
Research Article
Austin J Clin Ophthalmol. 2017; 4(2): 1081.
Does Age Influence the Effect of Povidone Iodine 5% on the Cornea?
Ridder III WH¹*, Oquindo C¹, Dhamdhere K² and Burke J²
¹Southern California College of Optometry, Marshall B. Ketchum University, Fullerton, CA 92831, USA
²Allergan, Inc., 2525 Dupont Dr. Irvine, CA 92623, USA
*Corresponding author: Ridder III WH, Southern California College of Optometry, Marshall B. Ketchum University, 2575 Yorba Linda Blvd, Fullerton, CA USA 92831, USA
Received: July 19, 2017; Accepted: August 10, 2017; Published: August 17, 2017
Abstract
Purpose: To determine if age influences the effects of 5% Betadine applied to the eye on visual function, corneal integrity and subjective complaints.
Methods: Twenty subjects were chosen to participate in this study (Ten younger: 25.8 +/- 2.94; and ten older: 58.2 +/- 5.59). LogMAR acuity, contrast sensitivity, corneal fluorescein staining, and subjective complaints were measured before and after 60μl of 5% Betadine was applied to one eye (baseline, 5, 30, and 60 minutes and 4 and 24 hours post-application). Contrast sensitivity at 14cpd was determined with a spatial two-alternative, forced choice procedure (BeethovenTM software). The NEI grid pattern was used to grade corneal staining with sodium fluorescein. Subjective complaints were monitored using the Schein dry eye questionnaire.
Results: The data were analyzed with an ANOVA (linear mixed-effects model). For all the subjects, logMAR acuity was significantly reduced from baseline at the 30 and 60 minute visits (all p values < 0.05) and contrast sensitivity was reduced from baseline at 5, 30, and 60 minutes after Betadine application (all p values < 0.0001). Total corneal staining and the Schein dry eye questionnaire were significantly different from baseline at every visit (all p values < 0.05). The age groups were only different at the 1 hour visit for logMAR acuities.
Conclusions: 5% Betadine application significantly decreases epithelial integrity of the cornea, decreases functional vision, and increases subjective complaints. Age has a minimal effect on the result.
Keywords: Betadine; Corneal staining; Vision; Contrast sensitivity; Symptoms
Introduction
A recently published study demonstrated that the application of povidone iodine 5% (brand name Betadine 5%, Alcon Laboratories, Inc., Fort Worth, TX) to the eyes of normal patients resulted in an increase in corneal staining and subjective complaints that lasted 24 hours and a loss in functional vision lasting 1 hour [1]. Povidone iodine 5% is commonly used pre-operatively to disinfect the ocular surface [2-5]. In these procedures, a few drops of povidone iodine are placed in the conjunctival sac and this significantly decreases the local bacterial concentration [6]. With the large increase in the number of intravitreal injections in the last decade (i.e., from about 3000 in 1999 to over 2 million in 2012 [7]) ocular disinfection has become extremely important [8,9].
Previous studies of povidone iodine 5% have not considered the effect of the patients age on the cornea or vision [1]. Since aging results in several changes in the cornea (e.g., increased epithelial cell permeability, impaired wound healing, decreased number of keratocytes, decreased endothelial cell density), povidone iodine 5% may be more toxic to the corneas of older patients [10,11]. The effect of age may also be significant because most patients undergoing intraocular injections or surgery are older. The mean age for patients undergoing cataract surgery is over 70 [12,13]. Thus, older individuals may exhibit a greater extent of corneal staining and functional vision problems than younger individuals in response to povidone iodine use.
Povidone iodine can reduce visual function either by disrupting the tear layer or producing corneal epithelial cell damage. The administration of any fluid (e.g., an artificial tear) to the ocular surface can disrupt the tear layer and interfere with vision [14-16]. Tear layer disruption results in a decrease in the modulation transfer function (MTF) for the eye [17,18]. The tear layer is the first refracting surface of the eye and its break up can cause a decrease in contrast sensitivity and visual acuity [19-21]. Artificial tears should have the least effect on visual function. Other agents, like povidone iodine, are not designed to replace or supplement the tear layer. Thus, povidone iodine may not mix well with the native tear layer and can significantly alter visual function due to tear layer disruption. This effect may be greater in older individuals since they are more likely to have an unstable tear layer and a dry eye [22].
Povidone iodine is more acidic than the tear layer and the free iodine it releases may cause corneal epithelial cell damage [23]. Applying Povidone iodine to the conjunctival sac of humans and rabbits produced severe epithelial damage [1,24]. The epithelial damage can then further disrupt the tear layer. If this occurs over the optic axis, this would interfere with vision. Thus, vision can be affected either by the application of povidone iodine disrupting the tear layer or the resulting corneal epithelial cell damage. Age related changes in the cornea may exacerbate the effects of povidone iodine. In this study, the effect of age on povidone iodine 5% use in the normal eye was determined.
Methods
Subjects
Twenty subjects free from ocular pathology were chosen (age range 22 – 68, average age ± SD = 42.0 ± 17.2). The subjects were divided into 2 age groups of 10 each: younger (age range 22 – 33, average age 25.8 ± 2.94) and older (age range 52 – 68, average age 58.2 ± 5.59). Subjects were recruited from the College community. Informed consent was obtained from all subjects after the testing procedure was explained to them and the procedures adhered to the tenets of the Declaration of Helsinki [25]. The procedures were approved by Sterling IRB (Atlanta, Georgia). The subjects were seen for an eligibility visit to determine if they met the inclusion and exclusion criteria.
Inclusion criteria
There were no requirements as to subject race, gender or occupation. All subjects met the following criteria:
- The informed consent document was read, signed and dated by the subject before conducting any procedures.
- Adult subjects, 18 years or older.
- Subjects were able and willing to follow study instructions.
- Subjects were able and willing to discontinue flexible (i.e., soft) contact lens wear for two (2) days prior to each visit.
- Subjects had best corrected visual acuity of 20/25 (0.1 logMAR) or better in the test eye.
- Subjects demonstrating any medical condition that may affect the results of this study were not enrolled. The following are specific conditions that excluded subjects from enrollment in this study.History or evidence of ocular or intraocular surgery or ocular trauma in the test eye.
- History or evidence of viral, bacterial, or fungal disease of the cornea and/or conjunctiva.
- Use of concomitant topical ocular medications during the study period.
- Subjects using systemic steroids, immunosuppressive agents and/or anti-cholinergics.
- Rigid (i.e., gas permeable) contact lens wearers.
- Individuals unwilling to discontinue soft contact lens wear for two (2) days prior to each visit.
- Pregnant women or women that are breast feeding.
- Allergic to iodine or fluorescein.
Exclusion criteria
Test procedures
Table 1 gives the visit schedule and the tests performed (i.e., visual acuity, contrast sensitivity, sodium fluorescein staining of the cornea, Schein questionnaire) at each visit.
Test Order
Eligibility Visit
Baseline Visit
5 Minute Visit
30 Minute Visit
1 Hour Visit
4 Hour Visit
24 Hour Visit
Survey
X
X
X
X
X
Acuity
X
X
X
X
X
X
X
CS
X
X
X
X
X
X
X
NaFl Stain
X
X
X
X
CS = contrast sensitivity.
Table 1: Visit schedule and tests performed at each visit.
Schein questionnaire: The Schein dry eye questionnaire was used to assess subjective eye comfort before and after the application of Betadine [26]. The subject was asked to answer the questions based on their current ocular symptoms. The questionnaire consists of 6 questions that are each given a score from 0 to 4 (i.e., never occurs (0), rarely occurs (1), sometimes occurs (2), often occurs (3), and occurs all of the time (4)). The maximum score is 24 (i.e., 6 questions times a maximum score of 4 each equals 24).
Visual acuity: Visual acuity was measured with the M&S Technologies, Inc. Smart System II PC-Plus projected chart. The ETDRS setting was used to obtain logMAR acuities. The letter-byletter scoring method was used so each letter correct decreased the logMAR acuity by 0.02.
Measurement of contrast sensitivity: The methods for the contrast sensitivity measurements were previously published [14,27] and are briefly described below. Subject training occurred during the eligibility visit.
The stimulus was produced with BeethovenTM software (Ryklin Software, Inc. New York; Version 754). The stimulus was a stationary, horizontally oriented, sine wave grating (14 cycles per degree; roughly equivalent to a 20/40 letter size). The sine wave grating was overlain with a Gaussian filter to produce a Gabor patch in which the stimulus edges were blurred. The visible stimulus diameter was 1.5 degrees viewed from 200cm on a ViewPixx monitor (VPixx Technologies, Inc.) under photopic conditions (screen luminance = 100cd/m2). The center of the stimulus was placed either 1 degree to the left or right of a fixation spot on the ViewPixx monitor. The subjects used their optimal spectacle correction for the testing. The contrast sensitivity was measured under monocular conditions.
A spatial two-alternative, forced choice procedure combined with a self-paced method of limits was used to determine the contrast threshold. The subject fixated the spot at the center of the ViewPixx monitor. The stimulus duration was 1.5 seconds. During a single run, the subject was required to correctly identify the location (i.e., slightly left or right of fixation) of the stimulus. The subject responded with a joystick. The subject was given 2 seconds to make a response. If no choice was made, the response was recorded as incorrect. Stimulus contrast was decreased by 0.05log units following correct responses. The same stimulus was repeated a second time if the response was incorrect. A threshold was operationally defined as 2 incorrect responses in a row. The contrast was increased by 0.3log units following a threshold and the above procedure repeated. Ten thresholds were obtained on each visit and these were averaged together for the final threshold.
Corneal sodium fluorescein staining: 2.0μl of 1.0% preservativefree sodium fluorescein (Greenpark Compounding Pharmacy, Houston, TX) was used. The sodium fluorescein was administered with a positive displacement pipette and sterile tip (Rainin Instruments, LLC, Oakland, CA). Staining was measured 3 – 5 minutes after fluorescein administration without anesthesia. The scoring of corneal staining was done dynamically when the patient was at the slit lamp. The corneal staining was evaluated using the NEI 5-sector grid pattern. A blue-free barrier filter was used to maximize the visibility of the staining [28]. Each sector of the grid was graded on a 0 – 3 scale in 0.1 increments. Thus, the maximum corneal staining score is 15 (5 sectors X 3 = 15).
Sodium fluorescein staining was performed at the eligibility visit but not the baseline visit (Table 1). Any substance applied to the tear layer can have a negative impact on vision (see Introduction). To avoid altering the visual acuity or contrast sensitivity results in the first few minutes after the application of Betadine, corneal staining with sodium fluorescein was not performed until the 1 hour visit and it was always the last test performed at the visit. Thus, the post-Betadine corneal staining results are compared to the staining obtained at the eligibility visit.
Betadine 5% application: On the day of the Betadine application, visual acuity, contrast sensitivity, and the Schein questionnaire were performed first (Table 1). After the baseline data were collected, a drop of anesthetic (0.5% proparacaine hydrochloride ophthalmic solution, Alcon Laboratories, Inc. Fort Worth, TX) was applied to the test eye (chosen randomly). One minute after the anesthetic was applied, Betadine (60 microliters administered with a positive displacement pipette with sterile tips, Rainin Instrument, LLC, Oakland, CA) was instilled in the subject’s eye on the superior conjunctiva. The 60 microliter drop size was chosen so that each subject received the same amount of Betadine. Furthermore, 60 microliters is close to the maximum drop size that will stay on the eye. None of the drop flowed onto the cheek after administration for any subject. Two to three minutes after Betadine was instilled, it was washed out with another drop of proparacaine hydrochloride.
Data analysis
The data are graphed as box plots. The box plots display the mean (thick horizontal lines in the box) and median (thin horizontal lines in the box) of the data, the interquartile range (IQR), and the 10th and 90th percentile ranges (i.e., the whiskers). Any data points displayed are outliers. If the data in these plots are the same for all the subjects, the boxes collapse to a single horizontal line (see the 1 hour data in Figure 4). Each set of data (i.e., visual acuity) were analyzed with an initial ANOVA (linear mixed-effects model) to determine if there were any differences between the age groups. The factors in the ANOVA were visit time, age group (young vs old), and the interaction of visit time by age group. A final ANOVA was run after insignificant variables were removed. Statistical significance for the ANOVA was set at a P value of 0.05.
Results
Figure 1 displays the loss in acuity data for the younger (N = 10), the older (N = 10) and all of the subjects (N = 20). The horizontal axis shows the visit time in hours after the Betadine application (e.g., 0.08 hours = 5 minutes) and the vertical axis shows the change in logMAR acuity from baseline (i.e., before Betadine application). Positive values indicate a decrease in visual acuity. The initial ANOVA indicated that there was a significant difference in acuity loss between the older and younger subjects (p = 0.044). This difference was driven by 2 older subjects with significant losses in acuity after Betadine application. A post-hoc 2 sample t-test indicated that the only visit that was significantly different between the age groups was the 1 hour visit (average acuity loss; young = 0.043logMAR, old = 0.128logMAR). The data at the 1 hour visit failed a normality test (Shapiro-Wilk, p < 0.05) so the Mann-Whitney rank sum test was performed (young vs old; p = 0.027).
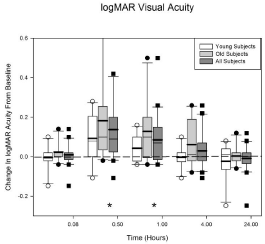
Figure 1: Average logMAR acuity loss (in box plot format, positive values
indicate acuity loss) after Betadine application for the young, old, and all
subjects. For all of the subjects, Betadine resulted in a decrease in acuity
at 0.5 (p < 0.0001) and 1.0 (p = 0.0137) hour after application. There was
a significant difference between the young and the old groups at the 1 hour
visit (p = 0.027). No other visit times were significantly different. The asterisks
indicate the visits in which all of the subjects are significantly different from
baseline. See text for further details.
Since age was a significant variable, the age groups were included as a factor in the final ANOVA model when all of the acuity data was analyzed. The other factor was visit. The interaction (visit by age group) was dropped because it was not significant (p = 0.63). The results indicate that the loss in acuity from baseline is significant at the 30 (p < 0.0001) and 60 minute (p= 0.0137) visits for all of the subjects (N = 20). The significant times are indicated in Figure 1 by the asterisks. A loss in acuity of 0.10 is equivalent to a 1 line or 5 letter loss on the logMAR chart. The greatest loss in acuity (0.14 ± 0.044) occurred at 30 minutes after Betadine application for all of the subjects.
Figure 2 displays the loss in contrast sensitivity for the subjects. The format is identical to Figure 1. There is not a significant difference between the younger and the older subjects for contrast sensitivity (p = 0.33). The ANOVA indicated there is a significant loss in contrast sensitivity across visits (p < 0.0001). There is a significant loss in contrast sensitivity at 5 (p < 0.0001), 30 (p < 0.0001), and 60 (p < 0.0001) minutes after Betadine application for all of the subjects. The loss is approximately 0.3log units at the 5, 30 and 60 minute visits.
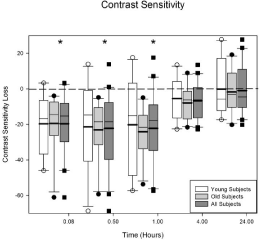
Figure 2: Average contrast sensitivity loss after Betadine application for the
young, old, and all subjects. There were no differences in contrast sensitivity
at any visit for the young and the old groups (p = 0.33). For all of the subjects,
Betadine resulted in a decrease in contrast sensitivity at 5, 30, and 60minutes
after application (all p values < 0.0001). The asterisks indicate the visits in
which the Betadine results for all of the subjects are significantly different
from baseline.
Figure 3 displays the Schein dry eye questionnaire data. The horizontal axis displays time and the vertical axis displays the total Schein score (maximum 24). The ANOVA indicated there was no difference between the age groups (p = 0.31) but there was a difference across the visits (p < 0.0001). The average baseline score (plotted at 0) for all the subjects is 3.15 ± 0.495. One hour after Betadine application the score increased to 10.7 ± 0.641. A score greater than 7 is considered significant in dry eye patients. There was a significant increase in the score at 1 (p < 0.0001), 4 (p = 0.0001), and 24 (p = 0.015) hours.
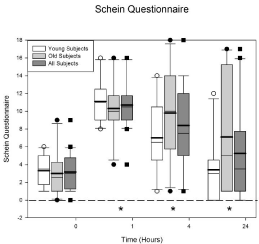
Figure 3: Schein dry eye questionnaire scores before and after Betadine
application. There were no differences in the Schein score at any visit for
the young and the old groups (p = 0.31). Betadine caused an increase in the
Schein score at 1 (p < 0.0001), 4 (p = 0.0001), and 24 (p = 0.015) hours after
application for all of the subjects. The asterisks indicate the visits in which the
Betadine results for all of the subjects are significantly different from baseline.
The total corneal staining scores are plotted in Figure 4. This figure plots the total staining score from all 5 NEI sectors. The maximum score is 15. The graph format is the same as Figure 3. There is no difference between the age groups (p = 0.15) but there is a difference across the visits (p < 0.0001) for all of the subjects. The average baseline staining score for all 20 subjects is 1.95 ± 0.502. At 1 hour the staining score increased to 14.9 ± 0.077. All subjects, except one, had a staining score of 15 (the maximum) at the 1 hour visit. The 1, 4, and 24 hour visits have significantly more staining than baseline (all p values < 0.0001).
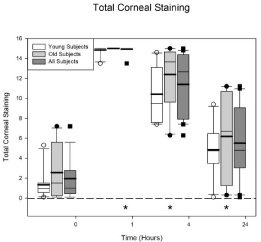
Figure 4: Total corneal staining scores (maximum = 15) before and after
Betadine application. There were no differences in the total corneal staining
scores at any visit for the young and the old groups (p = 0.15). The data
plotted at time 0 is from the eligibility visit. Betadine caused an increase in
corneal staining at 1, 4, and 24 hours after application for all of the subjects
(all p values < 0.0001). The asterisks indicate the visits in which the Betadine
results for all of the subjects are significantly different from the eligibility visit.
Discussion
The purpose of this study was to determine age effects with povidone iodine 5% (i.e., Betadine) application on the ocular surface, functional vision, and subjective symptoms in normal subjects. Povidone iodine is used to decrease the incidence of endophthalmitis following intraocular surgical procedures [5]. For all of the subjects, the application of 60μl of Betadine to the eye resulted in a loss in functional vision lasting 1 hour, an increase in subjective complaints lasting 24 hours and an increase in corneal staining lasting 24 hours. The age groups were only different for visual acuity at the 1 hour visit. There were no statistically significant differences between the age groups for contrast sensitivity, corneal staining, or the Schein questionnaire.
Povidone iodine is cytotoxic to various tissues in the eye (e.g., human corneal epithelial cells (HCE-T) and human cultured fibroblast) [23,29,30]. It has been shown to penetrate the corneas of human donor eyes [31]. Rabbit corneas demonstrated severe epithelial damage based on sodium fluorescein staining 30 minutes after application of 2.5% and 5% povidone iodine [24]. Intra-ocular application of povidone iodine also results in endothelial cell damage and corneal edema [24,32,33]. The cytotoxicity of povidone iodine may be the result of the free iodine released from the solution which results in its bacteriocidal effect [34]. The present results in humans agree with the previous report in rabbits [24].
There are many anatomical and biochemical changes that occur in the eye with age. These changes affect the tear layer and every layer of the cornea. The tear layer composition and the glands that produce the tear layer show age related changes [35,36]. Tear production decreases and tear composition changes with age [35]. The lacrimal gland displays an increase in ductal fibrosis and a decrease in secretion with age [36]. The Meibomian glands decrease in number and their ducts become keratinized with age. Age also results in a change in the lipids produced by the Meibomian glands [37].
The corneal epithelium becomes more permeable with age [11]. This may be the result of changes in integrin molecules which form the attachments between cells [38]. The basement membranes (i.e., Bowmans and Decemet’s) in the cornea also increase in thickness [39,40]. This may result in a disruption of the anchoring fibrils for the basal epithelial cells. Studies have also demonstrated that there is a decrease in corneal sensitivity and nerve density with age [41,42]. The structural and biochemical composition of the stroma changes and there is a decrease in the endothelial cell count with age [43-45].
Since aging results in several changes in the cornea (e.g., increased epithelial cell permeability, impaired wound healing, decreased number of keratocytes, decreased endothelial cell density), Betadine would be expected to be more toxic to the corneas of older than younger patients [10,11]. For example, since the epithelium becomes more permeable with age, [11] Betadine should penetrate and damage the corneas of older patients more than young patients. Also, Betadine should cause less irritation in older patients since they have a decrease in corneal nerve density and corneal sensitivity [41,42]. However, the results of this study indicate that the only difference between the two age groups was the visual acuity at the 1 hour time point after Betadine application. The similarity in the results for the two age groups may be explained based on the exclusion criteria. Anything that might cause corneal damage resulted in the subject’s exclusion from the study. Furthermore, corneal staining, a measure of corneal epithelial integrity, was the same for the two age groups at baseline. Betadine would more readily enter and damage the cornea if the epithelial barrier was compromised. Thus, patients that have compromised corneas would be expected to have more severe losses of visual function, greater corneal staining, and more subjective complaints after the use of Betadine than the patients in the present study.
Conclusions
Povidone iodine 5% is the most commonly used and accepted antiseptic before intraocular surgery. The results of the present study indicate that the application of Betadine 5% to the ocular surface significantly alters the epithelial corneal surface and affects vision and comfort. The results were not significantly different between the young and old age groups.
Acknowledgements
The statistical analysis (Minitab Software, Minitab, Inc.) was carried out by Andrew Loc Nguyen, PhD from the California State University at Fullerton. This study was funded by Allergan, Inc. Authors WR and CO received research grants from Allergan. Authors KD and JB are employees of Allergan. Some of this data have been previously published [1].
References
- Ridder WH, 3rd, Oquindo C, Dhamdhere K, Burke J. Effect of Povidone Iodine 5% on the Cornea, Vision, and Subjective Comfort. Optom Vis Sci. 2017; 94: 732-741.
- Papanikolaou T, Islam T, Hashim A, Mariatos G. Tolerability and safely profile of povodine iodine in pre-operative skin and eye disinfection prior to intraocular surgery. J Clin Exp Ophthalmol. 2011; 2: 125.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991; 98: 1769-1775.
- Olson RJ. Reducing the risk of postoperative endophthalmitis. Surv Ophthalmol. 2004; 49: S55-61.
- Nentwich MM, Ta CN, Kreutzer TC, Li B, Schwarzbach F, Yactayo-Miranda YM, et al. Incidence of postoperative endophthalmitis from 1990 to 2009 using povidone-iodine but no intracameral antibiotics at a single academic institution. J Cataract Refract Surg. 2015; 41: 58-66.
- Halachmi-Eyal O, Lang Y, Keness Y, Miron D. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refract Surg. 2009; 35: 2109-2114.
- Williams GA. IVT injections: health policy implications. Rev Ophthalmol. 2014; 14: 62-64.
- Ta CN. Minimizing the risk of endophthalmitis following intravitreous injections. Retina. 2004; 24: 699-705.
- Shimada H, Hattori T, Mori R, Nakashizuka H, Fujita K, Yuzawa M. Minimizing the endophthalmitis rate following intravitreal injections using 0.25% povidone-iodine irrigation and surgical mask. Graefes Arch Clin Exp Ophthalmol. 2013; 251: 1885-1890.
- Dawson DG, Ubels JL, Edelhauser HF. Cornea and Sclera. In: Levin LA, Nilsson SFE, Ver Hoeve J, Wu SM, editors. Adler’s Physiology of the Eye: Elsevier; 2011; 71-130.
- Chang SW, Hu FR. Changes in corneal auto fluorescence and corneal epithelial barrier function with aging. Cornea. 1993; 12: 493-499.
- O’Reilly P, Mahmoud U, Hayes P, Tormey P, Beatty S. Age and sex profile of patients having cataract surgery between 1986 and 2003. J Cataract Refract Surg. 2005; 31: 2162-2166.
- Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013; 39: 1383-1389.
- Ridder WH, LaMotte J, Hall JQ, Jr., Sinn R, Nguyen AL, Abufarie L. Contrast sensitivity and tear layer aberrometry in dry eye patients. Optom Vis Sci. 2009; 86: E1059-1068.
- Ridder WH, Lamotte JO, Ngo L, Fermin J. Short-term effects of artificial tears on visual performance in normal subjects. Optom Vis Sci. 2005; 82: 370-377.
- Ridder WH, 3rd, Tomlinson A, Paugh J. Effect of artificial tears on visual performance in subjects with dry eye. Optom Vis Sci. 2005; 82: 835-842.
- Himebaugh NL, Thibos LN, Bradley A, Wilson G, Begley CG. Predicting optical effects of tear film break up on retinal image quality using the Shack- Hartmann aberrometer and computational optical modeling. Adv Exp Med Biol. 2002; 506: 1141-1147.
- Himebaugh NL, Wright AR, Bradley A, Begley CG, Thibos LN. Use of retroillumination to visualize optical aberrations caused by tear film break-up. Optom Vis Sci. 2003; 80: 69-78.
- Timberlake GT, Doane MG, Bertera JH. Short term, low contrast visual acuity reduction associated with in vivo contact lens drying. Optom Vis Sci. 1992; 69: 755-760.
- Tutt R, Bradley A, Begley C, Thibos LN. Optical and visual impact of tear break-up in human eyes. Invest Ophthalmol Vis Sci. 2000; 41: 4117-4123.
- Thai LC, Tomlinson A, Ridder WH. Contact lens drying and visual performance: the vision cycle with contact lenses. Optom Vis Sci. 2002; 79: 381-388.
- Smith JA, Albeitz J, Begley C, Caffery B, Nichols K, Schaumberg DA, et al. The epidemiology of dry eye disease: report of the epidemiology subcommittee of the International Dry Eye Workshop (2007). The Ocular Surface. 2007; 5: 93-107.
- Shibata Y, Tanaka Y, Tomita T, Taogoshi T, Kimura Y, Chikama T, et al. Evaluation of corneal damage caused by iodine preparations using human corneal epithelial cells. Jpn J Ophthalmol. 2014; 58: 522-527.
- Jiang J, Wu M, Shen T. The toxic effect of different concentrations of povidone iodine on the rabbit’s cornea. Cutaneous and ocular toxicology. 2009; 28: 119-124.
- Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Med Assoc. 2000; 284: 3043-3045.
- Schein OD, Tielsch JM, Munoz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997; 104: 1395-1401.
- Ridder WH, 3rd, Tomlinson A. The effect of artificial tears on visual performance in normal subjects wearing contact lenses. Optom Vis Sci. 2003; 80: 826-831.
- Koh S, Watanabe H, Hosohata J, Hori Y, Hibino S, Nishida K, et al. Diagnosing dry eye using a blue-free barrier filter. Am J Ophthalmol. 2003; 136: 513-519.
- Lineaweaver W, McMorris S, Soucy D, Howard R. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985; 75: 394-396.
- Yanai R, Yamada N, Ueda K, Tajiri M, Matsumoto T, Kido K, et al. Evaluation of povidone-iodine as a disinfectant solution for contact lenses: antimicrobial activity and cytotoxicity for corneal epithelial cells. Contact lens & anterior eye: the journal of the British Contact Lens Association. 2006; 29: 85-91.
- Pels E, Vrensen GF. Microbial decontamination of human donor eyes with povidone-iodine: penetration, toxicity, and effectiveness. Br J Ophthalmol. 1999; 83: 1019-1026.
- ElKitkat RS, Ebeid WM, Habib EK, Shoukry Y. Safety of intracameral injection of minimal bactericidal concentration of povidone iodine on the corneal endothelium in a rabbit model. Cornea. 2016; 35: 72-76.
- Naor J, Savion N, Blumenthal M, Assia EI. Corneal endothelial cytotoxicity of diluted povidone--iodine. J Cataract Refract Surg. 2001; 27: 941-947.
- Siggia S. The chemistry of polyvinylpyrrolidone-iodine. Journal of the American Pharmaceutical Association American Pharmaceutical Association. 1957; 46: 201-204.
- McGill JI, Liakos GM, Goulding N, Seal DV. Normal tear protein profiles and age-related changes. Br J Ophthalmol. 1984; 68: 316-320.
- Obata H. Anatomy and histopathology of the human lacrimal gland. Cornea. 2006; 25: S82-89.
- Gipson IK. Age-related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci. 2013; 54: ORSF48-53.
- Trinkaus-Randall V, Tong M, Thomas P, Cornell-Bell A. Confocal imaging of the alpha 6 and beta 4 integrin subunits in the human cornea with aging. Invest Ophthalmol Vis Sci. 1993; 34: 3103-3109.
- Alvarado J, Murphy C, Juster R. Age-related changes in the basement membrane of the human corneal epithelium. Invest Ophthalmol Vis Sci. 1983; 24: 1015-1028.
- Johnson DH, Bourne WM, Campbell RJ. The ultrastructure of Descemet’s membrane. I. Changes with age in normal corneas. Arch Ophthalmol. 1982; 100: 1942-1947.
- Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007; 91: 1165-1169.
- Roszkowska AM, Colosi P, Ferreri FM, Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004; 218: 350-355.
- Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci. 1998; 39: 644-648.
- Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM. Ageing of the human corneal stroma: structural and biochemical changes. Biochim Biophys Acta. 1992; 1138: 222-228.
- Roszkowska AM, Colosi P, D’Angelo P, Ferreri G. Age-related modifications of the corneal endothelium in adults. Int Ophthalmol. 2004; 25: 163-166.