
Special Issue - Dental Treatment
J Dent App. 2022; 8(1): 477-487.
Periodontal Treatment Reduces Circulating Pro-Inflammatory Cytokine and Chemokine Levels in African American HIV+ Individuals with Virological Suppression
Sampath C1, Harris EP1,2, Berthaud V2, Tabatabai MA3, Wilus DM4, Crayton MA1, Moss K5, Webster-Cyriaque J6, Southerland JH7, Koethe JR8, Gangula PR1*
1Department of Oral Diagnostic Sciences & Research in Biochemistry Meharry Medical College, School of Dentistry, USA
2Meharry Community Wellness Center, USA
3Department of Biostatistics, School of Graduate Studies and Research, USA
4School of Graduate Studies and Research, USA
5Division of Oral and Craniofacial Health Sciences, University of North Carolina Adams School of Dentistry, USA
6University of North Carolina Adams School of Dentistry, USA
7University of Texas Medical Branch at Galveston, Galveston, USA
8Vanderbilt University Medical Center, USA
*Corresponding author: Pandu R Gangula, Department of ODS & Research, Meharry Medical College, School of Dentistry, 1005 Dr. D.B. Todd Jr. Blvd, Nashville, TN 37208, USA
Received: July 16, 2022; Accepted: August 03, 2022; Published: August 10, 2022
Abstract
Introduction: Periodontal Disease (PD), a chronic inflammatory disease, is highly prevalent among Persons Living With HIV (PLWH) and is characterized by microbial symbiosis and oxidative stress. Our hypothesis stipulates that periodontal therapy attenuates systemic inflammatory and bacterial burden while improving periodontal status in PLWH.
Methods: Sixteen African Americans (AA) with suppressed HIV viremia on long-term Antiretroviral Therapy (ART) were recruited to this study. Participants were placed into two groups, based on their dental care status: group 1 (In- Care, IC) and group 2 (Out of Care, OC). Periodontal health was investigated at baseline, 3 months, 6 months, and 12 months. Cytokine/chemokines, microbial phyla, and Asymmetric Dimethylarginine (ADMA, a marker for endothelial cell dysfunction) levels were assessed in the serum. Statistical comparisons between groups and at different visits were performed using multiple comparison tests.
Results: Across longitudinal visits, periodontal treatment significantly reduced the levels of several cytokines and chemokines. At baseline, the out of care group had significantly higher blood levels of ADMA and actinobacteria than the IC group. Periodontal treatment significantly altered the abundance of circulating genomic bacterial DNA for various phyla in out of care group.
Conclusions: Periodontal treatment interventions effectively attenuated circulating pro-inflammatory cytokines and altered microbial translocation, both critical drivers of systemic inflammation in PLWH.
Keywords: Periodontal disease; Cytokines; Microbial translocation; ADMA; Endothelial cells; HIV
Introduction
Periodontal Diseases (PD) include several forms; however, the most common ones are gingivitis and periodontitis [1]. The 2009- 2012 National Health And Nutrition Examination Survey (NHANES) estimated that 47 percent of the United States population had periodontitis, of which 37.1 and 8.9 percent suffered from mild-tomoderate and severe forms of the PD [2]. Similarly, the report of the third National Health And Nutrition Examination Survey (NHANES III) in the U.S. showed that individuals living in neighborhoods characterized by lower socioeconomic status were 1.8 times more likely to have periodontitis than those of higher status characterized neighborhoods [3]. Racial and ethnic disparities in socioeconomic status and geographic location seem to play a role in susceptibility to PD [3].
Persons Living With HIV (PLWH) are at a higher risk of severe PD according to the American Academy of Periodontics (AAP) with an incidence of up to 75% [2]. The occurrence of oral and periodontal infections in virally suppressed PLWH on Antiretroviral Therapy (ART) suggests that ART may have a limited impact on oral inflammation. African Americans remain at a greater risk of HIV infection as compared to Caucasians [3]. Lack of access to dental care and ongoing oral inflammation resulting in gingivitis and/or PD represent major concerns in PLWH, especially within the AA population. Tooth loss can be a severe consequence of advanced periodontitis [4].
Even in the context of sustained viral suppression, chronic inflammation remains a key component of HIV pathology. Triggering of inflammatory signaling pathways by pathogenic bacteria is crucial for the induction of the inflammatory process in the periodontium [5]. PD has largely been recognized as an inflammatory disease caused by bacteria and their by-products detected in the dental plaque [6]. Untreated PD can result in the systemic spread of proinflammatory mediators such as cytokines like interleukin (IL)-1a, IL-1β, IL-6, IL-12, Tumor Necrosis Factor (TNF)-a that play a vital role in the progression of the inflammatory process [7]. Furthermore, treatment of periodontitis decreased IL-6 in virally suppressed PLWH while CD4 count increased [8], suggesting a potential impact on HIV disease and the risk of cardiovascular disease and diabetes in PLWH. Therefore, it is important to explore the expression of a wide array of these biomarkers in HIV+ individuals. In this study, we investigated the hypothesis that periodontal treatment attenuates circulating bacteria and cytokines in PLWH on suppressive ART.
Materials and Methods
Study Population
Participant recruitment and sample collection followed the study protocol (#1410019) as approved by the Meharry Medical College Institutional Review Board. All study participants signed the IRBapproved informed consent prior to enrollment. All of them were recruited at the Meharry Community Wellness Center, a Tennesseedesignated AIDS Center of Excellence. Subjects were eligible to participate if they were African Americans, male and female aged between 18 to 55 years old and had undetected viral load (<50 copies/ ml). Participants were stratified into two groups of eight: group one (In-Care, IC) included participants who received regular dental care (at least two visits in past year,) and group two (Out-of-Care, OC) included subjects not in regular dental care (less than 2 visits in past year). We assessed periodontal status at baseline by oral examination and digital radiographs. At each study visit, plaque index was recorded. Periodontal therapy along with oral hygiene instructions was provided at baseline, 3, 6, and 12 months.
Blood Sampling and Preparation
Prior to dental treatment and examination at each visit, 10 mL of whole blood was collected via venipuncture from the participant’s arm into vacutainer tubes. Then, the tubes were centrifuged at 1026 × g for 20 minutes to separate the serum. The serum samples were transferred into a new tube and stored at -80°C until used, resulting in a total of 61 samples from 16 participants during a total of 4 study visits.
CD4+ T-cell Count and HIV Viral Load Specimens
CD4+ T cell count and HIV-1 RNA level were performed as standard of HIV care at a commercial laboratory (LabCorp Inc., Nashville, TN) using flow cytometry and the Roche™ PCR assay, respectively.
Microarray Cytokine/Chemokine Analysis in Serum Specimens
Serum RNA extraction was performed according to Braunstein et al. [9]. Total RNA was isolated from human serum samples using a single-step guanidine thiocyanate method with Trizol (Invitrogen, Carlsbad, CA). RNA quality was determined by NanoDrop™. To synthesize cDNA, we used the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA).To perform RT-qPCR amplification, we applied the SYBR-Green method (Bio-Rad, Hercules, CA). PCR arrays were performed using the PAHS-011ZD Human Inflammatory Cytokines & Receptors Pathway RT2 Profiler PCR Array (Qiagen, Germantown, MD). The list of genesis represented in (Supplementary Table 1).
Furthermore, qPCR was performed to validate the microarray findings from OC group. The validation of microarray was conducted with selected primers (Supplemental Table 2).
Microbial Screening of Serum Specimens
DNA was extracted from human serum samples using QIAmp DNA Mini Kit (Qiagen, Germantown, MD) according to the manufacturer’s protocol. The microorganism quantification was conducted with selected primers (Supplemental Table 3) for Real Time PCR in the CFX96 thermocycler. The amplification program consisted of an initial 95°C 3-minute denaturalization step, followed by 35 cycles starting with one 95°C 30-second step, followed by 30-second annealing step, and a final step of 72°C 30-second. The melt curve was obtained with a gradual 0.5°C increase starting from 54°C to 95°C. Relative quantification was analyzed with the ΔCq of each microorganism, using the 16S rDNA as reference gene. In addition to a positive control sample for bacterial DNA, each PCR experiment contained a negative control that consisted of all PCR reagents in experimental samples without bacterial DNA. This measure safeguarded against the potential contamination of stock PCR reagents with microbial DNA products, which could lead to false positive results.
Measurements of Asymmetric Dimethylarginine (ADMA) Levels in the Serum of PLWH
Serum ADMA levels were measured at baseline, 6- and 12-month visits using ELISA kit (Eagle Biosciences, Amherst, NH) per manufacturers’ guidelines.
Statistical Analysis
Quality control was conducted to ensure the integrity of the data and check for the presence of outliers. We used pair wise tests to compare group visits, matched by gene, Shapiro-Wilk test to check normality, and Levene’s test to assess the equality of variances of gene expression levels. If the assumptions for the t-test were not satisfied, then, the non-parametric Wilcoxon test was used. Cytokines were correlated using Spearman correlation for small sample size, with the respective phyla from the same group and visit [10]. Data analysis was conducted using RStudio, version 1.4.1106 and software packages, lawstat, ggplot2, openxlsx, mosaic, grid, devtools, ggbiplot, ggfortify, and reshape2.
Results
Study Participant Characteristics
The study population included African Americans only, ten men and six women, all over thirty-five years old with a median age of 40 (IQR, 13.5 – 14.5).The median CD4+ T-cell count was 509/mL (IQR, 328/mL) and 728/mL (IQR, 404/mL) at baseline (Supplementary Table 4). In addition, 50% of the participants were diagnosed with gingivitis (probing depth <4mm) and 50% with periodontitis (probing depth >4mm). As per ADA guidelines, prophylaxis was performed for subjects diagnosed with gingivitis and pocket depths less than 4 mm; scaling and root planning was implemented for subjects diagnosed with periodontitis and pocket depths greater than 4 mm. At baseline, the mean extent pocket depths >=4 mm and attachments loss >=3 mm were 7.285 for group 1 and 87.1 for group 2, and decreased post-treatment to 3.37 and 83.9, respectively. Measures of gingival inflammation revealing bleeding on probing levels were 49.7 for group 1 and 77.3 for group 2 as compared to 39.53 and 45.7, post treatment. Likewise, with the implementation of oral hygiene, local factors diminished for group 2 with mean plaque score of 69.4 at baseline compared to 45.04, post intervention.
Correlation between Cytokines and CD4 count
The baseline visits comparisons between the two groups revealed the expression of 80 cytokines/chemokines out of 84 up regulated in group 2 when compared to group one (Supplementary Table 5A, Figure 1A). Among the up regulated cytokines, only two (FASLG & IL 10RB) were reduced at the first visit in both the groups, along with one chemokine (CCL 17). At the 12-month visit, group two demonstrated a higher response rate as compared to group one: 19 chemokines and 26 cytokines were significantly down regulated as compared to 7 and 20 (Supplementary Table 5B, Figure 1B-D). Such abnormal cytokine production could contribute to the pathogenesis of HIV inflammation and reflect cell-mediated immunity.
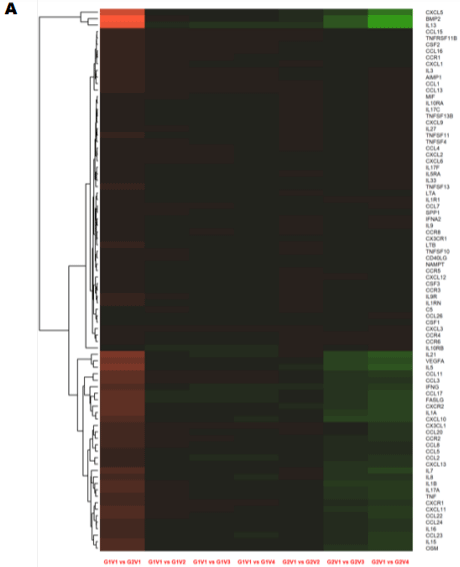
Figure 1: Longitudinal comparison of plasma cytokine/chemokine concentrations in PLWH before and after periodontal treatment. Heat map of differentially
expressed cytokine/chemokine array from PLWH at (A) Baseline (V1) comparisons of cytokines and chemokines between IC (In-Care) and OC (Out-of-Care)
PLWH. Data are presented as the average fold change at each visit compared to the baselines of their respective groups. Relative expression values from high
to low was shown by gradient of red to green in the heat map. Red indicates higher and green indicating lower expression of mRNAs. Volcano plot analysis of the
differentially abundant serum cytokine/chemokines in PLWH. The graphs represents the relative abundance of each metabolite against its statistical significance,
respectively reported as difference and -log10 (q-value), in IC in (Group 1); (B) Serum cytokine concentrations were reduced with periodontal treatments at different
regime (V2-3mo, V3-6mo, V4-12 months) when compared to baseline (V1) in IC (Group 1) PLWH; (C) Serum cytokine concentrations significantly reduced after
dental treatment in OC PLWH (Group 2) at different regime (V2-3mo , V3-6mo, V4-12 months). (D)Serum cytokine levels were significantly elevated in OC PLWH
(Group 2, V1 baseline) when compared to IC in (Group 1, V1 baseline). Wilcox on Signed Rank test was used for matched comparisons. (E-F) Sixteen candidate
serum mRNA markers selected by microarray analysis for qPCR validation. mRNA expression of eight cytokines and eight chemokines were highly expressed in
group 2 baseline corresponding to the microarray data. Expression levels were determined using the 2-ΔΔCt method (n=8). Legend: PLWH – people living with HIV;
IC - In-Care, OC - Out-of-Care; G1 - group 1(IC), G2 – group 2 (OC); V1 - baselineor visit 1, V2 – visit at 3 months, V3 – visit at 6 months, V4 – visit at 12 months;
ADMA - asymmetric dimethylarginine.
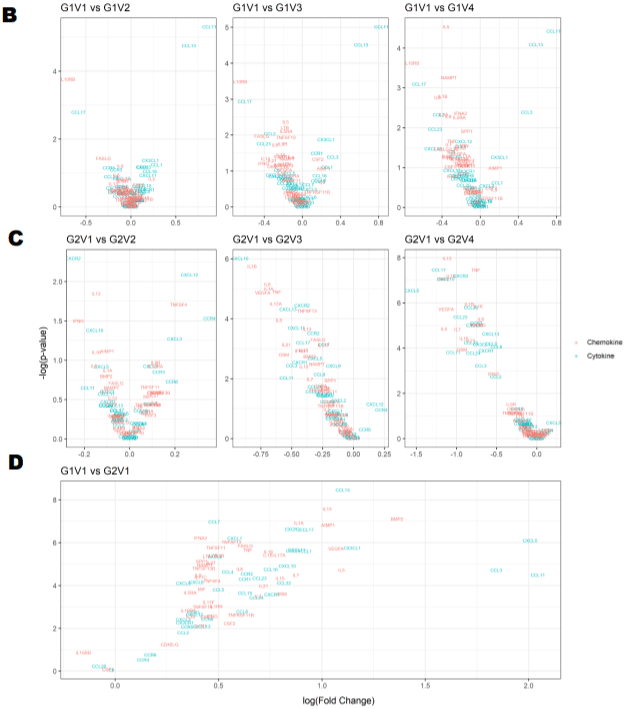
Figure 1B: Longitudinal comparison of plasma cytokine/chemokine concentrations in PLWH before and after periodontal treatment. Heat map of differentially
expressed cytokine/chemokine array from PLWH at (A) Baseline (V1) comparisons of cytokines and chemokines between IC (In-Care) and OC (Out-of-Care)
PLWH. Data are presented as the average fold change at each visit compared to the baselines of their respective groups. Relative expression values from high
to low was shown by gradient of red to green in the heat map. Red indicates higher and green indicating lower expression of mRNAs. Volcano plot analysis of the
differentially abundant serum cytokine/chemokines in PLWH. The graphs represents the relative abundance of each metabolite against its statistical significance,
respectively reported as difference and -log10 (q-value), in IC in (Group 1); (B) Serum cytokine concentrations were reduced with periodontal treatments at different
regime (V2-3mo, V3-6mo, V4-12 months) when compared to baseline (V1) in IC (Group 1) PLWH; (C) Serum cytokine concentrations significantly reduced after
dental treatment in OC PLWH (Group 2) at different regime (V2-3mo , V3-6mo, V4-12 months). (D)Serum cytokine levels were significantly elevated in OC PLWH
(Group 2, V1 baseline) when compared to IC in (Group 1, V1 baseline). Wilcox on Signed Rank test was used for matched comparisons. (E-F) Sixteen candidate
serum mRNA markers selected by microarray analysis for qPCR validation. mRNA expression of eight cytokines and eight chemokines were highly expressed in
group 2 baseline corresponding to the microarray data. Expression levels were determined using the 2-ΔΔCt method (n=8). Legend: PLWH – people living with HIV;
IC - In-Care, OC - Out-of-Care; G1 - group 1(IC), G2 – group 2 (OC); V1 - baselineor visit 1, V2 – visit at 3 months, V3 – visit at 6 months, V4 – visit at 12 months;
ADMA - asymmetric dimethylarginine.
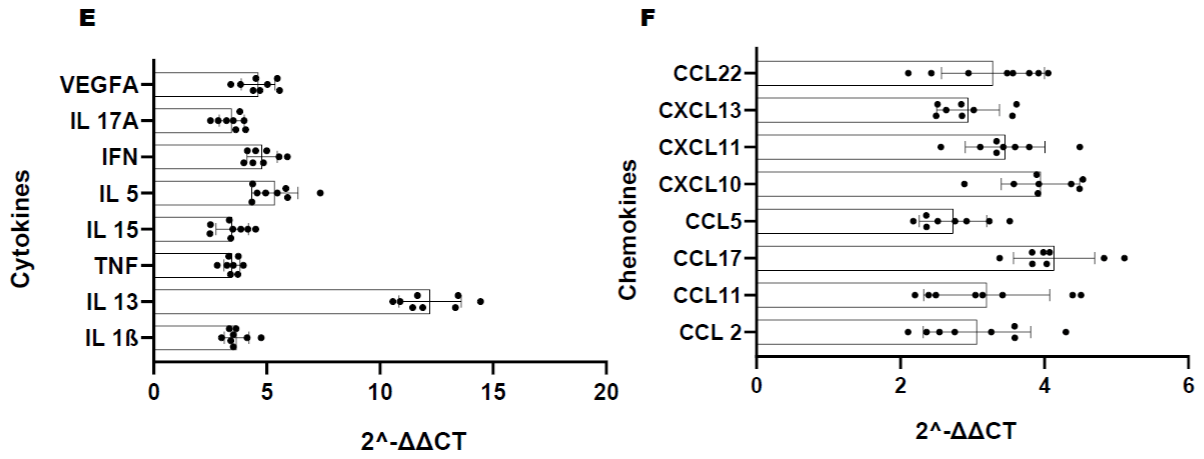
Figure 1C: Longitudinal comparison of plasma cytokine/chemokine concentrations in PLWH before and after periodontal treatment. Heat map of differentially
expressed cytokine/chemokine array from PLWH at (A) Baseline (V1) comparisons of cytokines and chemokines between IC (In-Care) and OC (Out-of-Care)
PLWH. Data are presented as the average fold change at each visit compared to the baselines of their respective groups. Relative expression values from high
to low was shown by gradient of red to green in the heat map. Red indicates higher and green indicating lower expression of mRNAs. Volcano plot analysis of the
differentially abundant serum cytokine/chemokines in PLWH. The graphs represents the relative abundance of each metabolite against its statistical significance,
respectively reported as difference and -log10 (q-value), in IC in (Group 1); (B) Serum cytokine concentrations were reduced with periodontal treatments at different
regime (V2-3mo, V3-6mo, V4-12 months) when compared to baseline (V1) in IC (Group 1) PLWH; (C) Serum cytokine concentrations significantly reduced after
dental treatment in OC PLWH (Group 2) at different regime (V2-3mo , V3-6mo, V4-12 months). (D)Serum cytokine levels were significantly elevated in OC PLWH
(Group 2, V1 baseline) when compared to IC in (Group 1, V1 baseline). Wilcox on Signed Rank test was used for matched comparisons. (E-F) Sixteen candidate
serum mRNA markers selected by microarray analysis for qPCR validation. mRNA expression of eight cytokines and eight chemokines were highly expressed in
group 2 baseline corresponding to the microarray data. Expression levels were determined using the 2-ΔΔCt method (n=8). Legend: PLWH – people living with HIV;
IC - In-Care, OC - Out-of-Care; G1 - group 1(IC), G2 – group 2 (OC); V1 - baselineor visit 1, V2 – visit at 3 months, V3 – visit at 6 months, V4 – visit at 12 months;
ADMA - asymmetric dimethylarginine.
The corresponding microarray data correlate with the validation results in group 2 serum from baseline visit (correlation coefficient to support this interpretation) (Figure 1E-F).
Serum Bacterial Phylum Composition
Both groups demonstrated a significant difference in the level of Actinobacteria phyla at baseline (Figure 2A). Following the periodontal intervention, we observed a significant increase in Proteobacteria at the 12-month visit in group 1 (Figure 2A). In group 2, Actinobacteria, Bacteroidetes and Proteobacteria phylum increased at 12-month posttreatment (Figure 2B). A significant decrease in Firmicutes was observed in the out of care group (Group 2) at 12-month visit (Figure 2B). Baseline comparisons between the groups are illustrated in (Figure 2C). The ratio between Firmicutes vs Bacteroidetes is as shown in (Figure 2D).
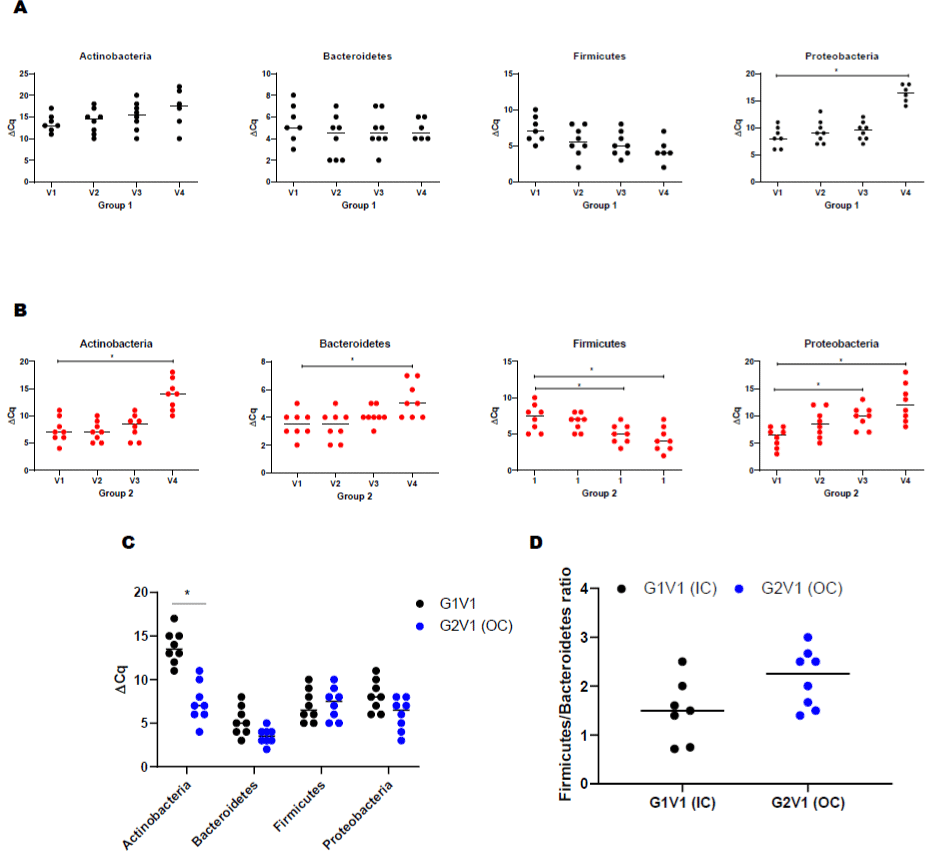
Figure 2: Serum bacterial populations assessment by qPCR. Bacterial phylum detections in PLWH. (A) Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria
in IC (Group 1) at different visits (V2-3mo, V3-6mo, V4-12 months); (B) Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria in OC (Group 1) at different
visits (V2-3mo, V3-6mo, V4-12 months); (C) Comparison of serum bacterial population between base line visits of IC (group 1) and OC (group 2) PLWH; (D) ratio
between Firmicutes and Results were expressed ΔCT values and are expressed as mean ± SD (n=61).Correlation of cytokines with bacterial phyla in PLWH using
Spearman correlation. (E) Correlation of cytokine/chemokines with Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria in IC (Group 1) at baseline visit
(V1); (F) Correlation of cytokine/chemokines Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria in OC (Group 1) at baseline visit (V1). The color green
represents significant correlations between phyla and cytokine/chemokines whereas red is not significant. (G) Effect of dental treatment on serum ADMA levels
between IC (G1) and OC (G2) PLWH. Change in serum ADMA levels shows a correlation between IC and OC PLWH. ADMA levels did not change after routine
dental treatment (6-, 12-month visit, V3, V4) in both IC and OC PLWH. This data suggests that elevated ADMA level may lead to increased cardiovascular risk
among PLWH. Data were expressed as mean ± SD. P<0.05 was compared with the baseline visits (V1) of each group. Legend: PLWH – people living with HIV;
IC - In-Care, OC - Out-of-Care; G1 - group 1(IC), G2 – group 2 (OC); V1 - baselineor visit 1, V2 – visit at 3 months, V3 – visit at 6 months, V4 – visit at 12 months.
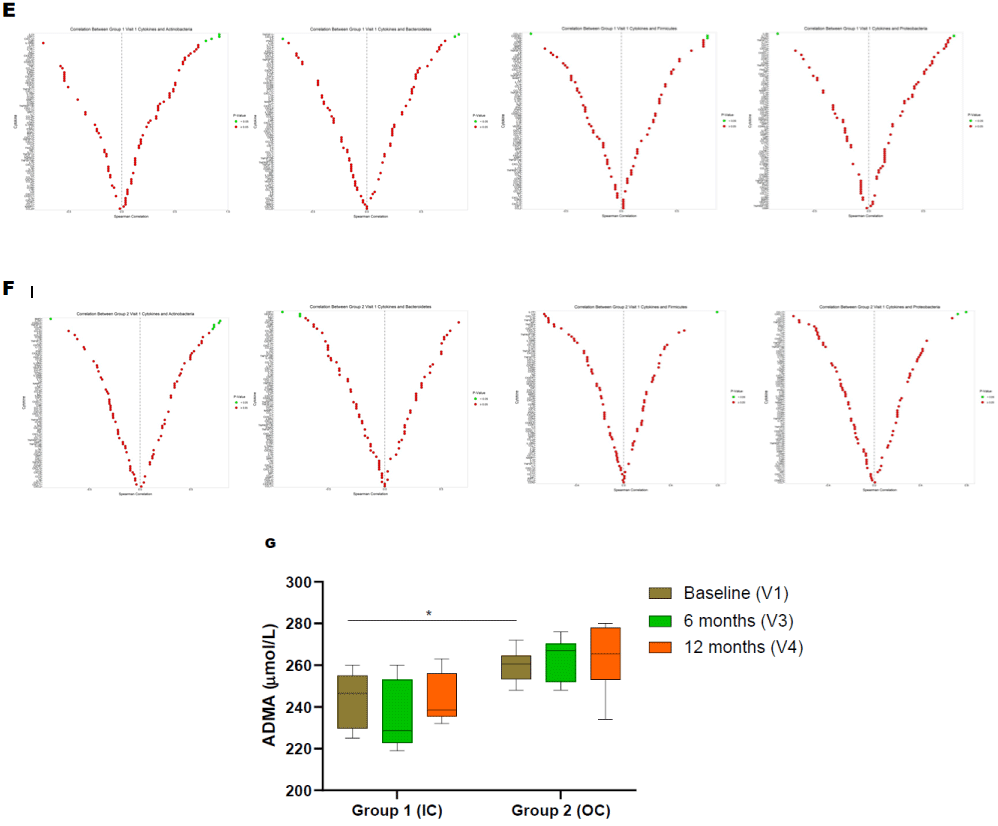
Figure 2b: Serum bacterial populations assessment by qPCR. Bacterial phylum detections in PLWH. (A) Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria
in IC (Group 1) at different visits (V2-3mo, V3-6mo, V4-12 months); (B) Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria in OC (Group 1) at different
visits (V2-3mo, V3-6mo, V4-12 months); (C) Comparison of serum bacterial population between base line visits of IC (group 1) and OC (group 2) PLWH; (D) ratio
between Firmicutes and Results were expressed ΔCT values and are expressed as mean ± SD (n=61).Correlation of cytokines with bacterial phyla in PLWH using
Spearman correlation. (E) Correlation of cytokine/chemokines with Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria in IC (Group 1) at baseline visit
(V1); (F) Correlation of cytokine/chemokines Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria in OC (Group 1) at baseline visit (V1). The color green
represents significant correlations between phyla and cytokine/chemokines whereas red is not significant. (G) Effect of dental treatment on serum ADMA levels
between IC (G1) and OC (G2) PLWH. Change in serum ADMA levels shows a correlation between IC and OC PLWH. ADMA levels did not change after routine
dental treatment (6-, 12-month visit, V3, V4) in both IC and OC PLWH. This data suggests that elevated ADMA level may lead to increased cardiovascular risk
among PLWH. Data were expressed as mean ± SD. P<0.05 was compared with the baseline visits (V1) of each group. Legend: PLWH – people living with HIV;
IC - In-Care, OC - Out-of-Care; G1 - group 1(IC), G2 – group 2 (OC); V1 - baselineor visit 1, V2 – visit at 3 months, V3 – visit at 6 months, V4 – visit at 12 months.
Correlation between Cytokines and the Circulating Microbial Phyla
Pair wise correlation tests were performed at baseline between microbial taxonomic compositions at the phylum level with each cytokine using Spearman correlation (Figure 2E-F). Each phylum was significantly associated with at least one potential cytokine/ chemokine interaction and association across all of the tested mediators. Interestingly, the group 2 and group 1 demonstrated distinct profiles. Actinobacteria were positively correlated with IL-1R1, CD40LG, TNSF-11, CCL-26, and CCL-7 in group 2 and positively correlated with IL-13, CSF-1, CXCL-12, IL-9 R in group 1. Bacteroidetes was positively correlated with TNSF11 and CXCL6 in the IC group. Similarly, Firmicutes was positively correlated with IL17F and chemokines such as CX3CR1 and CCR4 in group one. Proteobacteria demonstrated positive correlation with chemokines (CCL17 and CXCL13) in group two and with IL17Ain group one.
Asymmetric Dimethylarginine (ADMA) Levels in the Serum of PLWH
Among all participants, the median ADMA level was 254 μmol/L. At baseline, group 2 individuals were associated with significantly higher ADMA levels compared to those individuals from group 1 (Figure 2G). However, the periodontal intervention did not result in modification within ADMA levels for either group (Figure 2G).
Discussion
As expected, discrete systemic inflammatory profiles in PLWH were associated with being in or out of dental care. Our data demonstrate that AIMP1, BMP2, NAMPT, OSM, SPP1, TNF, TNFRSF 11B, TNFSF 11, VEGFA, IL-3, IL-33, IL-5, IL-5RA, IL-7, IL-8, IL-9, IL-9R, LTA, LTB, and MIF were significantly higher in ‘OC’ (group 2) compared to ‘IC’ (group 1) at baseline. Periodontal treatment lowered systemic inflammatory markers in ‘OC’ group compared to ‘in care’ PLWH at all-time points examined. Similarly, periodontal treatment significantly lowered the majority of circulatory chemokines (CCL8, CX3CL1, CXCL10, CXCL11, CCL11, CCL4, CCL20, CXCL5, CXCL6, and CCL23). Finally, our data show that genomic DNA for several phyla was correlated with changes in cytokine/chemokine levels in circulation. Our study findings suggest that periodontal treatment, which modulates the oral microbiome, may play an important role in suppressing inflammatory burden among AA-PLWH who are virally suppressed on ART.
Previous studies have demonstrated that persons with HIV carry a higher risk of increased periodontal disease severity [8,10,11]. In fact, inflammation in the periodontal connective tissue leads to attachment loss between bone and tooth, with epithelial cells proliferating apically along the root surface, thus leading to the formation of a deep pocket [12]. As the periodontal pocket deepens, so does the extent of the inflammatory infiltrate [12]. In our study population, periodontal treatment attenuated and reduced the levels of pro inflammatory cytokines and chemokines in circulation (Figure 3). Several pro-inflammatory cytokines including interleukins like IL 1, IL-6, IL-12, IL-17, IL-18, and IL-21, TNFa, and IFN-γ have been demonstrated to be involved in the pathogenesis of periodontitis. In this study, high levels of serum pro-inflammatory cytokines were detected at baseline in participants who were out of regular dental care, probably induced by monocytes and macrophages to initiate inflammation, which is essential for the development of innate immunity. IL-1 family members include IL-1a, IL-1β, IL-18, IL-33, IL- 36, IL-37, and IL-38 are involved in both innate and adaptive immune responses and proven to be related to increased risk of inflammation, autoimmunity, cardiovascular disorders, and cancer [7]. It has been reported that gene polymorphisms in IL-1B, IL-1R (which encodes the IL-1-associated receptor) and IL-1N (which encodes the receptor antagonist IL-1Ra) are related to a susceptibility to PD, indicating that IL-1β is involved in the pathogenesis of periodontitis [13]. In our study, we observed a reduction in IL-33, IL-1A, IL-1B and IL-1RN subsequent to periodontal treatment, with a larger effect size in ‘out of dental care’ PLWH.
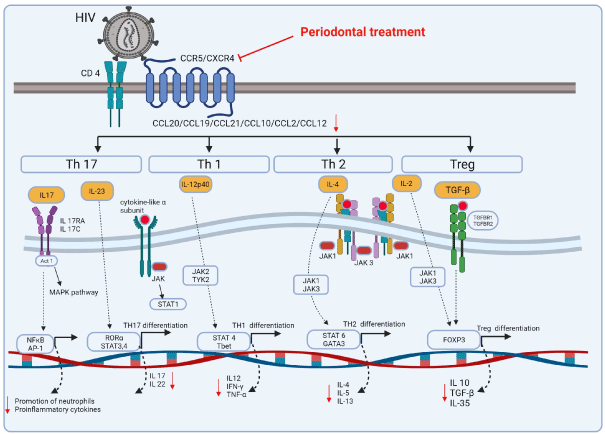
Figure 3: Proposed schematic representation of the signaling pathways of chemokines and cytokine affecting the modulation during Human Immunodeficiency
Virus (HIV) infection and Periodontal Disease (PD). Under stimulation by certain inflammatory cytokines, naive CD4+ T cells differentiate towards multiple directions,
including Th1 (IL-12) and Treg (IL-2 and TGF-β) cells, which mainly have protective effects, and Th17 (IL-23) and Th2 (IL-4) cells, which mainly have pleiotropic
effects. Downstream of IL-17 (secreted by Th17 cells) and IL-10 (secreted by Treg cells) are specific and of special significance to the periodontal host immune
response.
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) efficiently induces IL6 transcription after activation by LPS, IL-1, IL-17 and tumor necrosis factor-a (TNF-a) [14]. IL-17A and IL-17F, the family members of IL-17 are linked to the progression of chronic inflammation and pathogenesis of PD [7]. Our study shows that serum levels of IL-9, IL-5, and IL-21 which belongs to the γc family, were high at baseline levels and their levels reduced significantly with periodontal intervention. Salivary levels of proinflammatory cytokines, IL-5 and IL 8, which do not belong to IL-6 family, have been shown to be elevated in patients with periodontitis and have been confirmed to reflect systemic inflammation [12]. Our results suggest that PD treatment may alleviate systemic inflammatory burden in PLWH by reducing the levels of IL-5 and IL-8 in circulation. Our data demonstrate that levels of all of the above pro-inflammatory biomarkers, IL-5, IL-8, IL-9, IL-15, IL-21, IL-17, and TNF a, were significantly reduced in the circulation with local periodontal care and oral hygiene in virally suppressed PLWH.
Chemokines are categorized as a large subfamily of cytokines, which promote chemotaxis, interleukins (regulators of communication between white blood cells), interferons (regulators of innate immunity), and lymphokines and tumor necrosis factor (proinflammatory) [7]. Based on the lig and structures, chemokines consist of two major families: CXC and CC with their receptors and CXC chemokine receptor (CXCR) and CCR, respectively [15]. These receptors are expressed on Th1 cells express chemokine receptors CCR5 and CXCR3, Th2 cells mainly express CCR3 and CCR4, neutrophils, monocytes and macrophages express CCR5 and CCR2 [15]. CXCL10, a chemoattractant for activated Th1 cells, is found in inflamed gingival tissues and expressed by human gingival fibroblasts in response to interferon-γ, TNF-a, and IL-1β, suggesting this as a mechanism to recruit Th1 cells to inflammatory foci of periodontitis [16]. Higher CXCL1, CCL2, and CCL5 levels were found in human and rat gingiva from sites of periodontitis as compared with periodontally healthy sites [17]. Significantly higher concentrations of CXCL10 were found in saliva and serum of patients with chronic periodontitis [16]. CCL20 is involved in Th17 cell recruitment and may promote periodontal disease [15]. It has been reported that IL-22 increases CCL20 production in human gingival fibroblasts through an NF-κB-dependent mechanism [15]. Similarly, IL-6-stimulated periodontal ligament cells showed elevated secretion of CCL20 in a STAT3-dependent manner [15]. CXCL13, which is chemo attractant for B-cells, is highly expressed in diseased tissues, suggesting a role for this chemokine in the local humoral response to periodontal pathogens [18]. CCR4, a high affinity receptor for both CCL17 and CCL22, is expressed at higher levels in chronic periodontitis [18]. The effect of IL-8 is mediated by its two receptors CXCR1 and CXCR2 [19]. They are involved in multiple biological activities, such as initiation and amplification of acute inflammatory reaction, as well as tumor growth, angiogenesis, and metastasis [20]. Our data show that periodontal treatment significantly reduced gene expression for all of the above chemokines in the serum of PLWH (Figure 3).
Well-designed epidemiological studies have shown that people with periodontal diseases carry a higher risk for systemic inflammation [5,21]. Bacterial translocation from the periodontal pocket into the systemic circulation is common, as supported by detection of bacteremia subsequent to relatively minor periodontal events and/or procedures [22]. Chronic periodontitis is strongly associated with “red complex” Gram-negative bacteria, such as Tannerella forsythia, Porphyromonas gingivalis, and Treponema denticola [23]. In healthy gingival sulci; Proteobacteria, Firmicutes were more prominent, whereas Spirochaetes and Bacteroidetes, were associated with periodontal disease. Yamanaka et al., reported that periodontal treatment significantly reduced periodontal pathogens in gingival sulcito undetectable levels in patients with periodontitis [24]. In addition, these studies further demonstrated that periodontal treatment significantly attenuated the elevated levels of Fusobacterium and Kingella in gingival sulci which are known to be associated with periodontal infection [24]. However, none of the above studies could not detect red complex in the serum specimens.
Periodontal pathogens express a set of virulence factors. P. gingivalis produces Lys-and Arg-proteases (Kgp, RgpA and RgpB gingipains), which can activate complement system to generate C5 a anaphylotoxin [25]. Lipopolysaccharide (LPS) from P. gingivalis is another important virulence factor, shown to up regulate TLR-2 and TLR-4 on the host cell surface, leading to secrete IL-1, IL-6, IL-8, IL 18 and TNF-a expression [26,27]. This is followed by the release of inflammatory mediators such as CXCL8 and IL 6. Gingipains are well known to cleave CXCL8, as well as other cytokines and chemokines, such as IL-6, IL-6 receptor, CXCL10, TNF-a, CD14, IL-4, and IL-12 [28].
Specific bacteria have been associated with PD progression in PLWH. Significant associations between oral bacterial populations and serum inflammatory biomarkers has been reported [29]. The higher abundance of P. melaninogenica, Fusobacterium sp., V. parvula, P. endodontalis, P. pallens, S. anginosus, P. nigrescens, C. ureolyticus, P. nanceiensis, P. anaerobius are closely associated with elevated levels of interleukins 6, 8, TNF-a, GM-CSF, and IFN-γ [30]. In addition, a significant increase in the levels of Fusobacterium nucleatum, Prevotella intermedia, as well as IFNγ and TGFβ in gingival crevicular fluid has also been shown, as potential risk factors for rapid progression of periodontitis in PLWH [31]. Previous studies have shown that T. forsythia surface-lipoproteins activate host cells to release pro inflammatory cytokines (IL 6 and TNF a) and induce cellular apoptosis [32]. Treponema glycolipids and lipoteichoic acid from T. denticola stimulates the innate immune system through TLR2, which activates the production of TNF-a, IL-1β, IL-6, IL-10, and IL- 12 [33]. Our study showed that periodontal treatment alleviated the periodontal pocket depth and systemic proinflammatory cytokines/ chemokines levels, suggesting that oral health is critical in maintaining innate immune function among PLWH.
As reported by Sergio Serrano-Villar et al., PLWH display a distinct microbial signature in their blood, dominated by members of the families Pseudomonadaceae (Pseudomonas), Enterobacteriaceae, Comanodaceae, and Moraxellaceae (Acinetobacter) from the Proteobacteria phylum; the families Micrococcaceae (Arthrobacter) and Corynebacteriaceae (Corynebacterium) from Actinobacteria [34]. HIV increased alpha diversity in the blood, dominated by aerobic bacteria belonging to Micrococcaceae (Actinobacteria) and Pseudomonadaceae (Proteobacteria) families [34]. The composition of the blood microbiota was consistent with the bacterial signature as reported from other groups [35]. Proteobacteria was the predominant phylum detected in the serum of both study groups. Each of the phyla detected in our study correlated with the inflammatory biomarkers measured in serum, which suggests that the molecular crosstalk between the host and the translocated bacterial products could influence ART-mediated immune recovery.
Our results show that periodontal treatment did not affect ADMA level at 12-month visit. Larger sample size, extended study duration and periodontal care may be necessary to demonstrate a significant impact on ADMA level.
In conclusion, periodontal care appears to significantly attenuate local and systemic inflammatory cytokines suggesting a beneficial impact on systemic inflammation among African Americans with sustained viral suppression on ART. Our results suggest an association between deep periodontal pockets and marginal bone loss with systemic immuno-inflammatory biomarkers in PLWH. These study findings deserve further exploration in a larger and more diverse population.
Acknowledgements
We thank the MMC Molecular Core Laboratory (CRISALIS) for providing CFX96 thermocycler to complete RT-PCR assays.
Grants
This work is supported by the NIH/NIMHD Meharry Translational Research Centers Grant (#5U54MD007593-09), Meharry Community Wellness Center, HRSA Grant #H76HA01706-13, CFAR Vanderbilt/ Meharry: Tennessee Center for AIDS Research (P30 AI110527), Minority Institutions (RCMI) grant (National Institutes of Health (NIH) grant MD007586) and HRSA #H97HA07511 UNC, NIHThe National Institute of General Medical Sciences (NIGMS)-SC1 (GM121282).
Disclosure
The authors report no conflicts of interest related to this study.
Author Contributions
All authors have made substantial contributions to conception and design of the study. P.R.G. conceived and designed research; C.S., E.H., performed experiments; C. S., prepared figures; C.S., JWC, KM, and P.R.G., analyzed data; C. S., and P.R.G., interpreted results of experiments; C.S., M.C and P.R.G. drafted manuscript; V.B., J.S., J.W.C., J.K., E.H., and P.R.G., edited and revised manuscript; D.W and M.T performed statistical analysis; P.R.G. approved final version of manuscript. All authors have contributed to, seen, and approved the final, submitted version of the manuscript.
References
- Mann J, Bernstein Y, Findler M. Periodontal disease and its prevention, by traditional and new avenues. Experimental and Therapeutic Medicine. 2020; 19: 1504-1506.
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. Journal of periodontology. 2015; 86: 611-622.
- Borrell LN, Burt BA, Warren RC, Neighbors HW. The role of individual and neighborhood social factors on periodontitis: the third National Health and Nutrition Examination Survey. Journal of periodontology. 2006; 77: 444-453.
- Akintobi TH, Hoffman LM, McAllister C, Goodin L, Hernandez ND, Rollins L, et al. Assessing the Oral Health Needs of African American Men in Low- Income, Urban Communities. American Journal of Men’s Health. 2018; 12: 326-337.
- HASTURK H, KANTARCI A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontology 2000. 2015; 69: 255- 273.
- Valentine J, Saladyanant T, Ramsey K, Blake J, Morelli T, Southerland J, et al. Impact of periodontal intervention on local inflammation, periodontitis, and HIV outcomes. Oral diseases. 2016; 22: 87-97.
- Ramadan DE, Hariyani N, Indrawati R, Ridwan RD, Diyatri I. Cytokines and Chemokines in Periodontitis. European Journal of Dentistry. 2020; 14: 483- 495.
- Valentine J, Saladyanant T, Ramsey K, Blake J, Morelli T, Southerland J, et al. Impact of periodontal intervention on local inflammation, periodontitis, and HIV outcomes. Oral diseases. 2016; 22: 87-97.
- Braunstein M, Williamson M, Kusmenkov T, Landes J, Biberthaler P, Kanz K, et al. Significant Cytokine mRNA Expression Changes Immediately after Initiation of Cardiopulmonary Resuscitation. Mediators of Inflammation. 2017; 2017: 1-10.
- Groenewegen H, Bierman WF, Delli K, Dijkstra PU, Nesse W, Vissink A, et al. Severe periodontitis is more common in HIV- infected patients. The Journal of infection. 2019; 78: 171-177.
- Fricke U, Geurtsen W, Staufenbiel I, Rahman A. Periodontal status of HIVinfected patients undergoing antiretroviral therapy compared to HIV-therapy naive patients: a case control study. European Journal of Medical Research. 2012; 17: 2-2.
- Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. International Journal of Oral Science. 2019; 11.
- Lavu V, Venkatesan V, Lakkakula BVKS, Venugopal P, Paul SFD, Rao SR. Polymorphic regions in the interleukin-1 gene and susceptibility to chronic periodontitis: a genetic association study. Genetic testing and molecular biomarkers. 2015; 19: 175-181.
- Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014; 70: 11-20.
- Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. The Febs Journal. 2018; 285: 2944-2971.
- Aldahlawi S, Youssef A, Shahabuddin S. Evaluation of chemokine CXCL10 in human gingival crevicular fluid, saliva, and serum as periodontitis biomarker. Journal of Inflammation Research. 2018; 2018: 389-396.
- Rath-Deschner B, Memmert S, Damanaki A, Nokhbehsaim M, Eick S, Cirelli JA, et al. CXCL1, CCL2, and CCL5 modulation by microbial and biomechanical signals in periodontal cells and tissues—in vitro and in vivo studies. Clinical Oral Investigations. 2020; 24: 3661-3670.
- Sahingur SE, Yeudall WA. Chemokine Function in Periodontal Disease and Oral Cavity Cancer. Frontiers in Immunology. 2015; 6.
- Kaur M, Singh D. Neutrophil Chemotaxis Caused by Chronic Obstructive Pulmonary Disease Alveolar Macrophages: The Role of CXCL8 and the Receptors CXCR1/CXCR2. The Journal of Pharmacology and Experimental Therapeutics. 2013; 347: 173-180.
- Kavrikova D, Borilova Linhartova P, Lucanova S, Poskerova H, Fassmann A, Izakovicova Holla L. Chemokine Receptor 2 ( CXCR2 ) Gene Variants and Their Association with Periodontal Bacteria in Patients with Chronic Periodontitis. Mediators Inflamm. 2019; 2019: 1–8.
- Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, Flores-Ramírez R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart, lung & circulation. 2018; 27: 1327-1334.
- Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nature Reviews. Immunology. 2021; 21: 426-440.
- Tomita S, Kasai S, Ihara Y, Imamura K, Kita D, Ota K, et al. Effects of systemic administration of sitafloxacin on subgingival microflora and antimicrobial susceptibility profile in acute periodontal lesions. Microbial pathogenesis. 2014; 71-72: 1-7.
- Yamanaka W, Takeshita T, Shibata Y, Matsuo K, Eshima N, Yokoyama T, et al. Compositional Stability of a Salivary Bacterial Population against Supragingival Microbiota Shift following Periodontal Therapy. PLoS ONE. 2012; 7. doi:10.
- Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunology letters. 2014; 162: 22-38.
- Yee M, Kim A, Alpagot T, Düzgünes N, Konopka K. Porphyromonas gingivalis stimulates IL-18 secretion in human monocytic THP-1 cells. Microbes and infection. 2012; 14: 684-689.
- Savitri IJ, Ouhara K, Fujita T, Kajiya M, Miyagawa T, Kittaka M, et al. Irsogladine maleate inhibits Porphyromonas gingivalis -mediated expression of toll-like receptor 2 and interleukin-8 in human gingival epithelial cells. J Periodontal Res. 2015; 50: 486–93.
- Palm E, Khalaf H, Bengtsson T. Suppression of inflammatory responses of human gingival fibroblasts by gingipains from Porphyromonas gingivalis. Molecular oral microbiology. 2015; 30: 74-85.
- Iebba V, Zanotta N, Campisciano G, Zerbato V, Bella SD, Cason C, et al. Profiling of Oral Microbiota and Cytokines in COVID-19 Patients. Frontiers in Microbiology. 2021; 12.
- Rai AK, Panda M, Das AK, Rahman T, Das R, Das K, et al. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Archives of Microbiology. 2020; 203: 137-152.
- Alpagot T, Konopka K, Bhattacharyya M, Gebremedhin S, Düzgünes N. The Association Between Gingival Crevicular Fluid TGF-β1 Levels and Periodontal Status in HIV-1 + Patients. J Periodontol. 2008; 79: 123–30.
- Hasebe A, Yoshimura A, Into T, Kataoka H, Tanaka S, Arakawa S, et al. Biological Activities of Bacteroides forsythus Lipoproteins and Their Possible Pathological Roles in Periodontal Disease. Infection and Immunity. 2004; 72: 1318-1325.
- Ruby J, Martin M, Passineau MJ, Godovikova V, Fenno JC, Wu H. Activation of the Innate Immune System by Treponema denticola Periplasmic Flagella through Toll-Like Receptor 2. Infection and Immunity. 2017; 86.
- Serrano-Villar S, Sanchez-Carrillo S, Talavera-Rodríguez A, Lelouvier B, Gutiérrez C, Vallejo A, et al. Blood Bacterial Profiles Associated With Human Immunodeficiency Virus Infection and Immune Recovery. J Infect Dis. 2021; 223: 471–81.
- Li S, Leung RK, Guo H, Wei J, Wang J, Kwong K, et al. Detection and identification of plasma bacterial and viral elements in HIV/AIDS patients in comparison to healthy adults. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012; 18: 1126-1133.