
Research Article
Ann Depress Anxiety. 2014;1(7): 1033.
Erythrocyte Aggregability Enables the Distinction between Negative and Depressive Symptoms among Schizophrenia and Schizoaffective Disorder Patients
Alexander M Ponizovsky1,2*, Gregory Barshtein4, Yakov Nechamkin1, Michael Ritsner1,3, Saul Yedgar4 and Lev D Bergelson5
1Institute for Psychiatric Research, Sha’ar Menashe Mental Health Center, Israel
2Mental Health Services, Ministry of Health, Israel
3Department of Psychiatry, Bruce Rappaport Faculty of Medicine, Israel
4Department of Biochemistry, Hebrew University, Jerusalem
5Laboratory of Biological Membranes, Hebrew University, Jerusalem
*Corresponding author: Alexander M Ponizovsky, Mental Health Services, Ministry of Health, 39 Yirmiyahu St., POBox 1176, Jerusalem, 9446724, Israel
Received: November 04, 2014; Accepted: November 13, 2014; Published: November 18, 2014
Abstract
Objective: Based on the membrane-phospholipid hypothesis of schizophrenia, the authors tested the assumption that erythrocyte aggregation is differentially associated with negative and depressive symptoms of schizophrenia and schizoaffective disorder.
Methods: A cell flow properties analyzer was used to measure erythrocyte aggregation levels in 68 in patients with schizophrenia and schizoaffective disorder between ages 18 and 60 years and 30 normal comparison subjects without known neuropsychiatric disorders, proportionally matched for age and gender. Positive, negative and general psychopathological symptoms were quantified with the Positive and Negative Syndrome Scale (PANSS). Multiple regression analysis was used to examine the association of erythrocyte aggregation with clinical symptoms.
Results: There were no significant differences in erythrocyte aggregation levels between schizophrenic and schizoaffective disorder patients and normal control subjects. The erythrocyte aggregability directly and strongly correlated with the severity of negative syndrome, but inversely with affective components of the disorders. These findings were unrelated to gender, age at testing, age at onset and duration of the illnesses, body mass index, serum cholesterol and fibrinogen levels, smoking, and current medication.
Conclusion: The findings provide evidence that erythrocyte aggregation may serve as a potential endophenotype marker to distinguish negative and depressive features in schizophrenia and schizoaffective disorder patients.
Keywords: Schizophrenia; Schizoaffective disorder; Symptom dimensions; Erythrocyte aggregability; Peripheral marker
Abbreviations
RBC: Red Blood Cell; BMI: Body Mass Index; EPUFAs: Essential Polyunsaturated Fatty Acids; DSM-IV: Diagnostic Statistical Manual; PANSS: Positive and Negative Syndromes Scale; SCID: Structured Clinical Interview for DSM-IV; CGI: Clinical Global Impression scale; GAF: Global Assessment of Functioning scale; DDD: Defined Daily Dose; PBS: Phosphate Buffered Saline; AAS: Average Aggregate Size.
Introduction
Schizophrenia is a severe mental illness with polymorphic symptomatology, unknown etiology and complex pathophysiology. The core features of schizophrenia are conventionally separated, by behavioral criteria, into two major types: the positive (psychotic) syndrome (PS) characterized by hallucinations, delusions and thought disorders and the Negative Syndrome (NS) characterized by apathy, emotional blunting, avolition and alogia [1-4]. This dichotomy has important clinical and prognostic significance. While the positive symptoms are preponderant at onset of the illness or in phases of acute exacerbation and may be improved with drug therapy, the negative symptoms appear generally in the chronic course, are often treatment-resistant with conventional and even with atypical antipsychotics, and responsible for the bulk of disability caused by the disease.
Because the distinction between NS and PS is based exclusively on behavioral criteria and most individuals with schizophrenia display a mixed positive negative symptomatology, physiological and biochemical criteria have been sought for the assessment of the two syndromes [2,4,5]. Both schizophrenia subtypes were found to be associated with impaired regional cerebral blood flow (rCBF) [6-9], but after neuroleptic treatment this impairment could be correlated only with NS [10,11]. In accord with that it has been found that PANSS negative scores correlate with rCBF in the cingulate gyrus and other brain regions [12].
Recent biochemical, cerebral magnetic resonance spectroscopy, and molecular genetic findings support the membrane phospholipid hypothesis of schizophrenia [13]. This hypothesis suggests that phospholipid metabolism is disturbed in schizophrenia, and various abnormalities in the composition and structure of brain and blood cell membranes differentially correlate with negative and positive symptoms of the illness [14-16]. For instance, red blood cells (RBCs)
of schizophrenic patients with prominent negative features have been shown to have low levels of essential polyunsaturated fatty acids (EPUFAs; especially, arachidonic and docosohexanoic acids), while RBCs from patients at the active phase of the psychosis show the opposite trend [17-19]. Recently we found that on the average RBCs from schizophrenia patients with mainly negative symptoms display higher sphingomyelin and lower phosphatidylethanolamine levels than RBCs from patients with predominantly positive symptoms [20]. Neuroimaging techniques show reduced phosphomonoesters and increased phosphodiester levels in the frontal lobes of neuroleptic-treated as well as drug-naive schizophrenia patients [21- 24], consistent with a deficit in the function of prefrontal dopamine pathways (hypofrontality), which is involved in the pathology of the disorder [25].
The specific alterations of RBC membrane phospholipids in schizophrenia may be expected to result in changes of their hemodynamic properties, e.g., aggregability. The aggregability of RBCs, i.e., their ability to form multicellular aggregates, plays a major role in blood flow. RBCs in the presence of plasma proteins, most importantly fibrinogen, may aggregate to form rouleaux formations [26]. The extent of RBC aggregation is determined by opposing forces: the repulsive force between the negatively-charged cells, the cell-tocell adhesion induced by plasma proteins, and the disaggregating shear force generated by blood flow [27,28]. RBC aggregation is thus dependent both on plasma (extrinsic) factors and on cellular (intrinsic) factors. Normally, the blood flow is sufficient for dispersion of RBC aggregates, which is essential for normal tissue perfusion. However, in low flow states and other pathological conditions, increased RBC aggregation may contribute to circulatory disorders and, particularly in the microcirculation, to the occlusion of micro-vessels [29]. It is assumed that this process is dependent upon both the size of RBC aggregates and the cohesive forces within aggregates, expressed by the shear stress required for dispersing them.
Although increased RBC aggregability has been observed in various pathological states, such as cardiovascular diseases [30-33],diabetes [34], hyperlipidemia, sickle cell, hemorrhagic shock [35,36], Binswanger’s disease and other subtypes of dementia [37], only one recently published study explored this phenomenon in schizophrenia [38]. This study documented that patients meeting DSM-IV criteria for chronic schizophrenia with mainly negative symptoms, as measured by the Positive and Negative Syndrome Scale scores, had significantly increased aggregation of RBCs compared with that of normal controls. At the same time, the levels of RBC aggregation among schizophrenic patients with mainly positive symptoms were normal [38] (Figure 1).
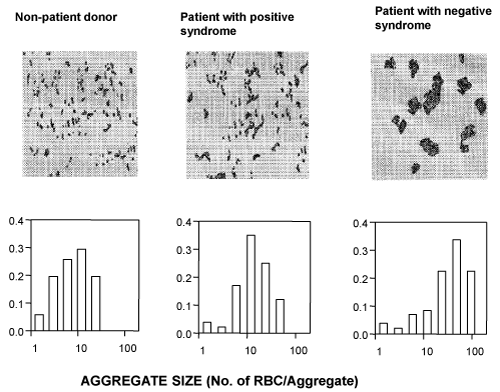
Figure 1: Representative aggregation images and corresponding aggregate
size distribution diagrams of RBCs (diagrams) of a nonpatient control subject
(left) and schizophrenic patients with mainly Positive Syndrome (middle) and
mainly Negative Syndrome (right).
The figure demonstrates that the aggregation of RBCs of the patient with mainly Negative Syndrome is markedly higher than that of both RBCs of the patient with mainly Positive Syndrome and the non-patient control subject. Moreover, while RBCs of the patient with Positive Syndrome and control RBCs show mainly small rouleaux-shaped aggregates, the RBCs of the patient with Negative Syndrome exhibit substantially larger amorphic aggregates.
RBC were isolated by centrifugation and resuspended in cell free autologous plasma at a concentration of 6%. The images were taken at a shear stress of 0.5 dyn/cm2 in the cell flow properties analyzer (see Methods).
In addition to the extent of RBC aggregation, in the patients with predominantly negative symptoms the strength of intercellular interaction as expressed by the aggregates resistance to disaggregation by the flow-induced shear stress was markedly enhanced. These differences in erythrocyte aggregability were found to be unrelated to antipsychotic treatments (haloperidol and risperidone), diet and body mass index, smoking, and plasma cholesterol and fibrinogen levels. This initial study was limited because of the relatively small numbers of patients and nonpatient control subjects.
The present study was undertaken to confirm and extend these earlier investigations. We extended our sample of schizophrenia patients and nonpatient controls, and added a number of patients with schizoaffective disorder, which is intermediate between schizophrenia and mood disorders. We hypothesized that RBC aggregation is associated with negative syndrome severity of both schizophrenia and schizoaffective disorder. We also hypothesized that because depressive features are often present in patients suffering from schizophrenia and schizoaffective disorders, and because some components of depression phenomenologically resemble negative symptoms, RBC aggregability could serve as a pathophysiological marker for the distinction between negative and depressive symptoms.
Methods
Subjects
Schizophrenia (n=58) and schizoaffective disorder (n=10) patients were recruited from Sha’ar Menashe Mental Health Center, Hadera, after providing written informed consent for participation in the study as approved by the Institutional Review Board for human studies. Diagnoses were made according DSM-IV criteria [39] by two senior psychiatrists using the Structured Clinical Interview for DSM-IV Axis I Disorders, Patients Edition (SCID) [40]. Patients with comorbid neurological or somatic disease, substance/alcohol abuse/ dependence, and clinically significant abnormalities in hematological and/or in biochemistry screening tests were not enrolled. Diagnostic types of schizophrenia patients included 31 patients with paranoid type (295.30), 17 with undifferentiated type (295.90), 3 with disorganized type (295.10), and 7 with residual type (295.60) of disease. Schizoaffective disorder patients included 4 patients with depressive type (295.70D) and 6 with maniac type (295.70M). The symptom presentations were assessed using the Positive and Negative Syndromes Scale (PANSS) [3,41]. The PANSS traditional tridimensional model (positive and negative syndromes, and general psychopathological symptoms) as well as the pentagonal structural model of schizophrenia symptoms (negative, positive, activation, dysphoric, and autistic preoccupation clusters) was analyzed [42]. In addition, the Clinical Global Impression Scale [43] and Global Assessment of Functioning scale [39] were employed.
Healthy volunteers (n=30) without known psychiatric history, and age/sexed matched to the patient group were recruited from staff members of the same hospital.
Table 1 presents basic characteristics of the patient and control groups. As can be seen, the study groups matched for gender and age, but not other sociodemographic characteristics. Unmarried subjects with lower education level were over-represented among the patients, and married and more educated among the controls. The index group included typical patients suffering from long-term severe mental illnesses (mean ± SD of illness duration=16.6±10.5 years) with significantly expressed positive (PANSS mean score ± SD=20.1±7.4) or negative symptoms (PANSS mean score ± SD =30.8±9.2). Most patients were disabled and socially isolated; they had low levels of functioning, and made clinical impression of markedly ill people. Since diet and smoking were shown to affect RBCs aggregation, all patients had similar (but not identical) dietary regime; 47 of them smoked 20 - 60 cigarettes/day regularly during several years. At the time of study all patients were receiving various antipsychotic medications at conventional doses including haloperidol (n=25), perphenazine (n=4), clozapine (n=4), risperidone (n=21), and olanzapine (n=13). Schizoaffective patients received additional antidepressants or anti-manic agents, and some patients received anticholinergic medications and benzodiazepines. All patients were exposed to prolonged antipsychotic medication. Dosages for all drugs were converted into Defined Daily Dose (DDD), which is the average maintenance dosage as defined by WHO Collaborative Centre for Drug Statistics (1997).
Characteristic
Patients (n=68)
Nonpatients (n=30)
Statistics
Sex, No. (%) male
50 (75.7)
23 (76.6)
z=.33, p=.74
Age, y
40.6 ± 10.8
41.0 ± 5.5
t=.24, p=.81
Marital status, No. (%)
Single
39 (59.1)
--
--
Married
13 (19.7)
24 (80.0)
z=5.73, p<.001
Divorced, separated, or widowed
14 (21.2)
6 (20.0)
z=.06, p=.95
Education, y
9.7 ± 2.7
14.7 ± 3.5
t=6.96, p<.001
Employment status, No (%) disabled
62 (93.9)
--
--
Age at onset of symptoms, y
23.8 ± 6.4
Length of illness, y
16.6 ± 10.5
Number of hospitalizations
6.6 ± 6.0
Length of hospital stay, mo
67.2 ± 86.9
Current medication dose, DDD*
2.5 ± 1.9
Smoking, cigarettes/day
34.5 ± 11.5
PANSS
Positive syndrome
20.1 ± 7.4
Negative syndrome
30.8 ± 9.2
General psychopathology
45.3 ± 11.1
Total score
96.1 ± 20.5
Clinical Global Impression scale
4.9 ± 1.1
Global Assessment of Functioning
39.5 ± 12.6
Mean scores ± SD are shown, unless otherwise indicated
* - Defined Daily Doses (DDD, World Health Organization Collaborative Center for Drug Statistics, 1997)
PANSS = the Positive and Negative Syndrome Scale
t - two-tailed t-test; z - Mann-Whitney two-sample (non-matched) test.
Table 1: Basic characteristics of patient and nonpatient comparison groups.
Determination of RBC aggregation
Blood samples (5 mL) were drawn from the antecubital vein and collected into EDTA- containing Vacutainers. All laboratory manipulations were performed within two hours of blood collection. Red blood cells were isolated by centrifugation (2000 rpm for 10 min), washed with phosphate buffered saline (PBS) pH 7.4, and resuspended to the desired hematocrit (6%) in autologous plasma as well as in PBS supplemented with 1 % bovine serum albumin (Sigma, St. Louis, MO) and 0.5 % dextran-T500 (Pharmacia Biotech, Upsala, Sweden), to induce the formation of RBC aggregates similar in size and shape to those formed in plasma [44]. This was done in order to differentiate between the contribution of cellular and plasmatic factors to the elevated aggregation, since the actual aggregation in blood is determined both by the properties of the cell membrane (aggregability) and the level of aggregation-inducing plasma components, mainly fibrinogen [45]. Accordingly, all experiments were performed in parallel with plasma and with PBC.
RBC aggregation was determined using the cell flow-properties analyzer described in detail previously [44]. In this computerized image analyzer, the RBC aggregation/disaggregation process is monitored visually under controllable flow conditions in a thermostatic (370C) narrow gap flow chamber (30 - 40 μm) placed under a microscope connected to a video camera. This transmits the RBC images to a computer equipped with software that provides the distribution of aggregate size (number of cells per aggregate) and Average Aggregate Size (AAS) under varying shears stress. Flow is induced by a pressure difference in the chamber, with a pump monitored by a pressure transducer. We measured RBC-aggregability at fixed level of shear stress 0.5 dyne/cm2. All measurements were done in triplicate and the average of the two closest values was used for the estimation of AAS. All measurements were performed blind to clinical information on the subjects.
Statistical methods
All statistical analyses were performed using the Number Cruncher Statistical System (NCSS-2000, NCSS Statistical Software, Kaysville, Utah). Means and standard deviations with 95% confidence intervals (95% CI) were computed. Between-group differences in means (±SD) and proportions were analyzed using two-tailed t-tests and Man-Whitney two-sample proportion (nonmatched) tests, respectively. Multiple regression analysis was employed to test whether or not the clinical symptoms (PANSS subscales) were correlated with erythrocyte aggregation (AAS) controlling for potentially confounding variables (age, length of illness, and current medication dose). To clarify which of the 5 symptom clusters (negative, positive, activation, dysphoric, and autistic preoccupation) [42] are associated with RBC aggregation, the PANSS cluster scores were compared between two subgroups of patients: a) those with low AAS levels (= 15 RBC/aggregate) and b) those having high AAS levels (>15 RBC/aggregate). We use this conservative cutoff based on data from previous studies, where the mean AAS score varied from 11.6 ± 1.3 to 14.3 ± 6.4 RBCs per aggregate under normal conditions [34- 36]. Moreover, of several cutoff points tested, we found the 15 RBCs/ aggregate point to fit best the two PANSS models (tridimensional and pentagonal) used in the present study (data for other cutoffs tested available on request). The relationships between continuous variables (age at testing, age at onset of the illness, body mass index, and serum cholesterol and fibrinogen levels) and RBC aggregation were tested by computing Pearson’s correlation coefficients. Analysis of variance (ANOVA) was used to examine the main effects of categorical parameters (gender and tobacco smoking) on the variation in AAS. For all analyses, the level of statistical significance was defined as α<.05.
Results
Mean AAS of RBCs from schizophrenia patients (15.04±7.5, 95% CI 13.2-16.8, n=58) did not significantly differ from those of schizoaffective disorder patients (11.0±5.0, 95% CI 7.4-14.6, n=10) (t1,68=1.89, P=.06) and healthy control subjects (12.3±6.8, 95% CI 8.2-12.4, n=30) (t1,88=1.70, P=.09). Since the schizophrenia and schizoaffective disorder patients had comparable AAS levels, we combined the two patient subgroups for further analyses (Table 2).
Predictor
Regression Coefficient (b)
Standard Error
t-value (b=0)
Probability Level
Total % of Variance Accounted For
Positive Syndrome
0.01
.09
0.10
.92
3.8
Negative Syndrome
0.58
.07
4.09
.0001
9.1
General psychopathology
-0.51
.07
-3.32
.0016
3.4
Age
0.22
.08
1.20
.23
2.0
Length of illness
-0.19
.09
-1.06
.29
0.3
Current medication dose
0.07
.04
0.64
.52
0.2
* AAS = Average Aggregate Size (RBC/aggregate)
# PANSS - the Positive and Negative Syndrome Scale
F68 = 3.90, P = .002, R2 = .28; Adjusted R2 = .21
Table 2: Summary of multiple regression analysis for predicting AAS* from PANSS# scores for 68 patients with schizophrenia and schizoaffective disorder.
The results of multiple regression analysis showed that only two of the PANSS dimensions had predictive power (F68 = 3.90, P = .002, R2 = .28; Adjusted R2 = .21) for the variance in individual levels of RBC aggregation: negative syndrome severity appeared to be associated with increased AAS scores, while general psychopathological symptoms were associated with reduced RBC aggregation (Table 2). The subscales accounted for 9.1% and 3.4%, respectively, of the total variance in AAS scores, when potential confounders (age, length of illness, and current medication doses) were controlled.
Among the three PANSS dimensions, only Negative syndrome mean scores were significantly higher in the subgroup of patients with high AAS than in the subgroup with low AAS values (35.8±6.7 vs. 29.5±5.5; t=3.94, P<0.001). When the PANSS pentagonal model was analyzed (Table 3), negative cluster scores were significantly higher (t=6.83, P<0.001) and dysphoric cluster scores were lower (t=3.86, P<0.001) in the high AAS subgroup compared with the low AAS subgroup. The negative symptom cluster included the PANSS items such as lack of spontaneity, blunted affect, emotional withdrawal, poor rapport, apathetic/social withdrawal, motor retardation, mannerisms, uncooperativeness, impaired volition, and impulsivity. The dysphoric cluster comprised the symptom items of anxiety, tension, guilt, depression, and somatic concerns.
PANSS
Low AAS group (n=44)
High AAS group (n=24)
t-value
P
Mean ± SD 95% CI
Mean ± SD 95% CI
Cluster
Negative
25.9 ± 6.2 24.0 27.8
36.1 ± 5.7 33.7 38.5
6.83
.001
Positive
18.5 ± 5.6 16.8 20.2
19.1 ± 4.9 17.0 21.2
0.46
.65
Activation
6.5 ± 2.3 5.8 7.2
7.0 ± 2.5 5.9 8.1
0.81
.42
Dysphoric
6.1 ± 2.1 5.5 6.7
4.4 ± 1.5 3.8 5.0
3.86
.001
Autistic preoccupation
5.9 ± 1.8 5.4 6.4
6.1 ± 2.0 5.3 6.9
0.41
.68
PANSS - the Positive and Negative Syndrome Scale
AAS - Average Aggregate Size:
Low AAS - £ 15 RBC/aggregate
High AAS - >15 RBC/aggregate
Table 3: Differences in PANSS pentagonal model clusters and individual item scores between the groups of patients with low and high RBC aggregation.
The AAS of RBC was not associated significantly with current age and age at onset of the illness (both r=.05), body mass index (r=.02), and serum-cholesterol and fibrinogen levels (r=.07 and r=.11, respectively; all P>.05). Likewise, no significant effects of gender (oneway ANOVA; F1,68=0.23, P=.62), and tobacco smoking (F1,68=0.07, P=.81) on AAS scores were found.
Discussion
This study tested the hypothesis that RBC aggregation is differentially associated with the severity of negative and affective symptoms of schizophrenia and schizoaffective disorder. The obtained results support this hypothesis demonstrating a significant relationship between increased RBC aggregation and the severity of key elements of the negative syndrome. In addition, we found a significant inverse association of RBC aggregation with affective symptom cluster of the disorders. These findings appeared to be unrelated to gender, age at testing, age at onset and length of the illness, body mass index, serum cholesterol and fibrinogen levels, smoking, and current medication dose. Thus, the findings provide evidence that RBC aggregation may serve as a physiological marker for the distinction between negative and dysphoric symptom clusters in schizophrenia and schizoaffective disorders.
Despite ample evidence for the membrane phospholipid hypothesis, an important issue for clinicians is whether it has any useful implication for diagnosis and treatment. A depressive syndrome as an independent facet of schizophrenia could conceivably masque as negative features [46,47]. Indeed, the characteristic symptoms of negative schizophrenia, such as blunted affect, emotional withdrawal, and cognitive deficits [48,49] are most likely confounded with depressive features, such as anhedonia, diminished motivation (apathy), ideation and motor activity reduction [50]. Moreover, drug-induced depression resembling the core features of the negative syndrome is common in schizophrenic patients receiving antipsychotic treatments [51].The different levels of RBC aggregation established in this study might help clinicians to differentiate primary from secondary negative symptoms associated with depression, if a clinically useful test will be created.
Although several biological (endophenotype) markers for schizophrenia have been proposed recently [52,53], no one of them appears to be specific. This, of course, is true also for RBC aggregability, which is enhanced in many pathological conditions. This nonspecificity makes it unlikely that RBC aggregability is a primary event involved in the pathogenesis of schizophrenia. Rather, we suggest that mechanism(s) underlying the observed phenomenon modifies the course of schizophrenia and contributes to a deteriorating course and development of the negative syndrome.
This mechanism(s) of RBC aggregability is yet not known. As mentioned in the Introduction, RBC aggregation is the result of opposing forces: aggregation induced by the presence of plasma proteins (or other macromolecules), especially fibrinogen and disaggregation induced by repulsive (electrostatic) forces and flowinduced shear stress [54]. The patients included in this study had a normal blood level of fibrinogen that was not correlated with RBC aggregate size. This is in accord with previous reports that no differences in plasma fibrinogen and immunoglobulin levels were found between medicated schizophrenic and bipolar patients when compared to normal control subjects [55]. This suggests that the relationship between RBC aggregation and the negative syndrome was not due to extracellular factors, but rather to changes in the cell membrane. As shown, RBCs from schizophrenic patients do not differ from normal controls in their protein patterns [56], but several important abnormalities are observed in their lipid composition [16,20], some of which differentially correlate with the dimensions of schizophrenia [14,17,38]. These alterations in turn can influence the rheological properties of erythrocytes, which contribute to a circulatory slowdown and may result in worsening the condition of the cerebral white matter in different brain regions.
This study had several strengths. First, the research included a relatively large and clinically homogeneous group of individuals, thus providing data on a cohort of chronic hospitalized schizophrenia and schizoaffective disorder patients. Second, a large number of genderand age-matched nonpatient comparison subjects from the same hospital staff were studied in the same way.
There also are several limitations to the study. We were unable to include in our study unmediated patients, and, therefore, drug effects on RBC-aggregation cannot be ruled out. Such influence, however, seems to be unlikely for the following reasons: 1) RBC AAS did not significantly differ between patients and control subjects, yet the former were exposed to prolonged medication, and the latter not; 2) RBC AAS were similar in the patients with schizophrenia and schizoaffective disorder, although the subgroups received different types and classes of drugs; and 3) multiple regression analysis showed no effect of current medication dose on RBC aggregation. Unfortunately, the size of our sample permitted us to control the effects of only 5 variables on erythrocyte aggregation by multiple regression analysis, whereas the remaining putative confounders were tested with a simple correlation without adjusting for their mutual influence. Although as in almost all cases with cutoffs, our cutoff point (15 RBCs/aggregate), used to separate the groups with high and low aggregation was chosen somewhat arbitrarily, it was shown to fit better both PANSS models employed in this study. In the future studies, the groups of patients with high syndrome scores could be contrasted with those with low severity scores by levels of RBC aggregation, and thus the patients could serve as control for themselves. Likewise, because of negative symptoms may mediate food intake, dietary patterns should be controlled for in the future study.
In conclusion, the findings presented here suggest that enhanced RBC aggregation is linked to the negative syndrome of schizophrenia and might serve as a valuable peripheral marker for negative symptoms in genetic studies of schizophrenia.
Independent replication investigation including a larger number of schizoaffective and mood disturbed patients and taking into account other limitations of the study would be warranted.
Acknowledgment
We would like to dedicate this paper to the memory of Lev D. Bergelson, DSc, the prime mover of this study, who unfortunately passed away before the work was published. This study was supported in part by the Israel Ministry of Absorption (Dr Ponizovsky); and by grants 1460-1-99 from the Israel Ministry of Sciences and 482/96- 3 from the Israel Science Foundation and the Aachen University of Applied Sciences (Dr Yedgar), the Szold Foundation (Keren Yissumit of the Hebrew University) and the Israel Friends of the Hebrew University (“Ezvanot”, DrsYedgar and Barshtein), and 4165 (DrBarshtein) from the Israel Ministry of Health. We wish to thank Dr A. Gibel for his assistance with data collection.
References
- Crow TJ. The two-syndrome concept: origins and current status. Schizophr Bull. 1985; 11: 471-486.
- Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982; 39: 789-794.
- Kay SR. Positive and Negative Syndromes in Schizophrenia. Assessment and Research. New York, NY: Brunner/Mazel, Inc. 1987.
- Schultz SK, Andreasen NC. Schizophrenia. Lancet. 1999; 353: 1425-1430.
- Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004; 363: 2063-2072.
- Russell JM, Early TS, Patterson JC, Martin JL, Villanueva-Meyer J, McGee MD. Temporal lobe perfusion asymmetries in schizophrenia. J Nucl Med. 1997; 38: 607-612.
- Owega A, Klingelhofer J, Sabri O, Kunert HJ, Albers M, Sass H. Cerebral blood flow velocity in acute schizophrenic patients. A transcranial Doppler ultrasonography study. Stroke. 1998; 29: 1149-1154.
- Min SK, An SK, Jon DI, Lee JD. Positive and negative symptoms and regional cerebral perfusion in antipsychotic-naive schizophrenic patients: a high-resolution SPECT study. Psychiatry Res. 1999; 90: 159-168.
- Erkwoh R, Sabri O, Willmes K, Steinmeyer EM, Bull U, Sass H. Active and remitted schizophrenia: psychopathological and regional cerebral blood flow findings. Psychiatry Res. 1999; 90: 17-30.
- Sabri O, Erkwoh R, Schreckenberger M, Cremerius U, Schulz G, Dickmann C, et al. Regional cerebral blood flow and negative/positive symptoms in 24 drug-naive schizophrenics. J Nucl Med. 1997; 38: 181-188.
- Erkwoh R, Sabri O, Willmes K, Steinmeyer EM, Bull U, Sass H. [Aspects of cerebral connnectivity in schizophrenia. A comparative CBF study on treated schizophrenics before and after medication]. Fortschr Neurol Psychiatr. 1999; 67: 318-326.
- Ashton L, Barnes A, Livingston M, Wyper D; Scottish Schizophrenia Research Group. Cingulate abnormalities associated with PANSS negative scores in first episode schizophrenia. Behav Neurol. 2000; 12: 93-101.
- Horrobin DF, Glen AI, Vaddadi K. The membrane hypothesis of schizophrenia. Schizophr Res. 1994; 13: 195-207.
- Glen GJ, Glen EM, Horrobin DF, Vaddadi KS, Spellman M, Morse-Fisher N, et al. A red cell membrane abnormality in a subgroup of schizophrenic patients: evidence for two diseases. Schizophr Res. 1994; 12: 53-61.
- Walker NP, Fox HC, Whalley LJ. Lipids and schizophrenia. Br J Psychiatry. 1999; 174: 101-104.
- Fenton WS, Hibbeln J, Knable M. Essential fatty acids, lipid membrane abnormalities, and the diagnosis and treatment of schizophrenia. Biol Psychiatry. 2000; 47: 8-21.
- Horrobin DF, Glen AJM, Skinner F. Red cell membrane fatty acids in schizophrenic patients with mainly positive or mainly negative symptoms. Schizophr Res. 1993; 9: 221-226.
- Yao JK, van Kammen DP, Welker JA. Red blood cell membrane dynamics in schizophrenia. II. Fatty acid composition. Schizophr Res. 1994; 13: 217-226.
- Doris AB, Wahle K, MacDonald A, Morris S, Coffey I, Muir W, et al. Red cell membrane fatty acids, cytosolic phospholipase-A2 and schizophrenia. Schizophr Res. 1998; 31: 185-196.
- Ponizovsky AM, Modai I, Nechamkin Y, Barshtein G, Ritsner MS, Yedgar S, et al. Phospholipid patterns of erythrocytes in schizophrenia: relationships to symptomatology. Schizophr Res. 2001; 52: 121-126.
- Pettegrew JW, Keshavan MS, Minchev NJ. A 31P nuclear magnetic resonance spectroscopy study of neurodevelopment and schizophrenia. Schizophr Bull. 1993; 19: 36-53.
- Stanley JA, Williamson PC, Drost DJ, Carr TJ, Rylett RJ, Malla A, et al. An in vivo study of the prefrontal cortex of schizophrenia patients at different stages of illness via phosphorus magnetic resonance spectroscopy. Arch Gen Psychiatry. 1995; 52: 399-407.
- Shioiri T, Someya T, Murashita J, Kato T, Hamakawa H, Fujii K, et al. Multiple regression analysis of relationship between frontal lobe phosphorus metabolism and clinical symptoms in patients with schizophrenia. Psychiatry Res. 1997; 76: 113-122.
- Molina Rodriguez V, Montz AR, Perez CMJ, Gutierrez LR, Ferre NF, Carreas DJL, et al. Cerebral perfusion correlates of negative symptomatology and parkinsonism in a sample of treatment-refractory schizophrenics: an exploratory 99mTc-HMPAO SPECT study. Schizophr Res. 1997; 25: 11-20.
- Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004; 110: 243-256.
- Puniyani RR. Clinical Hemorheology: New Horizons. New Delhi, India: Popal New Age. 1996.
- Chien S. Blood rheology in myocardial infarction and hypertension. Biorheology. 1986; 23: 633-653.
- Lim B, Bascom PA, Cobbold RS. Simulation of red blood cell aggregation in shear flow. Biorheology. 1997; 34: 423-441.
- Chen S, Gavish B, Zhang S, Mahler Y, Yedgar S. Monitoring of erythrocyte aggregate morphology under flow by computerized image analysis. Biorheology. 1995; 32: 487-496.
- Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, et al. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11a substudy. Thrombolysis in Myocardial Infarction. J Am CollCardiol. 1998; 31: 1460-1465.
- De Jong K, Geldwerth D, Kuypers FA. Oxidative damage does not alter membrane phospholipid asymmetry in human erythrocytes. Biochemistry. 1997; 36: 6768-6776.
- ICSH recommendations for measurement of erythrocyte sedimentation rate. International Council for Standardization in Haematology (Expert Panel on Blood Rheology) J Clin Pathol. 1993; 46: 198-203.
- Cicco G, Vicenti P, Stingi GD, Tarallo, Pirrelli A. Hemorheology in complicated hypertension. Clin Hemorheol Microcirc. 1999; 21: 315-319.
- Foresto P, D'Arrigo M, Carreras L, Cuezzo RE, Valverde J, Rasia R. Evaluation of red blood cell aggregation in diabetes by computerized image analysis. Medicina (B Aires). 2000; 60: 570-572.
- Yedgar S, Hovav T, Barshtein G. Red blood cell intercellular interactions in oxidative stress states. Clin Hemorheol Microcirc. 1999; 21: 189-193.
- Chen S, Eldor A, Barshtein G, Zhang S, Goldfarb A, Rachmilewitz E, et al. Enhanced aggregability of red blood cells of beta-thalassemia major patients. Am J Physiol. 1996; 270: H1951-1956.
- Chen C, Jia H, Ma H. [Changes of erythrocyte and platelet membrane lipid pattern in different subtypes of dementia]. Zhonghua Yi Xue Za Zhi. 1998; 78: 771-773.
- Barshtein G, Ponizovsky AM, Nechamkin Y, Ritsner M, Yedgar S, Bergelson LD. Aggregability of red blood cells of schizophrenia patients with negative syndrome is selectively enhanced. Schizophr Bull. 2004; 30: 913-922.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Press. 1994.
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM IV I Disorders, Patient Edition (SCID P) and version 2. New York State Psychiatric Institute, Biometrics Research. 1995.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13: 261-276.
- White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. The PANSS Study Group. Psychopathology. 1997; 30: 263-274.
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, et al. Mental health surveillance among children--United States, 2005-2011. MMWR Surveill Summ. 2013; 62 Suppl 2: 1-35.
- Chen S, Gavish B, Zhang S, Mahler Y, Yedgar S. Monitoring of erythrocyte aggregate morphology under flow by computerized image analysis. Biorheology. 1995; 32: 487-496.
- Ami RB, Barshtein G, Zeltser D, Goldberg Y, Shapira I, Roth A, et al. Parameters of red blood cell aggregation as correlates of the inflammatory state. Am J Physiol Heart Circ Physiol. 2001; 280: H1982-1988.
- Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA. Stability of the diagnosis of deficit syndrome in schizophrenia. Am J Psychiatry. 1999; 156: 637-639.
- Kelley ME, van Kammen DP, Allen DN. Empirical validation of primary negative symptoms: independence from effects of medication and psychosis. Am J Psychiatry. 1999; 156: 406-411.
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001; 58: 165-171.
- Kay SR, Murrill LM. Predicting outcome of schizophrenia: significance of symptom profiles and outcome dimensions. Compr Psychiatry. 1990; 31: 91-102.
- Zisook S, McAdams LA, Kuck J, Harris MJ, Bailey A, Patterson TL, et al. Depressive symptoms in schizophrenia. Am J Psychiatry. 1999; 156: 1736-1743.
- Peralta V, Cuesta MJ, Martinez-Larrea A, Serrano JF. Differentiating primary from secondary negative symptoms in schizophrenia: a study of neuroleptic-naive patients before and after treatment. Am J Psychiatry. 2000; 157: 1461-1466.
- Ben-Shachar D, Zuk R, Gazawi H, Reshef A, Sheinkman A, Klein E. Increased mitochondrial complex I activity in platelets of schizophrenic patients. Int J Neuropsychopharmacol. 1999; 2: 245-253.
- Ilani T, Ben-Shachar D, Strous RD, Mazor M, Sheinkman A, Kotler M, et al. A peripheral marker for schizophrenia: Increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci U S A. 2001; 98: 625-628.
- Skalak R, Zarda PR, Jan KM, Chien S. Mechanics of Rouleau formation. Biophys J. 1981; 35: 771-781.
- Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997; 66: 1-11.
- Fritze J, Koronakis P, Riederer P. Erythrocyte membrane proteins in psychiatric disorders and controls. J Affect Disord. 1988; 15: 187-190.