypothesis put out by the research was that PZQ contributed to the enhancement of channel availability for current conduction, interfering with the interaction between the 1 and subunits in these channels. As a result, this alteration affected the way intracellular calcium levels were regulated [37].The tegument of schistosomes has also been shown to undergo morphological alterations, according to studies. The early observations of surface blebbing and vacuole growth inside the tegument were made by [23,38]. An enhanced presentation of antigens on the parasite's surface is the outcome of the morphological changes mentioned in the research by [39]. According to [39], PZQ's lipophilic character, which makes it easier for it to interact with the hydrophobic cores of the tegument, is the method by which it affects the exposed antigens.
Trematodes have a poor digestive system, yet Schistosoma species may survive long periods of in vitro incubation without depending on gastrointestinal food absorption [40,41]. In the early stages of the trematode's life cycle, when the gut is still developing, glucose absorption in trematodes is shown [42]. The primary source of energy for both juvenile and adult schistosomes is plasma glucose acquired from the host. According to physiological investigations [42,43], a process mediated by carriers facilitates the entrance of glucose into the tegument.
The Schistosome Tegument: Exploring Potential Molecular Targets
There are several targets on the tegument's surface. To effectively target the male schistosomes' tegument surface, engineered drug-loaded nanoparticles need to contain Acetylcholinesterase (AChE), Schistosoma Japonicum Tegument Protein 1 (SGTP1), Schistosoma Japonicum Tegument Protein 4 (SGTP4), and a nicotinic type of acetylcholine receptor (nAChR). Among other components, dynein, aquaporins, and tetraspanins are additional significant surface proteins found on the tegument that may be targeted. The substances found on the tegument's surface are essential because they serve as the main molecular targets for the development of novel therapeutic compounds and vaccines that target the Schistosoma parasite.
Potential molecular targets for nano-delivery systems in glucose transporters
Numerous studies [44-46] have shown that the availability of energy in the form of glucose is necessary for the survivability of Schistosoma parasites. The primary site of energy (glucose) consumption is the tegument, not the intestinal cecum. According to [44], the presence of glucose transporters on the tegument facilitates the process of absorption. In their work, [44] they found and examined three distinct cDNAs. The protein sequences of these cDNAs were found to be structurally and logically similar to those of facilitated diffusion transporters found in mammals, microbes, and plants. Two distinct glucose transporters, SGTP1 and SGTP4, were identified in the tegument.
The study also found that the SGTP 1 and 4 genes are expressed in adult and larval schistosomes of both sexes, enabling efficient glucose uptake from the host organism. Only two of the four encoded glucose transporters found in Schistosoma mansoni's genome, according to [47], were shown to help in glucose diffusion. Their research's findings also showed that Schistosoma mansoni's class 1 glucose transporters were unable to facilitate glucose transfer, and that this function originated independently in the schistosome-specific glucose transporter.
The glucose transport routes of schistosome platyhelminthes-specific transporters and those found in humans were significantly different, according to. The sequences of SGTP1 and 4 share 60% of the same letters, according to earlier studies. In their investigation, [47] employed electron microscopy techniques to spatially locate the different locations of these transporters on the tegument. The scientists observed that SGTP1 was mostly present in the basal lamina, with a secondary and less significant presence beneath the muscle cells. Unbound glucose may be more likely to cross the epidermis and enter the fluids around the parasite's internal organs as a result of this process. According to [47], SGTP4 was shown to be evenly distributed throughout the dorsal and ventral surfaces of both male and female teguments, each of which had a distinctive double lipid bilayer structure. According to [44,45], SGTP4 is thought to have a role in the transfer of glucose from the host circulation into the parasite tegument due to its specific localization on the outer tegumental membrane.
Additionally, the transformation of free-living cercariae into schistosomula is mediated by SGTP4. The host satisfies the parasite's need for high glucose absorption during both the schistosomula stage and maturity, as shown by [44,45]. As a consequence, [46] findings support the idea that SGTP proteins might be used in nano-delivery systems. In their research, the overexpression of the SGTP4 and SGTP1 genes was reduced in the schistosomula and adult worm life stages via RNA interference (RNAi). The purpose of the present investigation was to assess how important these proteins were to the parasite. The ability of glucose to be transported was diminished when SGTP4 or SGTP1 were downregulated in comparison to the control. The study also discovered that, in comparison to downregulating just one SGTP gene, downregulating both SGTP1 and SGTP4 at the same time lowered the parasite's capacity to aid glucose transport. Furthermore, there were no obvious phenotypic differences between the suppressed parasites and the control group after an extended period of incubation on a nutrient-rich medium.
The role of SGTP1 and SGTP4 in facilitating the transfer of exogenous glucose from the mammalian host, which contributes to the parasite's regular development, was lastly hypothesized to be crucial. The aforementioned finding was made as a consequence of an empirical analysis of parasites with reduced SGTPs, which significantly reduced their ability to survive within infected experimental animals [46,48]. Found that the Akt/Protein kinase B signaling pathway controls glucose absorption in Schistosoma mansoni, which provided evidence in favor of the aforementioned theory. The study found that the host's natural amino acid, L-arginine, may activate Akt. In addition, it has been shown that insulin effectively raises Akt signaling in both adult and schistosomula teguments. During host invasion, the upregulation and maturation of SGTP4 on the surface of the parasite's larval stage were reduced due to the downregulation of Akt. In mature worms, the observed inhibition of SGTP4 overexpression in the tegument was associated with a reduction in glucose uptake.
Therefore, a more potent alternative to current anti-schistosomal therapy may be achieved by conjugating nanoparticles with specific agents such as antibodies, aptamers, antibody-like ligands, peptides, and small molecules with great selectivity for SGTP proteins. This plan would enable the targeted delivery of anti-schistosomal medications via nanotechnology. The development of nanoparticulate systems made possible by nanotechnology has enabled scientists to produce nanocarriers with the necessary selectivity for medication delivery. These nanocarriers may include pharmaceuticals, increasing their efficacy and enabling more precise delivery. Overexpressed receptor molecules, particularly SGTP proteins, which act as binding sites for possible therapeutic medications, are to blame for the behavior that has been seen. It is theoretically possible to enhance the localization of therapeutic medications within certain organs and tissues by modifying drug-containing nano-delivery systems with ligands that target specific receptors. Therefore, a promising method for the creation, development, and administration of anti-schistosomal drugs is the use of nano-delivery systems with ligands specific for Schistosoma Granulocyte Transmembrane Proteins (SGTPs) as receptors.
It's crucial to comprehend the Praziquantel drug's mechanism of action while looking for novel schistosomiasis chemotherapy treatment options. The underlying mechanism of PZQ has been the subject of a great deal of study and examination. A PZQ binding site was discovered by [49] in the voltage sensor-like region of a schistosome transient receptor potential melastatin ion channel (Sm.TRPMPZQ) using ligand- and target-based approaches. This binding site was located in a juxtamembrane cavity. The authors demonstrated that PZQ may open the Sm.TRPMPZQ channel, causing calcium to influx and the paralysis of the parasitic flatworm. Le Clec'h et al. also demonstrated that the transient receptor potential channel in S. mansoni had a role in PZQ response variation by using genome-wide association to pinpoint the loci responsible for PZQ response variance. [50] Suggested that nanomolar concentrations of PZQ might activate this channel. The Park group created 43 praziquantel derivatives, including the enantiomers (R)-PZQ, (S)-PZQ, and the primary trans-(R)-4-OH PZQ metabolite, in order to investigate the pharmacological specificity of the schistosome TRP channel produced by PZQ.
The cyclohexyl moiety (R group, as depicted in Figure 1A) in PZQ is essential to its efficacy, according to research into the structure-activity connections of these analogs. Analogs with diminished activity or potency were produced by significant changes to this molecule [49]. The findings were in line with other studies suggesting a potential connection between the cyclohexyl group's structural characteristics and its antitischistosomal activities [5]. PZQ undergoes fast metabolism, which leads to the production of a substantial trans-cyclohexanol metabolite, as identified by [51]. However, it was discovered that this metabolite was much less efficient than PZQ. Trans-cyclohexanol metabolite ketone oxidation products, as well as other analogues, were produced by [52] and had increased metabolic stability. The action of these substances against young S. japonicum and S. mansoni is minimal to moderate.
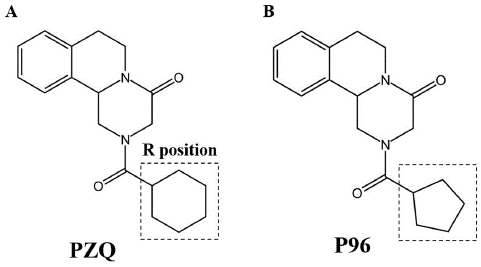
Figure 1: Chemical structures of praziquantel and P96. https://doi.org/10.1371/journal.pntd.0011215.g001
The R site underwent a number of modifications to enhance the schistosomicidal activity (Figure 1A). In contrast to PZQ's schistosomicidal activity, the majority of these drugs had limited to moderate efficiency [53]. But [49] has made us aware of a specific Praziquantel derivative known as compound 5. The research group referred to this particular molecule as P96, and it has piqued our interest. Sm.TRPMPZQ was shown to be activated by the compound designated as 5, in which cyclopentyl is substituted for cyclohexyl (as shown in Figure 1B). However, [49] found that it has an apparent affinity that is nearly six times lower than PZQ.
In the field of medicinal chemistry, utilizing a computational approach to screen and evaluate possible therapeutic compounds with the ability to interact with specific molecular receptors has shown to be an effective strategy. The present study will look at the effects of several anti-schistosomal medicines, such as praziquantel (as a control), Licochalcone A, Licarin, Harmonine, and P96, on a protein identified in the tegument of Schistosoma species. [36] revealed a number of intriguing targets for therapeutic and vaccine development. These targets include specific proteins and molecular receptors found on the schistosome's tegument. The authors, for example, underlined that, as previously reported by [45], schistosome glucose transporters 1 (SGTP1) and 4 (SGTP4) are present in all schistosome variations. According to [54] and [52] acetylcholinesterase (AChE) and a nicotinic type of acetylcholine receptor (nAChR) were identified on the surface of male schistosomes.
Dynein is a distinct protein in Schistosoma mansoni that is specifically targeted and present on the surface of the tegument [52]. This study's target protein was SGTP4, which is located on both the ventral and dorsal sides of the teguments of male and female schistosomes and has a distinctive double lipid bilayer structure. According to various studies, Schistosoma parasites need a particular quantity of energy/glucose to live [46].
Instead of the caecum of the gut, the tegument of schistosomes absorbs glucose or energy first. Schistosome glucose transporters, which are found in the schistosome tegument, help in the absorption of glucose [46]. Due to its specific placement on the membrane tegument, the SGTP4 protein may operate as a transporter, assisting glucose to move from the host circulation into the parasite's tegument [45]. Furthermore, it has been shown that SGTP4 is required for managing the different stages of schistosome development. It is particularly important in the transformation of free-living cercariae into schistosomula. This protein meets the parasites' demands for adequate glucose absorption both when they initially infect the human host as schistosomula and during their whole adult life [45].
The purpose of this study is to look at the many elements of licochalcone A, licarin, p96, and harmine's anti-schistosomal action against the SGTP4 protein receptor. In addition, molecular docking with the SGTP4 protein receptor was used to compare the medicine praziquantel, which is widely accepted as the only treatment option for schistosomiasis.
Materials and Methods
SGTP4 Homology Modeling and Drug Target Preparation
The SGTP4 sequence, identified by the accession number Q26581_SCHMA, was retrieved in FASTA format from the Uniprot database. Given the unavailability of the crystal structure of SGTP4 in the Protein Data Bank (PDB), a blast search was performed on the PDB database to select an appropriate template for the purpose of homology modeling. The investigation conducted by our team resulted in the identification of the solute carrier family 2, facilitated glucose transporter member 3 (4ZW9) structures. This structure was employed as a reference to generate the three-dimensional model of SGTP4 using the Schrodinger Suite.
The process of aligning the target and template sequences was executed, and further adjustments were made to various PRIME parameters. Following that, we proceeded to optimize the loops and employed the resultant homology model for additional investigations. In order to ensure the quality of the model, a comprehensive preparation process was undertaken in Maestro. The PropKa optimization process was conducted, followed by the generation of hetero states using EPIK. Steric incompatibilities were resolved by applying the OPLS_2005 force field, and any missing atoms were repaired using PRIME.
The stability and overall quality of the SGTP4 homology model were evaluated by the utilization of RAMPAGE, in addition to its Ramachandran plot obtained from PDBSum and PROCHECK.
Selection and Preparation of Ligands as SGTP4 Inhibitors
In this study, a selection of five ligands with known schistosomicidal capabilities or potential schistosomicidal properties was utilized. Additionally, one extra ligand, P96, was included in the analysis. The prediction of P96 was based on a machine learning method or Molecular Modeling (MM) which was further supported by a modification experiment conducted by [55]. Among the six ligands considered in this study, praziquantel (PubChem ID-4891) was selected as the control molecule. The decision was reached based on its designation as the only chemotherapeutic treatment now accessible that demonstrates efficacy against all adult variations of Schistosoma spp. Consequently, it serves as a standard against which other options can be evaluated. The other ligands, obtained from a previous investigation documented in PubChem [54] consist of licochalcone A (PubChem ID-5318998), licarin (PubChem ID-73548), harmonine (PubChem ID-14825482), and P96, which is currently not, assigned a PubChem number.
It is important to highlight that a critical aspect of generating reliable and stable protein-ligand docked complexes entails the elimination of salt ions and the optimization of hydrogen atom locations. In order to accomplish this, the 3-D structural format of each ligand was subjected to a procedure referred to as ligand preparation, utilizing LigPrep inside the Schrodinger Maestro suite. During the course of this experimental procedure, the ligands underwent optimization using Epik software at a pH range of 7±2. Furthermore, measures were implemented to tackle ring conformations, eliminate the presence of salts in the ligands, address stereochemical considerations, and develop tautomeric forms, all with the objective of attaining a minimum of 32 conformations for each ligand.
Tox/Admet Screening
In the field of pharmaceutical research, the process of finding new compounds with desirable pharmacokinetic and ADME/Tox characteristics provides a considerable obstacle. As a result, the use of computational methods to forecast these characteristics has emerged as a crucial stage in the pursuit of discovering novel chemicals that are well-tolerated and demonstrate negligible or absent detrimental impacts on the human body [36,56,57].
In this investigation, an assessment of the pharmacokinetic properties of the ligands, specifically praziquantel, licochalcone A, licarin, and harmonine, was undertaken. The evaluation employed the QikProp tool within the Schrodinger-2019-4 software suite. Within the scope of this study, a computational analysis served as a predictive tool for several crucial characteristics. These encompassed acute rat acute toxicity, acute oral toxicity, carcinogenicity, and Ames toxicity, with a focus on mutagenicity assessment.
The importance of forecasting ADME qualities cannot be emphasized, given that an estimated 40% of drug candidates fail during clinical trials due to insufficient ADME characteristics. The occurrence of late-stage failures plays a substantial role in the increasing costs linked to the development of new pharmaceuticals. As a result, the capacity to detect candidates with potential issues in the early stages can significantly minimize the expenditure of time and resources, hence optimizing the overall efficiency of the drug development process.
It is noteworthy to mention that QikProp possesses a broad spectrum of predictive skills that span several aspects pertinent to the field of pharmaceuticals. The parameters under consideration encompass octanol/water and water/gas partition coefficients (log Ps), aqueous solubility (log S), blood-brain barrier penetration (log BB), overall Central Nervous System (CNS) activity, cell permeability through Caco-2 and MDCK cell lines, affinity for human serum albumin (log Khsa), and inhibition of the HERG K+-channel (log IC50). The extensive examination facilitates well-informed choices concerning the appropriateness of a molecule for subsequent advancement, hence promoting a more comprehensive and efficient evaluation of possible pharmaceutical candidates.
Grid Generation and Molecular Docking of SGTP4 with Respect to the Ligands
The ligand used in this study was imported from the Schrodinger suite after undergoing initial preparations, and it was readied for docking analysis using AutoDockTools version 4 through a series of essential steps:
1. Removal of Water Molecules: All water molecules were removed from the protein structure prior to conducting docking simulations. This step serves to streamline calculations, focus on biologically relevant interactions, and enhance the accuracy and efficiency of ligand binding predictions.
2. Repairing Missing Atoms: Missing atoms in the protein structure, which often occur in x-ray crystallography data, were addressed. This was achieved by identifying and repairing the missing atoms within the protein. In this process, a total of 445 residues were fixed.
3. Addition of Polar Hydrogens: Polar hydrogens were added to the protein structure. This step is crucial for ensuring the accuracy and reliability of predictions by considering essential hydrogen bonding interactions, structural flexibility, and accurate energetic calculations, all of which are vital for understanding the protein-ligand binding process.
4. Incorporation of Kollman Charges: Kollman charges were introduced to the protein molecule. These charges contribute to a more realistic representation of electrostatic interactions, accurate energy calculations, and improved identification of binding sites, ultimately leading to more reliable predictions of ligand-protein binding interactions. The net Kollman charge was determined to be 5.506 and redistributed to address any uneven distribution, resulting in a new net charge of 5.9999.
5. Addition of Ligands: The ligands were added to the prepared protein structure. During this step, charges were automatically assigned to the ligands, and the ligand's rotatable bonds were detected using the Ligand torsion tool. The number of rotatable bonds is important for setting parameters in subsequent docking simulations.
6. Grid Generation: Grids for docking simulations were created based on a blind docking approach, covering the entire molecule. This process was repeated for all ligands, with grids locked in by running the grid command.
7. Docking Setup: The docking process involved selecting the macromolecule (protein) and ligand with default parameters. The search parameters were configured to use a Genetic algorithm, a dependable and modern method for docking simulations. Universal parameters of 50 runs and a population of 300 were set for all ligands, except for Harmonine and Licarin, which had specific settings due to their different numbers of rotatable bonds. The output was configured to use the Lamarckian hybrid of local and simulated values for optimal results.
8. Docking Results Analysis: The obtained docking results were further analyzed using web servers for visualization and data manipulation. Specifically, PLIP was employed to generate 3D images, Protein Plus was utilized for in-depth analysis and 2D images, and LigPlot Plus provided a different variation of 2D images. These tools aided in the comprehensive assessment and interpretation of the docking results.
These meticulous steps in ligand preparation and docking analysis contribute to the robustness and reliability of the study's findings in the context of drug discovery and molecular interactions.
Results and Discussions
Given the difficulties associated with the only current medicine, Praziquantel, the urgent need for a new schistosomicide cannot be stressed. The development of a structure-based medication with great effectiveness has the potential to overcome the challenges that the current therapy faces. Traditional inhibitor identification and development strategies are both capital and time expensive. As a result, computational approaches have emerged as important instruments in this quest in recent years, as indicated by various research [36,56,57].
The following steps were taken in the docking analysis:
1. Importing Autodock Results: The Autodock results in .dlg format were imported into the docking analysis panel, where the macromolecule was overlaid with the ligand. The best conformation among the 50 runs was then selected, and the value of this best conformation was obtained from the first row of the RMSD Table in the .dlg file output. Subsequently, the complex was saved as a .pdbqt file.
Run with the best results
Protein
Compound
RMSD
Binding Energy (Kcal/Mole)
Inhibition Constant(Ki)
35
SGTP4
PZQ
91.23
-12.08
1.39 nm
14
SGTP4
Licochalcone
88.87
-11.83
2.12 nm
38
SGTP4
P96
91.29
-11.52
3.60 nm
26
SGTP4
Licarin
87.03
-9.79
66.91 nm
21
SGTP4
Harmonine
89.78
-8.21
963.11 nm
Table 1: Docking Simulation Results for Protein-Ligand Interactions.
Harmonine
Hydrophobic Interractions
Index
Residue
AA
Distance
Ligand Atom
Protein Atom
1
22A
PHE
3.77
4447
185
2
193A
LEU
3.92
4433
1751
3
194A
PRO
3.66
4437
1761
4
196A
ILE
3.66
4439
1775
5
197A
LEU
4
4439
1782
6
200A
ALA
3.79
4432
1811
Hydrogen Bonds
Index
Residue
Distance H-A
Distance D-A
Donor Angle
Protein donor?
Side chain
Donor Atom
Acceptor Atom
1
15A
THR
2.65
3.15
113.36
No
Yes
4454 [O3]
131 [O3
Table (2-6): Molecular Interactions in Protein-Ligand Complexes.
Licochalcone
Hydrophobic Interractions
Index
Residue
AA
Distance
Ligand Atom
Protein Atom
1
60A
THR
3.26
4513
543
2
67A
VAL
3.7
4497
620
3
286A
ASN
3.36
4503
2671
4
290A
TYR
3.6
4500
2708
5
291A
TYR
3.28
4501
2727
6
416A
VAL
3.67
4503
3852
7
420A
PHE
3.53
4501
3882
Hydrogen Bonds
Index
Residue
Distance H-A
Distance D-A
Donor Angle
Protein donor?
Side chain
Donor Atom
Acceptor Atom
1
35A
GLU
1.94
2.78
143.96
None
Yes
4518 [O3]
309 [O3]
2
36A
LYS
3.07
3.67
105.4
Yes
Yes
319 [N3+]
4518 [O3]
3
64A
SER
2.65
3.04
105.17
Yes
Yes
594 [O3]
4510 [O2]
π-Stacking
Index
Residue
AA
Distance
Angle
Offset
Stacking Type
Ligand Atoms
1
63A
TRP
4.78
73.63
1.73
T
4512, 4513, 4514, 4515, 4516, 4517
2
290A
TYR
4.91
24.72
0.67
P
4493, 4494, 4495, 4496, 4497, 4498
Table 3:
Licarin
Hydrophobic Interractions
Index
Residue
AA
Distance
Ligand Atom
Protein Atom
1
254A
GLN
3.98
4494
2359
2
255A
VAL
3.69
4505
2370
3
399A
PRO
3.6
4507
3699
4
400A
ALA
3.43
4507
3706
Hydrogen Bonds
Index
Residue
Distance H-A
Distance D-A
Donor Angle
Protein donor?
Side chain
Donor Atom
Acceptor Atom
1
82A
GLY
3.57
4.01
109.76
No
No
4511 [O3]
747 [O2]
2
86A
ASN
2.68
3.67
155.51
Yes
Yes
785 [Nam]
4509 [O3]
3
255A
VAL
2.19
3.44
156.27
Yes
None
2366 [Nam]
4516 [O3]
Table 4:
P96
Hydrophobic Interractions
Index
Residue
AA
Distance
Ligand Atom
Protein Atom
1
287A
ALA
3.24
4512
2682
2
291A
TYR
3.2
4514
2727
3
416A
VAL
3.9
4512
3852
4
420A
PHE
3.13
4513
3882
5
421A
PRO
3.01
4506
3895
Table 5:
PZQ
Hydrophobic Interractions
Index
Residue
AA
Distance
Ligand Atom
Protein Atom
1
287A
ALA
3.41
4515
2682
2
291A
TYR
3.12
4513
2727
3
416A
VAL
3.74
4515
3852
4
420A
PHE
3.46
4513
3882
5
421A
PRO
2.94
4506
3895
6
421A
PRO
3.13
4508
3894
Hydrogen Bonds
Index
Residue
Distance H-A
Distance D-A
Donor Angle
Protein donor?
Side chain
Donor Atom
Acceptor Atom
1
64A
SER
2.72
3.55
138.82
Yes
No
584 [N3]
4494 [O2]
π-Stacking
Index
Residue
AA
Distance
Angle
Offset
Stacking Type
Ligand Atoms
1
291A
TYR
5.48
87.86
0.68
T
4500, 4505, 4506, 4507, 4508, 4509
Table 6:
2. Format Conversion: The format of the macromolecule was changed to a .pdb file format using the Open Babel application.
Homology Modelling
Because there is no resolved 3-D structure of the SGTP4 protein in the Protein Data Bank (PDB), a homology modeling method was used. The following steps were included in the procedure:
Sequence Alignment: The BLAST P algorithm was employed to conduct a search within the Protein Data Bank (PDB) for protein structures that possess homologous sequences to SGTP4. A crystal structure bearing the identifier 4ZW9: A was discovered and chosen as the template due to its sequence similarity of 37.83% to the SGTP4 protein sequence. It is important to highlight that a minimum sequence similarity of 25% is generally considered necessary for the successful application of homology modeling techniques [58].
Homology Modeling: The PRIME module, which is a component of the Schrodinger suite, was employed for the purpose of doing homology modeling. Figure 2A depicts the modeled structure of the SGTP4 protein alongside the overlay template.
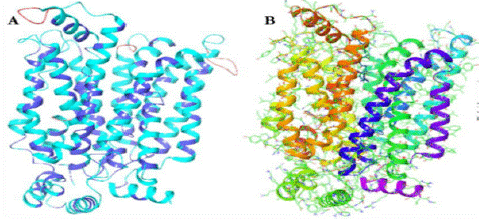
Figure 2: (A and B): depicting the raw and prepared SGPT4 protein.
Model Refinement: The Maestro Protein Preparation Wizard was used to further enhance the homology structure. The initial stage of this process was the introduction of hydrogen bonds to enhance the stability of the protein, followed by structural optimization and energy minimization. It is probable that Figure 2b depicts this particular procedure.
Quality Validation: PROCHECK was utilized to evaluate the structural integrity of the modeled SGTP4 structure. PROCHECK is a software application utilized for the purpose of structure validation, which relies on the analysis of the Ramachandran plot. The findings presented in Figure 2c indicate that a significant proportion of amino acid residues, specifically 93.2%, are located inside the most preferred regions. Additionally, 6.6% of residues are found in extra and generously authorized regions, while a little 0.2% are situated in banned regions. The findings suggest that the constructed model has a high level of quality, as evidenced by the exceptional distribution of amino acids within the permissible zones.
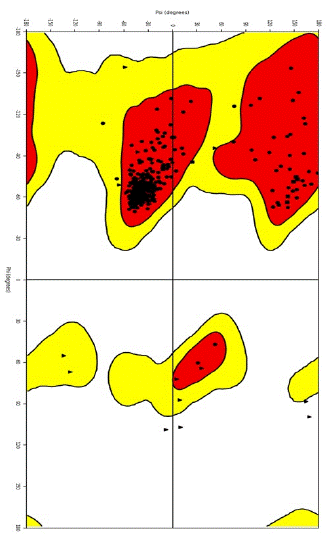
Figure 2C: Ramachandran plot of the SGTP4 protein that had been prepared.
The utilization of homology modeling and subsequent validation in this comprehensive strategy guarantees the reliability and high quality of the SGTP4 protein model employed in the study. This establishes a robust basis for subsequent molecular docking and drug discovery investigations.
Docking
By employing the genetic algorithm and in silico molecular docking research, the binding affinity of each candidate anti-schistosomal chemical to the SGTP4 protein was assessed. All of the druggable molecules' three-dimensional structures were located in a 3D sketcher and imported into the AutoDocksTools workspace for setup and optimization (Figure 3a–e).
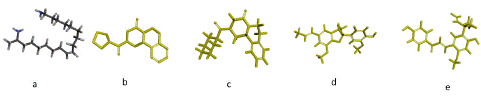
Figure 3: 3D Molecular Structures of Compounds (a) Harmonine, (b) P96, (c) PZQ, (d) Licarin, and (e) Licochalcone A.
Firstly, the Root Mean Square Deviation (RMSD) is a measurement employed to gauge how closely the predicted position of a ligand aligns with the actual experimental structure. Think of it as a way to quantify the similarity between the docked ligand's arrangement and the real arrangement in the crystal structure. Smaller RMSD values signify a better match, indicating that the predicted pose is in good agreement with the experimental data.
Secondly, we have the Binding Energy, expressed in units of Kcal per Mole. This value signifies the change in energy that accompanies the formation of a complex between the ligand and the protein target. In simpler terms, it reflects how strongly the ligand is sticking to the protein. A more negative binding energy implies a tighter, more stable binding interaction. This value is often employed to rank different ligands or different poses of the same ligand, helping researchers identify which ones are likely to have a stronger binding affinity.
Lastly, the Inhibition Constant (Ki) is a parameter that measures how strongly a ligand binds to a protein. Specifically, it represents the concentration of a ligand required to inhibit the protein's activity by 50%. Though typically determined through experiments, in docking studies, predicted Ki values can be estimated based on factors like docking scores or binding energies. A lower Ki value indicates a higher affinity, suggesting a stronger binding interaction between the ligand and the protein.
The docking result for PZQ shows a relatively high RMSD of 91.23, indicating that the predicted pose of the compound in the protein's binding site deviates significantly from the crystallographic pose. However, the substantial negative binding energy of -12.08 kcal/mol suggests a strong interaction between PZQ and the protein. The low Ki value of 1.39 nm further supports the high affinity of PZQ for the SGTP4 protein.
These results suggest that PZQ has a favorable binding mode and strong inhibitory potential against the target.Licochalcone displays a slightly lower RMSD of 88.87 compared to PZQ, indicating a somewhat better alignment of the predicted pose with the crystallographic pose. The negative binding energy of -11.83 kcal/mol suggests a strong binding affinity between Licochalcone and SGTP4. The Ki value of 2.12 nm indicates that Licochalcone exhibits moderate inhibitory potency against the target.
These findings suggest that Licochalcone could be a promising compound for further investigation.Similar to PZQ, P96 has a relatively high RMSD of 91.29, indicating a notable deviation in its predicted pose. However, the negative binding energy of -11.52 kcal/mol implies a favorable interaction between P96 and SGTP4. The Ki value of 3.60 nm suggests that P96 possesses moderate inhibitory activity against the target. While the RMSD is higher, the strong binding energy indicates that P96 could still be a viable compound for further evaluation.Licarin demonstrates a relatively low RMSD of 87.03, indicating a closer alignment with the crystallographic pose. The negative binding energy of -9.79 kcal/mol suggests a favorable binding interaction, although it is not as strong as observed for the previous compounds.
The higher Ki value of 66.91 nm indicates that Licarin has weaker inhibitory potency compared to the previous compounds. This result suggests that Licarin might require further optimization for improved binding.Harmonine's RMSD of 89.78 indicates moderate alignment with the crystallographic pose. The negative binding energy of -8.21 kcal/mol suggests a binding interaction, but the energy is notably weaker than that of the other compounds. The high Ki value of 963.11 nm suggests weak inhibitory activity of Harmonine against SGTP4. These results indicate that Harmonine might have a lower affinity for the target compared to the other compounds.
Overall, PZQ, Licochalcone, and P96 show strong binding affinities and inhibitory potentials against the SGTP4 protein, as indicated by their lower binding energies and Ki values. Among them, PZQ exhibits the highest binding energy and the lowest Ki value, suggesting the strongest interaction. Licarin displays moderate binding energy and inhibitory potency, while Harmonine shows weaker binding and inhibitory effects.
In conclusion, the docking results suggest that PZQ, Licochalcone, and P96 are promising candidates with strong interactions and inhibitory potential against SGTP4. Licarin displays moderate activity, and Harmonine exhibits relatively weaker binding and inhibition.
The docking analysis of Harmonine with the desired protein revealed a notable interaction profile. Hydrophobic interactions were observed with key amino acids such as PHE, LEU, ILE, and ALA, among others, suggesting a favorable binding characteristic. Moreover, Harmonine formed a hydrogen bond with the amino acid THR (15A). Notably, π-stacking interactions were also observed with TRP (63A) and TYR (290A), further stabilizing its binding conformation. These interactions collectively indicate that Harmonine exhibits a strong potential as a drug candidate for the desired protein, with THR being a critical residue for hydrogen bonding.
The docking results for Licochalcone demonstrated a robust interaction profile with the desired protein. Licochalcone engaged in hydrophobic interactions with residues including THR, VAL, ASN, TYR, VAL, and PHE. Furthermore, it formed hydrogen bonds with GLU (35A), LYS (36A), and SER (64A), indicating a strong binding affinity. π-stacking interactions were also identified with TRP (63A) and TYR (290A), enhancing its stability in the binding site. Overall, Licochalcone exhibits a promising profile as a potential drug candidate, with GLU, LYS, and SER being pivotal amino acids for hydrogen bonding.
The docking analysis of Licarin revealed favorable interactions with the desired protein. Hydrophobic interactions were observed with amino acids GLN, VAL, PRO, and ALA. Additionally, Licarin formed hydrogen bonds with GLY (82A), ASN (86A), and VAL (255A), indicating a strong binding potential. These interactions collectively suggest that Licarin holds promise as a drug candidate, with GLY, ASN, and VAL playing essential roles in hydrogen bonding.
The docking results for P96 primarily indicated hydrophobic interactions with the desired protein, suggesting its potential as a drug candidate. A more comprehensive analysis of specific interactions would be beneficial to determine its suitability in drug development.
PZQ displayed a robust interaction profile with the desired protein. It engaged in hydrophobic interactions with amino acids ALA, TYR, VAL, PHE, and PRO. Additionally, PZQ formed a hydrogen bond with SER (64A) and exhibited π-stacking interactions with TYR (291A). These interactions collectively highlight PZQ's potential as a drug candidate for the desired protein, with SER and TYR playing significant roles in key interactions.
A visual representation of the interactions between the ligands and the protein are represented in figures 4 through 5.
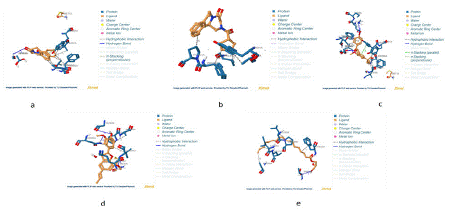
Figure 4: 3D Molecular Interactions in Ligand-Protein Complexes (a) Licarin, (b) P96, (c) Licochalcone A, (d) PZQ, and (e) Harmonine.
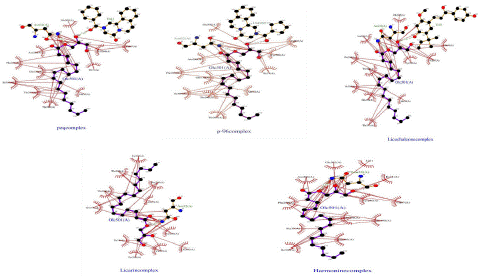
Figure 5: 2D Structures of Ligand-Protein Complexes.
Analysis of PZQ: This compound has a reasonable molecular weight, dipole moment, and surface area. It has suitable logP values for different solvents, suggesting moderate lipophilicity. The compound's logS value suggests potential solubility issues. The QPlogHERG score indicates low risk for HERG channel inhibition. The QPlogBB score suggests limited blood-brain barrier penetration. The QPPMDCK value is high, indicating good P-gp substrate. The compound's logKhsa score suggests it may bind to human serum albumin. However, the human oral absorption score is low, which might affect its oral bioavailability.
Analysis of P96: This compound has similar properties to PZQ, including reasonable molecular weight, dipole moment, and surface area. Its logP values suggest moderate lipophilicity. The logS value suggests potential solubility challenges. The QPlogHERG score indicates low risk for HERG channel inhibition. The QPlogBB score suggests limited blood-brain barrier penetration. The QPPMDCK value is high, indicating good P-gp substrate. The QPlogKhsa score suggests binding to human serum albumin. The human oral absorption score is low, affecting oral bioavailability, similar to PZQ.
Analysis of Licochalcone: This compound has a higher molecular weight, moderate dipole moment, and larger surface area. The high number of rotatable bonds suggests structural flexibility. The CNS value is negative, indicating limited central nervous system penetration. The QPpolrz and logP values suggest moderate lipophilicity. The QPlogHERG score indicates low risk for HERG channel inhibition. The QPlogBB score suggests limited blood-brain barrier penetration. The QPPMDCK value indicates it's a P-gp substrate. The QPlogKhsa score suggests binding to human serum albumin. The compound shows good oral absorption.
Analysis of Licarin: This compound has a molecular weight in a reasonable range, a moderate dipole moment, and a relatively large surface area. The number of rotatable bonds suggests flexibility. The CNS value is neutral, indicating potential central nervous system penetration. The QPpolrz and logP values suggest moderate lipophilicity. The QPlogHERG score indicates a low risk for HERG channel inhibition. The QPlogBB score suggests limited blood-brain barrier penetration. The QPPMDCK value indicates it's a P-gp substrate. The QPlogKhsa score suggests binding to human serum albumin. The compound shows good oral absorption.
Analysis of Harmonine: This compound has a relatively low molecular weight and a low dipole moment. It has a large surface area and a high number of rotatable bonds, suggesting flexibility. The CNS value is neutral. The QPpolrz and logP values indicate moderate lipophilicity. The QPlogHERG score suggests potential HERG channel inhibition. The QPlogBB score suggests limited blood-brain barrier penetration. The QPPMDCK value suggests it's a P-gp substrate. The QPlogKhsa score suggests binding to human serum albumin. The compound has lower human oral absorption, which might affect its bioavailability.
Conclusion
Docking Analysis
From the docking analysis, it is evident that the ligand-protein interactions play a pivotal role in determining the potential of compounds as candidates for further study. Licochalcone stands out as the most promising candidate due to its comprehensive interaction profile, featuring multiple strong hydrogen bonds and π-stacking interactions. PZQ closely follows, demonstrating robust interactions albeit with a slightly less diverse profile. Harmonine and Licarin exhibit moderate potential, with favorable interactions but not reaching the level of the top two candidates. P96 appears to be the least favorable due to its limited interaction profile.
ADMET Simulation Results
In-depth ADMET analysis established a ranking of the investigated compounds, with Licochalcone emerging as the most promising candidate. It possesses a reasonable molecular weight, a relatively low dipole moment, and a substantial surface area. Furthermore, Licochalcone adheres to Lipinski's Rule of Five and Rule of Three, indicating favorable drug-like properties, and exhibits a high human oral absorption score, suggesting good bioavailability. Despite a high number of rotatable bonds and a negative CNS score, its overall characteristics make it a strong candidate for further exploration.
Following Licochalcone, Licarin ranks as the next most promising compound, with favorable properties in terms of molecular weight, dipole moment, and surface area. It also complies with Lipinski's criteria and achieves a good human oral absorption score, positioning it as a potential candidate for drug development.
Praziquantel , the control compound, exhibits properties within acceptable ranges but faces challenges in terms of solubility and human oral absorption score, which may impact its bioavailability. However, it is essential to consider PZQ's established pharmacological activity against schistosomiasis when evaluating it alongside other compounds.
P96 shares similarities with PZQ in terms of molecular properties but also inherits some limitations, including low solubility and a low human oral absorption score. Although it shows potential, its properties do not significantly surpass those of PZQ. Harmonine ranks the lowest among the compounds analyzed, indicating limited structural complexity and concerns about bioavailability and safety.
Promising Candidates
These rankings and assessments provide valuable insights into the potential of these compounds for further drug development, with Licochalcone and Licarin emerging as the most promising candidates based on their favorable properties.
P96 Potential: Recent studies have highlighted the potential of P96, particularly with structural modifications. Substituting cyclohexyl with cyclopentyl has led to remarkable efficacy against immature stages of the parasite S. japonicum. Further research is needed to understand P96's precise mechanism of action and in vivo metabolic processes.
Licochalcone A: Licochalcone A, belonging to the chalconoid group of natural phenols isolated from liquorice root, demonstrates high promise with excellent drug-worthy properties. Notably, chalconoids have shown antimalarial, anticancer, antibacterial, and antiviral properties in vitro.
Future Research
In view of the challenges connected with Praziquantel , recent research on natural compounds from several chemical groups as potential anti-schistosomal medications is promising. In terms of binding affinity and stability of identified ligands against SGTP4, a druggable protein on the surface of the schistosome tegument, our computational findings closely reflect those of PZQ. This discovery has the potential to accelerate the development of schistosomiasis medications. To completely appreciate how these ligands exert their inhibitory effects on SGTP4, it is critical to underline that more experimental validations are required.
Author Statements
Consent for Publication
As the sole Author of the publication, I give consent for this research work to be published.
Competing Interests
The author declares no conflicts of interests regarding this submission. The research work has not been published nor under consideration elsewhere.
Authors Contributions
I, Peredy Khwesa, am the sole author of this work. All aspects of this document, including its conception, research, writing, and revision, are the result of my individual efforts and contributions.
Acknowledgements
I wish to thank Dr. Getachew Gizaw, AAU for his orientation throughout the research work.
References
- Fonseca BP, Albuquerque PC, Zicker F. Neglected tropical diseases in Brazil: lack of correlation between disease burden, research funding and output. Trop Med Int Health. 2020; 25: 1373-84.
- Abe EM, Tambo E, Xue J, Xu J, Ekpo UF, Rollinson D, et al. Approaches in scaling up schistosomiasis intervention towards transmission elimination in Africa: leveraging from the Chinese experience and lessons. Acta Trop. 2020; 208: 105379.
- Fuss A, Mazigo HD, Mueller A. Malacological survey to identify transmission sites for intestinal schistosomiasis on Ijinga Island, Mwanza, north-western Tanzania. Acta Trop. 2020; 203: 105289.
- Onasanya A, Bengtson M, Oladepo O, Van Engelen J, Diehl JC. Rethinking the top-down approach to schistosomiasis control and elimination in sub-Saharan Africa. Front Public Health. 2021; 9: 622809.
- da Silva VBR, Campos BRKL, de Oliveira JF, Decout JL, do Carmo Alves de Lima M. Medicinal chemistry of antischistosomal drugs: praziquantel and oxamniquine. Bioorg Med Chem. 2017; 25: 3259-77.
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006; 368: 1106-18.
- Available from: http://www.who.int. Schistosomiasis (bilharzia). [cited Jan 1 2023]. Available from: https://www.who.int/health-topics/schistosomiasis#tab=tab_1.
- Amoah AS, Hoekstra PT, Casacuberta-Partal M, Coffeng LE, Corstjens PLAM, Greco B, et al. Sensitive diagnostic tools and targeted drug administration strategies are needed to eliminate schistosomiasis. Lancet Infect Dis. 2020; 20: e165-72.
- Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388: 1603-58.
- Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014; 383: 2253-64.
- Nation CS, Da’dara AA, Marchant JK, Skelly PJ. Schistosome migration in the definitive host. PLOS Negl Trop Dis. 2020; 14: e0007951.
- Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014; 383: 2253-64.
- McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. 2018; 4: 13.
- Eyayu T, Zeleke AJ, Worku L. Current status and future prospects of protein vaccine candidates against Schistosoma mansoni infection. Parasite Epidemiol Control. 2020; 11: e00176.
- Caffrey CR. Chemotherapy of schistosomiasis: present and future. Curr Opin Chem Biol. 2007; 11: 433-9.
- Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg. 1994; 51: 83-8.
- Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, et al. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002-2008. Parasitology. 2009; 136: 1719-30.
- Olliaro P, Delgado-Romero P, Keiser J. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer). J Antimicrob Chemother. 2014; 69: 863-70.
- Wang W, Wang L, Liang YS. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res. 2012; 111: 1871-7.
- Crellen T, Walker M, Lamberton PHL, Kabatereine NB, Tukahebwa EM, Cotton JA, et al. Reduced efficacy of praziquantel AgainstSchistosoma mansoniIs associated with multiple rounds of mass drug administration. Clin Infect Dis. 2016; 63: 1151-1159.
- Couto FF, Coelho PMZ, Araújo N, Kusel JR, Katz N, Jannotti-Passos LK, et al. Schistosoma mansoni: a method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem Inst Oswaldo Cruz. 2011; 106: 153-7.
- Frye J. International drug price indicator guide; 2022. Available from: https://haiweb.org/wp-content/uploads/2019/12/International-Drug-Price-Indicator-Guide.pdf.
- Cioli D, Pica-Mattoccia L. Praziquantel. Parasitol Res. 2003; 90: S3-9.
- Botros S, Sayed H, Amer N, El-Ghannam M, Bennett JL, Day TA. Current status of sensitivity to praziquantel in a focus of potential drug resistance in Egypt. Int J Parasitol. 2005; 35: 787-91.
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007; 357: 1018-27.
- Pink R, Hudson A, Mouriès MA, Bendig M. Opportunities and challenges in antiparasitic drug discovery. Nat Rev Drug Discov. 2005; 4: 727-40.
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006; 6: 411-25.
- Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986; 61: 294-303.
- Utzinger J, Keiser J. Schistosomiasis and soil-transmitted helminthiasis: common drugs for treatment and control. Expert Opin Pharmacother. 2004; 5: 263-85.
- Abdulla MH, Lim KC, Sajid M, McKerrow JH, Caffrey CR. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLOS Med. 2007; 4: e14.
- Oliveira MF, d’Avila JC, Tempone AJ, Soares JB, Rumjanek FD, Ferreira-Pereira A, et al. Inhibition of heme aggregation by chloroquine ReducesSchistosoma mansoniInfection. J Infect Dis. 2004; 190: 843-52.
- Haas BJ, Berriman M, Hirai H, Cerqueira GG, LoVerde PT, El-Sayed NM. Schistosoma mansoni genome: closing in on a final gene set. Exp Parasitol. 2007; 117: 225-8.
- Ramirez B, Bickle Q, Yousif F, Fakorede F, Mouries MA, Nwaka S. Schistosomes: challenges in compound screening. Expert Opin Drug Discov. 2007; 2: S53-61.
- Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, et al. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLOS Negl Trop Dis. 2009; 3: e350.
- Bergquist NR. Schistosomiasis Consortium for Operational Research and Evaluation. Mission accomplished. Am J Trop Med Hyg. 2020; 103: 1-2.
- Adekiya TA, Kondiah PPD, Choonara YE, Kumar P, Pillay V. A review of nanotechnology for targeted anti-schistosomal therapy. Front Bioeng Biotechnol. 2020; 8: 32.
- Kohn AB, Anderson PAV, Roberts-Misterly JM, Greenberg RM. Schistosome calcium channel β subunits. J Biol Chem. 2001; 276: 36873-6.
- Becker B, Mehlhorn H, Andrews P, Thomas H, Eckert J. Light and electron microscopic studies on the effect of praziquantel onSchistosoma mansoni, Dicrocoelium dendriticum, andFasciola hepatica (Trematoda) in vitro. Z Parasitenkd. 1980; 63: 113-28.
- Harnett W, Kusel JR. Increased exposure of parasite antigens at the surface of adult male Schistosoma mansoni exposed to praziquantel in vitro. Parasitology. 1986; 93: 401-5.
- Isseroff H, Read CP. Studies on membrane transport—VIII. Absorption of monosaccharides by Fasciola hepatica. Comp Biochem Physiol A. 1974; 47: 141-52.
- Popiel I, Basch PF. Reproductive development of female Schistosoma mansoni (Digenea: Schistosomatidae) following bisexual pairing of worms and worm segments. J Exp Zool. 1984; 232: 141-50.
- Uglem GL, Lee KJ. Proterometra macrostoma (Trematoda: Azygiidae): functional morphology of the tegument of the redia. Int J Parasitol. 1985; 15: 61-4.
- Rogers SH, Bueding E. Anatomical localization of glucose uptake by Schistosoma mansoni adults. Int J Parasitol. 1975; 5: 369-71.
- Skelly PJ, Shoemaker CB. Rapid appearance and asymmetric distribution of glucose transporter SGTP4 at the apical surface of intramammalian-stage Schistosoma mansoni. Proc Natl Acad Sci U S A. 1996; 93: 3642-6.
- Skelly PJ, Shoemaker CB. The Schistosoma mansoni host-interactive tegument forms from vesicle eruptions of a cyton network. Parasitology. 2001; 122: 67-73.
- Krautz-Peterson G, Simoes M, Faghiri Z, Ndegwa D, Oliveira G, Shoemaker CB, et al. Suppressing glucose transporter gene expression in schistosomes impairs parasite feeding and decreases survival in the mammalian host. PLOS Pathog. 2010; 6: e1000932.
- Zhong C, Skelly PJ, Leaffer D, Cohn RG, Caulfield JP, Shoemaker CB. Immunolocalization of aSchistosoma mansonifacilitated diffusion glucose transporter to the basal, but not the apical, membranes of the surface syncytium. Parasitology. 1995; 110: 383-94.
- McKenzie M, Kirk RS, Walker AJ. Glucose uptake in the human pathogen Schistosoma mansoni is regulated through Akt/protein kinase B signaling. J Infect Dis. 2018; 218: 152-64.
- Park SK, Friedrich L, Yahya NA, Rohr CM, Chulkov EG, Maillard D, et al. Mechanism of praziquantel action at a parasitic flatworm ion channel. Sci Transl Med. 2021; 13: eabj5832.
- Le Clec’h W, Chevalier FD, Mattos ACA, Strickland A, Diaz R, McDew-White M, et al. Genetic analysis of praziquantel response in schistosome parasites implicates a transient receptor potential channel. Sci Transl Med. 2021; 13: eabj9114.
- Lima RM, Ferreira MAD, Ponte T, Marques MP, Takayanagui OM, Garcia HH, et al. Enantioselective analysis of praziquantel and trans-4-hydroxypraziquantel in human plasma by chiral LC–MS/MS: application to pharmacokinetics. J Chromatogr B. 2009;877(27):3083-8. doi: 10.1016/j.jchromb.2009.07.036.
- Dong Y, Chollet J, Vargas M, Mansour NR, Bickle QD, Alnouti Y, et al. Praziquantel analogs with activity against juvenile Schistosoma mansoni. Bioorg Med Chem Lett. 2010; 20: 2481-4.
- Guglielmo S, Cortese D, Vottero F, Rolando B, Kommer VP, Williams DL, et al. New praziquantel derivatives containing NO-donor furoxans and related furazans as active agents against Schistosoma mansoni. Eur J Med Chem. 2014; 84: 135-45.
- MacDonald K, Buxton S, Kimber MJ, Day TA, Robertson AP, Ribeiro P. Functional characterization of a novel family of acetylcholine-gated chloride channels in Schistosoma mansoni. PLOS Pathog. 2014; 10: e1004181.
- Xu J, Dong LL, Sun H, Huang P, Zhang RZ, Wang XY, et al. Small change, big difference: A promising praziquantel derivative designated P96 with broad-spectrum antischistosomal activity for chemotherapy of schistosomiasis japonica. PLOS Negl Trop Dis. 2023; 17: e0011215.
- Aruleba RT, Adekiya TA, Oyinloye BE, Masamba P, Mbatha LS, Pretorius A, et al. PZQ therapy: how close are we in the development of effective alternative anti-schistosomal drugs? Infect Disord Drug Targets. 2019; 19: 337-49.
- Aruleba RT, Adekiya TA, Molefe PF, Ikwegbue PC, Oyinloye BE, Kappo AP. Insights into functional amino acids of ULBP2 as potential immunogens against cancer. Sci Afr. 2020; 10: e00581.
- Yadav M, Khandelwal S. Homology modeling and molecular dynamics dimulation study of β carbonic anhydrase of Ascaris lumbricoides. Bioinformation. 2019; 15: 572-8.