
Research Article
Austin J Clin Ophthalmol. 2015;2(4): 1053.
Cost-Effectiveness of Laboratory Testing for Uveitis
Gupta SK¹, Bajwa A¹*, Wanchek T² and Reddy AK¹
11Department of Ophthalmology, University of Virginia, Charlottesville, USA
22Department of Public Health Sciences, University of Virginia, Charlottesville, USA
*Corresponding author: Asima Bajwa, Department of Ophthalmology, University of Virginia, 1300 Jefferson Park Avenue, Charlottesville, VA 22908, USA
Received: April 24, 2015; Accepted: June 30, 2015; Published: July 07, 2015
Abstract
Purpose: Lab tests are often required to reach a definitive diagnosis in uveitis and guide management. The purpose of this study is to evaluate the diagnostic value of these tests in an incremental cost perspective.
Methods: A revised Bayes’ theorem statistical analysis was performed to determine the most cost-effective testing based on review of the rheumatologic literature of 16 common causes of uveitis for epidemiological data, laboratory testing, sensitivities and specificities, positive and negative predictive values, pretest probability for disease, and Medicare/Medicaid reimbursements. Etiologies were subsequently ranked by Cost-Effectiveness Units (CEU).
Results: For Medicare patients, Rheumatoid Arthritis (RA) was the most cost-effective diagnostic evaluation, average 10.4 CEU, followed by syphilis, Bartonella, granulomatosis with polyangiitis, and polyarteritis nodosa. For Medicaid patients, (HLA-A29) was the most effective initial investigation, average 5.5 CEU, followed by HLA-B27, RA, toxoplasmosis and toxocariasis.
Conclusions: Stepwise process of elimination of lab tests based on CEU may enable the ophthalmologists to arrive at a diagnosis within resource constraints.
Keywords: Uveitis diagnostic tests; Cost effectiveness; Laboratory tests
Abbreviations
CEU: Cost-Effectiveness Units; CMS: Centers for Medicare and Medicaid Services; SLE: Systemic Lupus Erythematosus; ESR: Erythrocyte Sedimentation Rate; CRP: C-reactive Protein; ANA: Antinuclear Antibody; RF: Rheumatoid Factor; GPA: Granulomatosis with Polyangiitis; PAN: Polyarteritis Nodosa; BSCR: Birdshot Choreoretinopathy; RA: Rheumatoid Arthritis
Introduction
Uveitis has an extensive differential, including inflammatory and infectious etiologies [1]. It can be vision-threatening and accounts for an estimated 10% of legal blindness in the United States [1,2]. Significant morbidity exists as children and working young adults are often affected. Some cases of uveitis represent isolated ocular disease, while others may present as ocular manifestations of systemic illnesses [3].
Uveitis can be classified by location, based on the structure(s) of the eye most affected (anterior uveitis, intermediate uveitis, posterior uveitis, or panuveitis) or based on the presence or absence of granulomatous disease [4]. Anterior uveitis constitutes a significant proportion of the morbidity experienced by uveitis patients, with HLA-B27 associated uveitis accounting for the majority of these diagnoses, both in community-based and university referral settings [4,5]. Many of the HLA-B27 associated cases may also present with systemic findings attributable to the seronegative spondyloarthropathies; however, this is not always the case. In certain instances, anterior uveitis may be the sentinel presentation of a seronegative spondyloarthropathy [6]. Less commonly, other autoimmune diseases, such as systemic lupus erythematosus (SLE), sarcoidosis, Sjogren’s syndrome, and juvenile idiopathic arthritis may also be diagnosed based on laboratory testing [4,7,8].
Investigational testing can be useful in determining the etiology of uveitis; however, each test has associated costs, specificity, and sensitivity. In cases without pathognomonic clinical features to guide testing, a costly and inefficient patient work-up may result. Furthermore, non-specific testing practices may result in inaccuracies and false-positives and introduce delay to diagnosis and potential harm to patients [2]. As healthcare providers are increasingly encouraged to approach uveitis efficiently and economically, we developed an interest in evaluating commonly ordered testing in terms of diagnostic value and cost-effectiveness. Based on disease prevalence in the United States, available sensitivity and specificity data for individual tests, pretest likelihood of disease, and available Medicare and Medicaid cost data, we aimed to evaluate the laboratory and imaging investigational methods frequently ordered in the workup of uveitis and create a cost-analysis to determine practices which promote the highest yield and lowest costs for patients [9-12].
Methods
Institutional Review Board approval was not required for this study as it did not involve the examination or treatment of patients or a review of patient records. Diagnostic testing for classic and common etiologies of uveitis was specified by a uveitis specialist (AKR). A systematic literature review for the prevalence of each disease entity in the United States was conducted utilizing MEDLINE, the online bibliographical database, using the search terms “uveitis,” “prevalence,” “United States,” and individual disease entities. The bibliographies of these articles were reviewed and epidemiological data were extracted. A systematic literature review of the diagnostic value of each testing modality as well as sensitivity and specificity, was conducted utilizing the search terms, “uveitis,” “sensitivity,” and “specificity,” for each disease entity and the diagnostic test. English language articles were selected. The bibliographies of these articles were reviewed and diagnostic accuracy and values for tests were recorded [13-51].
A cost determination was made for Medicare and Medicaid reimbursement. Medicare reimbursement cost structures of diagnostic tests in Virginia for fiscal year 2014 were obtained from the 2000 American Medical Association Current Procedural Terminology (CPT) codebook and the Centers for Medicare and Medicaid Services (CMS) Searchable Medicare Physician Fee Schedule via the CMS website [11]. Medicaid reimbursement cost structures were determined using Virginia’s Department of Medical Assistance Services online searchable database [12].
Cost Effectiveness Units (CEU) were determined by dividing the total cost by the total effectiveness. To obtain the CEU, each etiology was paired with its appropriate diagnostic studies. The measure of effectiveness was constructed using each diagnostic test’s sensitivity and specificity for detecting disease as well as the disease prevalence in the United States as a measure of disease probability.
Figure 1 provides an example of one branch of the decision tree used to calculate and compare CEUs. There are two test procedures that could be used to test for Granulomatosis with polyangiitis, C_ANCA (anti neutrophilic cytoplasmic antibody) and PR-3 (anti proteinase-3 antibody). Each test procedure has a sensitivity and specificity and there is also a probability that the patient has granulomatosis with polyangiitis. The end of each branch includes the cost, in this case the Medicare cost is $17.66, and the effectiveness, which is either 1 if the test gave a correct result and 0 if the test gave an incorrect result (Figure 1).
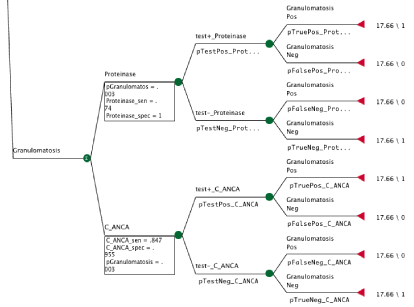
Figure 1: The formulas for the probability of a false negative using C_ANCA (pFalseNeg_C_ANCA), false positive (pFalsePos_C_ANCA), true negative
(pTrueNeg_C_ANCA), and true positive (pTruePos_C_ANCA) are provided below. The probability of granulomatosis with polyangiitis (pGranulomatosis) and the
sensitivity (C_ANCA_sen) and specificity (C_ANCA_spec ) of C_ANCA were identified from the literature.
• pFalseNeg_C_ANCA = (pGranulomatosis*(1-C_ANCA_sen))/((pGranulomatosis*(1-C_ANCA_sen))+((1-pGranulomatosis)*C_ANCA_spec))
• pFalsePos_C_ANCA = ((1-pGranulomatosis)*(1-C_ANCA_spec))/((pGranulomatosis*C_ANCA_sen)+((1-pGranulomatosis)*(1-C_ANCA_spec)))
• pTrueNeg_C_ANCA = ((1-pGranulomatosis)*C_ANCA_spec)/((pGranulomatosis*(1-C_ANCA_sen))+((1-pGranulomatosis)*C_ANCA_spec))
• pTruePos_C_ANCA = (pGranulomatosis*C_ANCA_sen)/((pGranulomatosis*C_ANCA_sen) + ((1-pGranulomatosis)*(1-C_ANCA_spec)))
• The formula for the probability that the test came back negative (pTestNeg_C_ANCA) and positive (pTestPos_C_ANCA) is given by the following formulas:
• pTestNeg_C_ANCA = ((pGranulomatosis*(1-C_ANCA_sen))+((1-pGranulomatosis)*C_ANCA_spec))
• pTestPos_C_ANCA = ((pGranulomatosis*C_ANCA_sen)+((1-pGranulomatosis)*(1-C_ANCA_spec)))
The CEU was calculated for each etiology and subsequently ranked from lowest to highest CEU (the lower the cost and the higher the accuracy, the more costeffective
the test).
Results
A total of 16 diagnoses were considered for this analysis. The average cost effectiveness for each diagnosis was determined and the diagnoses were ranked by most cost-effective. The rankings for Medicare payments are demonstrated in Table 1 and Medicaid payments are demonstrated in Table 2.
Rank
Etiology
Testing
Cost [11,12]
Prevalence (per 100,000)
Average Cost Effectiveness
Sensitivities
Specificities
Effectiveness
1
Rheumatoid arthritis [29]
Rheumatoid factor
$7.74
397
10.46
75%
74%
0.74
2
Syphilis [34-37]
Syphilis IgG
$41.68
2.4
14.49
91%
99%
2.88
VDRL
78-86% primary, 100% secondary, 95-98% latent
85-99%
RPR
78-86% primary, 100% secondary, 95-98% latent
85-100
MHATP
95%
99%
FTA-ABS
84% primary, 100% other stages
96%
3
Bartonella [52]
Bartonella Henslae serological antigen
$13.89
7100
15.62
2-50% IgM
86-100% IgM
0.89
14-100% IgG
34-100% IgG
4
Granulomatosis with polyangiitis [28]
C-ANCA
$35.32
3
18.07
85%
95%
1.95
Proteinase-3
74% ELISA/ 87.5% capture ELISA
100%
5
Polyarteritis Nodosa [31-33]
P-ANCA
$35.32
3.3-6.3
19.01
19%
92%
1.86
MPO
20%
94%
6
Toxocariasis [49,50]
Toxacara Antigen serological test
$17.75
14000
20.29
91%
86%
0.87
7
HIV/AIDS [47,48]
HIV ELISA
$59.66
355
20.65
100%
100%
2.89
HIV Western Blot
100%
89%
Rapid HIV
100%
100%
8
Toxoplasmosis [41-43]
Toxoplasma IgM
$39.29
10,8000-22,500
20.79
93-100%
78-99%
1.89
Toxoplasma IgG
97%
98%
9
Lyme Disease [44-46]
ELISA
$44.37
78
22.31
100%
99%
1.99
Western Blot
32% IgM
100% IgM
83% IgG
95%IgG
10
SLE [13-15]
Anti-nuclear antibody (ANA)
$35.23
52
23.18
93%
57%
1.52
Anti-double-stranded DNA (DSDNA)
70%
95%
11
Sjogren syndrome [13-16]
SSA
$48.92
420
25.24
70-96%
100%
1.94
SSB
60-91%
94%
12
Birdshot Chorioretinopathy [26,27]
HLA-A29 Molecular typing
$35.21
0.14
37.06
97%
95%
0.95
13
HLA B27 Uveitis [24,25]
HLA-B27 Molecular typing
$35.21
100
38.27
90%
92%
0.92
14
Tuberculosis [38-40]
Quantiferon
$316.69
3617
89.7
70%
92%
3.53
Purified Protein Derivative
67%
79%
Chest X-Ray
78%
51%
Chest CT
88%
88%
15
Sarcoidosis [21-23]
ACE
$269.76
48
176.16
73%
83%
1.53
Lysozyme
60%
70%
Chest X-Ray
70%
NA
Chest CT
78%
NA
16
Multiple Sclerosis [30]
MRI Scan
$336.73
159.8
387.56
53%
87%
0.87
Abbreviations: VDRL: Venereal Disease Research Laboratory; RPR: Rapid Plasmin Reagin; MHATP: Microhemagglutination assay for Treponema Pallidum; FTA-ABS: fluorescent treponemal antibody absorption test; C-ANCA: Cytoplasmic anti-neutrophil cytoplasmic antibodies; P-ANCA: Perinuclear Anti-neutrophil antibody test; MPO: Myeloperoxidase; ELISA: Enzyme linked immunosorbent assay; SSA: Sjogren’s Syndrome antigen A; SSB: Sjogren’s Syndrome antigen B; HLA: Human Leukocyte Antigen; ACE: Angiotensin Converting Enzyme
Table 1: Diagnoses ranked by average cost-effectiveness for Medicare.
Rank
Etiology
Testing
Cost
Prevalence
Average Cost Effectiveness (CEU)
Sensitivities
Specificities
Effectiveness
1
Birdshot Chorioretinopathy [26,27]
HLA-A29 Molecular typing
$5.20
0.14
5.47
97%
95%
0.95
2
HLA B27 Uveitis [24,25]
HLA-B27 Molecular typing
$5.20
100
5.65
90%
92%
0.92
3
Rheumatoid arthritis [29]
Rheumatoid factor
$7.46
397
10.08
75%
74%
0.74
4
Toxoplasmosis [41-43]
Toxoplasma IgM
$20.90
10,8000-22,500
11.06
93-100%
78-99%
1.89
Toxoplasma IgG
97%
98%
5
Toxocariasis [49,50]
Toxacara Antigen serological test
$10.45
14000
11.95
91%
86%
0.87
6
Bartonella [52]
Bartonella Henslae serological antigen
$10.74
7100
12.08
2-50% IgM
86-100% IgM
0.89
14-100% IgG
34-100% IgG
7
Syphilis [34-37]
Syphilis IgG
$41.15
2.4
14.3
91%
99%
2.88
VDRL
78-86% primary, 100% secondary, 95-98% latent
85-99%
RPR
78-86% primary, 100% secondary, 95-98% latent
85-100
MHATP
95%
99%
FTA-ABS
84% primary, 100% other stages
96%
8
Granulomatosis with polyangiitis [28]
C-ANCA
$32.83
3
16.79
85%
95%
1.95
Proteinase-3
74% ELISA/87.5% capture ELISA
100%
9
HIV/AIDS [47,48]
HIV ELISA
$50.82
355
17.59
100%
100%
2.89
HIV Western Blot
100%
89%
Rapid HIV
100%
100%
10
Polyarteritis Nodosa (MPA)
P-ANCA
$33.49
3.3-6.3
18.03
19%
92%
1.86
MPO
20%
94%
11
Lyme Disease [44-46]
Lyme ELISA
$41.23
78
20.73
100%
99%
1.99
Lyme Western Blot
32% IgM
100% IgM
83% IgG
95%IgG
12
Sjogren syndrome [13-16]
SSA
$41.80
420
21.56
70-96%
100%
1.94
SSB
60-91%
94%
13
SLE [13-15]
Anti-nuclear antibody (ANA)
$33.91
52
22.31
93%
57%
1.52
Anti-double-stranded DNA (DSDNA)
70%
95%
14
Tuberculosis [38-40]
Quantiferon
$217.66
3617
61.65
70%
92%
3.53
Purified Protein Derivative
67%
79%
Chest X-ray
78%
51%
Chest CT
88%
88%
15
Sarcoidosis [21-23]
ACE
$246.70
48
161.11
73%
83%
1.53
Lysozyme
60%
70%
Chest X-Ray
70%
NA
Chest CT
78%
NA
16
Multiple Sclerosis [30]
MRI Scan
$413.46
159.8
475.87
53%
87%
0.87
Table 2: Diagnoses ranked by average cost-effectiveness for Medicaid.
For Medicare patients, Rheumatoid Arthritis (RA) was the most effective first diagnostic evaluation, with an average cost effectiveness of 10.4 CEU. This was followed by syphilis (14.5 CEU), Bartonella infection (15.6 CEU), granulomatosis with polyangiitis (18.1 CEU), and polyarteritis nodosa (19.0 CEU).
For Medicaid patients, birdshot chorioretinopathy (HLA-A29) was the most effective initial investigation, with an average cost effectiveness of 5.5 CEU. This was followed by HLA-B27-positive anterior uveitis (5.7 CEU), RA (10.1 CEU), toxoplasmosis (11.1 CEU), and toxocariasis (11.9 CEU).
Discussion
Uveitis represents a significant portion of ophthalmology clinic visits to both community-based and academic centers [2,4,5]. The diagnostic evaluation for patients presenting with uveitis can be challenging and costly, and the approach to patients can be highly variable [3]. Determining the etiology of uveitis is of great importance as treatment regimens can be distinct for certain entities, and delay to appropriate therapy may lead to further morbidity for the patient related both to the original uveitic disease and the adverse effects of an ineffective treatment regimen. Thus, based on the prevalence of certain conditions and associated expense, not all testing is equally cost-effective.
In many cases, patients present with pathognomonic features (i.e. panuveitis with a rash of the palms and soles in syphilis or acute, recurrent anterior uveitis in a young man with HLA-B27 uveitis), in which case a complex algorithmic approach is not indicated and generally only confirmatory testing is necessary. The costeffectiveness of confirmatory testing in such situations is high. For patients without pathognomonic features at presentation, the analysis conducted shows that CEU can be utilized to guide a diagnostic work-up. Stepwise testing can lead to the exclusion of individual diagnoses until a positive test elucidates the origin of disease. It would be helpful to note that this step-wise approach would be particularly useful following a clinician’s assessment of pre-test probability based on history and physical exam. It doesn’t seem likely that the diagnosis will be reached solely based on exclusion by CEU (and may be less cost-effective overall) given the importance of the clinical exam in creating a differential diagnosis. Employing this particular method can assist ophthalmologists in preparing an effective diagnostic evaluation while remaining cognizant of costs to patients and the overall healthcare system.
As with any analysis based on literature review, limitations and weaknesses exist due to uncontrollable factors or unavailable data. One particular limitation faced at each step of the analysis was the separation of diagnostic testing from the clinical history and exam. Relevant historical data about a particular patient, such as exposure to infectious agents, family history of inflammatory conditions, demographic and racial factors, and a birth history complicated by congenital illnesses will likely lead a clinician down a more focused diagnostic path. A number of the disease processes responsible for causing uveitis may be evident from a patient’s past medical history. As patients present in a myriad of ways, clinical features were not accounted for in our analysis.
Another limiting factor included the lack of available prevalence studies for particular uveitis etiologies in parts of the United States. We chose the national prevalence of these entities for our calculations because their prevalence among patients with uveitis is not wellestablished. A complementary analysis with calculations based on the prevalence of these conditions among patients with uveitis will be published in a subsequent paper. Much of the epidemiological data in the literature includes small population studies, which are thought to be representative of the US population as a whole. The prevalence of certain conditions (especially infections such as syphilis and tuberculosis) are more common in some parts of the country than others, which affects determination of cost-effectiveness. Although estimates may be accurate in terms of demographical data, they may limit broad application of an algorithm derived from CEU. Our conclusions are based on the best calculations possible given what is known about the prevalence of these conditions in the United States.
A specific difficulty encountered in this particular analysis was with determination of the effectiveness of testing for sarcoidosis, specifically with imaging studies. As these studies can be read and interpreted with significant inter-reporter variability [52], specificity data for this parameter was not available and had to be inferred as zero.
A common issue facing all cost-effectiveness analyses is in the production of standardized data. Our results have been reported in cost-effectiveness units, which is a measure of total cost divided by total effectiveness. However, many other cost-effectiveness analyses performed use standardized units, such as quality-adjusted life-years. At present, no utilization data exists for this particular analysis, so we are unable to present the data as such. Etiologic differences also exist among the pediatric population versus the adult population. Our study was limited to the adult population, but further sub-group analysis should be performed for the diagnostic evaluation of uveitis in the pediatric population.
Previous studies of cost analysis have been performed for the diagnostic work-up of uveitis, but not in the United States. A Canadian study published in 2008 reported investigational patterns of Canadian ophthalmologists for anterior uveitis through survey and found that diagnostic practices varied significantly among providers. The authors concluded that ophthalmologists consistently ordered more tests than recommended by evidence-based guidelines. Furthermore, they asserted that a number of the tests ordered by ophthalmologists for diagnostic work up are non-specific and offered very little aid in determining the etiology of uveitis. The authors concluded that adherence to clinical practice guidelines and refraining from ordering extraneous tests reduced costs with no loss in sensitivity in diagnostic work-up. These tests included the Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP), Antinuclear Antibody (ANA), Rheumatoid Factor (RF). When applied to the Canadian population, adherence to clinical practice guidelines would have resulted in potential cost savings of $600,000 per year in the Canadian Healthcare System [9]. Algorithmic approaches to uveitis have been pursued, but tend to neglect cost-utility [53,54].
Conclusion
In conclusion, CEU can be used to guide diagnostic evaluation in uveitis to minimize costs to patients and the health economy, which is increasingly important as accountable care and economics play a larger role in medical decision making. As epidemiologic data on uveitis is collected and reported by a growing number of centers, our ability to apply CEU to guide evaluations for ocular inflammation will improve. Future studies may build on this data and explore costminimization models for various clinical scenarios in uveitis.
References
- Nussenblatt R, Whitcup S. Uveitis: Fundamentals and Clinical Practice. 3rd edn. Philadelphia: Mosby. 2004; 81: 651.
- Kahn HA, Moorhead HB. Statistics on blindness in the model reporting area: 1969-1970. Bethesda: National Eye Institute; 1973. US Department of Health, Education and Welfare Publication. NIH 73:427.
- Forooghian F, Gupta R, Wong DT, Derzko-Dzulynsky L. Anterior uveitis investigation by Canadian ophthalmologists: insights from the Canadian National Uveitis Survey. Can J Ophthalmol. 2006; 41: 576-583.
- Rodriguez A, Calonge M, Pedroza-Seres M, Akova YA, Messmer EM, D'Amico DJ, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996; 114: 593-599.
- McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996; 121: 35-46.
- Pato E, Bañares A, Jover JA, Fernández-Gutiérrez B, Godoy F, Morado C, et als. Undiagnosed spondyloarthropathy in patients presenting with anterior uveitis. J Rheumatol. 2000; 27: 2198-2202.
- Kanski JJ. Anterior uveitis in juvenile rheumatoid arthritis. Arch Ophthalmol. 1977; 95: 1794-1797.
- Smith JA, Mackensen F, Sen HN, Leigh JF, Watkins AS, Pyatetsky D, Tessler HH. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009; 116: 1544-155, 1551.
- Noble J, Hollands H, Forooghian F, Yazdani A, Sharma S, Wong DT, et al. Evaluating the cost-effectiveness of anterior uveitis investigation by Canadian ophthalmologists. Can J Ophthalmol. 2008; 43: 652-657.
- Vakil N, Rhew D, Soll A, Ofman JJ. The cost-effectiveness of diagnostic testing strategies for Helicobacter pylori. Am J Gastroenterol. 2000; 95: 1691-1698.
- Centers for Medicare and Medicaid Services. Physician Fee Schedule. 2014.
- Virginia Department of Medical Assistance Services. Procedure Fee Files and CPT Codes. 2014.
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 2008; 58: 15-25.
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 2008; 58: 26-35.
- Solomon DH, Kavanaugh AJ, Schur PH. American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum. 2002; 47: 434-444.
- Franceschini F, Cavazzana I. Anti-Ro/SSA and La/SSB antibodies. Autoimmunity. 2005; 38: 55-63.
- Sacks JJ, Helmick CG, Luo YH, Ilowite NT, Bowyer S. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001-2004. Arthritis Rheum. 2007; 57: 1439-1445.
- Macaubas C, Nguyen K, Milojevic D, Park JL, Mellins ED. Oligoarticular and polyarticular JIA: epidemiology and pathogenesis. Nat Rev Rheumatol. 2009; 5: 616-626.
- Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis why does it vary so much? J Rheumatol. 2002; 29: 1520-1530.
- Wong KO, Bond K, Homik J, Ellsworth JE, Karkhaneh M, Ha C, et al. Antinuclear Antibody, Rheumatoid Factor, and Cyclic-Citrullinated Peptide Tests for Evaluating Musculoskeletal Complaints in Children. Comparative Effectiveness Review No. 50 (Prepared by the University of Alberta Evidence-based Practice Center under Contract No. HHSA 290 2007 10021 I). AHRQ Publication No. 12-EHC015-EF. Rockville, MD: Agency for Healthcare Research and Quality.
- Erdal BS, Clymer BD, Yildiz VO, Julian MW, Crouser ED. Unexpectedly high prevalence of sarcoidosis in a representative U.S. Metropolitan population. Respir Med. 2012; 106: 893-899.
- Bonfioli AA, Orefice F. Sarcoidosis. Semin Ophthalmol. 2005; 20: 177-182.
- Nosal A, Schleissner LA, Mishkin FS, Lieberman J. Angiotensin-I-converting enzyme and gallium scan in noninvasive evaluation of sarcoidosis. Ann Intern Med. 1979; 90: 328-331.
- Darrell RW, Wagener HP, Kurland LT. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol. 1962; 68: 502-514.
- Suhler EB, Martin TM, Rosenbaum JT. HLA-B27-associated uveitis: overview and current perspectives. Curr Opin Ophthalmol. 2003; 14: 378-383.
- Boisgerault F, Khalil I, Tieng V, Connan F, Tabary T, Cohen JH, et al. Definition of the HLA-A29 peptide ligand motif allows prediction of potential T-cell epitopes from the retinal soluble antigen, a candidate autoantigen in birdshot retinopathy. Proc Natl Acad Sci USA. 1996; 93: 3466–3470.
- David C Gritz, Francis I. Proctor Foundation for Research in Ophthalmology, University of California, San Francisco and Kaiser Permanente Medical Group, Northern California Region; presented at ‘‘Birdshot Retinochoroidopathy: An International Workshop’’; October 15-17;2002; UCLA Conference Center, Lake Arrowhead.
- Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum. 2000; 43: 414-419.
- Bas S, Perneger TV, Seitz M, Tiercy JM, Roux-Lombard P, Guerne PA. Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors, Rheumatology (Oxford). 2002; 41: 809-814.
- Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985-2000. Neurology. 2003; 61: 1373-1377.
- Mahr A, Guillevin L, Poissonnet M, Aymé S. Prevalences of Polyarteritis nodosa, microscopic polyangiitis, Wegener's granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: A capture–recapture estimate. Arthritis Rheum. 2004; 51: 92-99.
- Lightfoot RW Jr, Michel BA, Bloch DA. The American college of rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990; 33: 1088-1093.
- Mohammad AJ, Jacobsson LT, Mahr AD, Sturfelt G, Segelmark M. Prevalence of Wegener's Granulomatosis, Microscopic Polyangiitis, Polyarteritis Nodosa and Churg Strauss Syndrome within a Defined Population in Southern Sweden. Rheumatology (Oxford). 2007; 46: 1329-1337.
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013; 40: 187-193.
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2012.
- U.S. Preventive Services Task Force [Guideline]. Screening for Syphilis Infection. Recommendation Statement. 2004.
- Young H, Moyes A, Seagar L, McMillan A. Novel recombinant-antigen enzyme immunoassay for serological diagnosis of syphilis. J Clin Microbiol. 1998; 36: 913-917.
- Bennett DE, Courval JM, Onorato I, Agerton T, Gibson JD, Lambert L, et al. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999-2000. Am J Respir Crit Care Med. 2008; 177: 348-355.
- Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006; 28: 24-30.
- Tozkoparan E, Deniz O, Ciftci F, Bozkanat E, Bicak M, Mutlu H, et al. The roles of HRCT and clinical parameters in assessing activity of suspected smear negative pulmonary tuberculosis. Arch Med Res. 2005; 36: 166-170.
- Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am J Trop Med Hyg. 2007; 77: 405-410.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004; 363: 1965-1976.
- Novatec-ID. Toxoplasma gondii 2nd Generation.
- Centers for Disease Control and Prevention. Final 2010 reports of nationally notifiable infectious diseases. MMWR Morb and Mortal Wkly Rep. 2011; 60:1088.
- Centers for Disease Control and Prevention. CDC provides estimate of Americans diagnosed with Lyme disease each year.
- Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for lyme disease. Clin Infect Dis. 2008; 47: 188-195.
- Centers for Disease Control and Prevention (CDC). HIV prevalence estimates--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008; 57: 1073-1076.
- Recommendation Summary. U.S. Preventive Services Task Force. September 2014.
- Won KY, Kruszon-Moran D, Schantz PM, Jones JL. National seroprevalence and risk factors for Zoonotic Toxocara spp. infection. Am J Trop Med Hyg. 2008; 79: 552-557.
- Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991; 29: 1831-1835.
- Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics. 2008; 121: e1413-1425.
- Reger RB, Petersen MR, Morgan WK. Variation in the interpretation of radiographic change in pulmonary disease. Lancet. 1974; 1: 111-113.
- Herbort CP. Appraisal, work-up and diagnosis of anterior uveitis: a practical approach. Middle East Afr J Ophthalmol. 2009; 16: 159-167.
- Grillo A, Levinson RD, Gordon LK. Practical diagnostic approach to uveitis. Expert Rev of Ophthalmol. 2011; 6: 449-459.