
Research Article
Austin J Clin Neurol. 2025; 12(1): 1173.
Evaluation of Safety and Efficacy of Various Treatments for Migraine: A Systematic Review and Network Meta-Analysis
Venkateswarlu M, Bharti SK and Bansal D*
Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research (NIPER), S.A.S. Nagar, Mohali, Punjab, India
*Corresponding author: Dr, Dipika Bansal, MD, DM, Associate Professor, Department of Pharmacy Practice National Institute of Pharmaceutical Education and Research (NIPER), S.A.S Nagar, Punjab-160062, India Email: dipikabansal079@gmail.com
Received: May 29, 2025 Accepted: June 05, 2025 Published: June 10, 2025
Abstract
Introduction: Migraine is a common neurological illness characterized by recurrent moderate to severe headaches that significantly reduce quality of life. Effective management requires a combination of pharmacological and nonpharmacological treatments, each varying in efficacy. This study uses indirect comparisons to evaluate and rank different treatments according to their efficacy and safety.
Methods: A systematic examination of RCTs was conducted by searching PubMed, Scopus, EMBASE, and ClinicalTrials.gov from its beginning to August 2024. Primary outcomes included headache days, visual analogue scale, headache impact test, and migraine disability assessment. Analyses employed a frequentist random-effects model with P-score
Results: A total of 24 studies were included in the analysis, encompassing 8,541 participants, of whom 80.49% were female. Topiramate 100 mg significantly reduced monthly headache days (MD: -5.49), while Lidocaine 2% improved HIT scores (MD: -4.50). BoNTA 25 U provided the greatest VAS reduction (MD: -3.50), and BoNTA 200 U showed the highest efficacy for MIDAS (MD: -13.56). P-scores ranked Lidocaine 2%, BoNTA 25 U, and BoNTA 200 U highest for headache days, pain severity, and disability, respectively.
Conclusion: This network meta-analysis highlights Lidocaine 2% and BoNTA formulations as effective migraine treatments, aiding clinicians and policymakers in optimizing treatment strategies and resource allocation.
Keywords: Migraine disorder, Pharmacological interventions, Nonpharmacological, Systematic Review, Frequentist Network Meta-Analysis.
Key Message: This network meta-analysis identifies Lidocaine 2% as the most effective for reducing headache days and BoNTA formulations as superior for alleviating pain severity and disability, offering valuable guidance for optimizing chronic migraine management.
PROSPERO Registration Number: CRD42024480139
Introduction
Migraine is a persistent neurological illness that results in recurring headaches with moderate to severe pain intensity [1-3]. As stated by the "Global Burden of Disease” study, migraine is the sixth most frequent reason for disability and the second most frequent condition linked to years lived with disability (YLD) [4]. Migraine affects up to 25% of women and 9.4% of men globally. It has a profound impact on individuals, families, and society with a significant rise in healthcare costs than non-affected families. Migraines have an annual around 11 billion dollars in direct costs and 11 billion dollars indirect costs in the United States [5,6]. For migraines, Nonsteroidal Anti- Inflammatory Drugs are the primary line of treatment, with triptans coming in second. Ditans and Gepants in the third line [6]. Beta blockers and Topiramate are employed in the first line of preventive treatment, followed by Candesartan, Flunarizine, Amitriptyline, Sodium valproate, and CGRP monoclonal antibodies in the second and third lines, respectively [7]. Both an open-label and a doubleblind, placebo-controlled trial have revealed that lidocaine works rapidly [8]. The surgical therapy of migraine headaches currently consists of surgery decompression of four major peripheral trigger sites, however other less common possible sites of compression exist, operative intervention is possible in four well-known therapeutic zones: frontal, temporal, occipital, and endonasal [9]. A recent meta-analysis on surgical interventions reveals a significant overall reduction in migraine intensity, migraine headache index, and migraine elimination [10]. The number of patients who experience a 50% or more reduction in monthly migraine days when compared to Placebo is increased by Gepants, Topiramate, and Monoclonal antibodies acting on the Calcitonin Gene Related Peptide or its receptor and the number of patients who experience a 50% moderate reduction in monthly migraine days while compared to control is increased by beta-blockers, valproate, and amitriptyline [11]. These medications have been tested for migraine in clinical trials, but a direct comparison to surgery is not possible due to a paucity of headto- head trials. While many studies have shown that pharmacological and non-pharmacological treatments for chronic migraines are more effective than placebo, no research has been compared the safety and efficacy of pharmacological and other non-pharmacological treatment modalities. We conducted a systematic review and network meta-analysis to make it simpler to compare pharmacological and other non-pharmacological treatments for migraine management
Materials and Methods
The study commenced upon the review protocol had a prospective PROSPERO registration (CRD42024480139) and followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines [12].
Search Strategy
The preliminary search was conducted on the PubMed, Scopus, and Embase databases from their foundation to March 2025, using the following key terms: "surgery," "pharmacological," "nonpharmacological," and "chronic migraine". There are no geographical or linguistic limits. We manually searched Google Scholar and reviewed reference lists of included studies for pertinent studies and Clinicaltrials.gov to find potential studies. A detailed search strategy given in ANNEXURE 1.
Study Eligibility
Trials were selected if they fulfilled all of the subsequent criteria are: Population: International Headache Society or International Classification of Headache Disorders was used to diagnose chronic migraine in the study participants. Intervention: Both pharmaceutical and non-pharmacological therapy were employed in the trial and comprehensive descriptions of the protocols, frequency, duration, and intensity of the interventions were provided. Comparator: Control using a placebo; Outcomes: The efficacy and safety of the trial were its main outcomes. Study design: A randomized parallel design with a control group was employed in this investigation. The following studies were excluded: narrative, scoping and systematic reviews, cohort studies, letters, case control, comments, posters, pilot studies, and conference presentations.
Data Extraction
Two investigators independently confirmed the data once it had been extracted into standardized forms. Two review authors (MV & SKB) extracted information from included studies independently and cross-checked it to eliminate errors. The following information was retrieved from the studies included using an information extraction spreadsheet produced in Microsoft Excel: publication year, research setting, participant demographics, baseline characteristics, intervention details, reported outcomes and the country where the study was conducted, treatment duration and dose, total sample size. Disagreements or discrepancies between both of the reviewers were resolved through discussions with the third author.
Quality Assessment
To evaluate the quality of the selected studies, the updated Cochrane risk-of-bias tool for randomized trials (RoB 2) was used [13]. Each item was labelled as having a low, moderate, or high risk of bias. Bias was classified into three levels: low, unclear, and high based on factors such as blinding, allocation concealment and random sequence generation to outcome results, inadequate outcome data, select reporting.
Statistical Analysis
We performed Frequentist Network Meta-Analysis on NMAstudio 2.0. Descriptive statistics characterize the study characteristics. Heterogeneity is assessed using the τ² statistic. Random-effects model is often used to account for variability between studies. Network diagrams visually represent the network of interventions and their direct and indirect comparisons aiding in understanding the scope of the evidence, inconsistencies between direct and indirect evidence are checked by node-splitting analysis. A league table summarizes relative effect sizes for all possible comparisons, P Score heat map to determine the relative ranking probabilities among all treatment effects on outcomes.
Results
Study Selection
The preliminary search identified 2317 studies Out of them, 24 studies were included in our analysis following an initial screening of abstracts and titles and a full-text analysis while the remainder (n = 2293) were excluded since they didn't fit the requirements for inclusion. Figure 1 displays a PRISMA flowchart that shows the numbers at each level and the subsequent study selection.
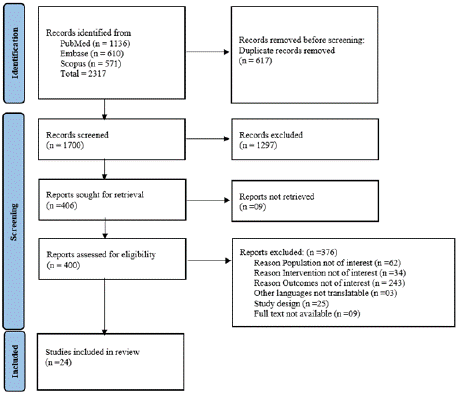
Figure 1: PRISMS Flow chart.
Characteristics of the Included Studies
The included studies listed in Table 1 [14-37] assessed three nonpharmacological therapies: TMS, Acupuncture, and Surgery, as well as four pharmaceutical interventions with varying dose categories, these included BoNTA at dosages of 25 U, 100 U, 155 U, and 195 U, as well as Lidocaine 2%, Topiramate 100 mg, and Bupivacaine 2%. The International Headache Society's or the ICHD criteria were used to enrol migraine patients for the studies. Prior the process of randomization most studies comprised a run-in period in which participants kept headache records to describe their symptoms. Of the total 8541 patients, 6875 (80.49%) were female and 1221 (14.29%) were male.
The patients' mean age was 39.04 ± 14.52 years. These studies were published in multiple countries between 2005 and 2023, despite most of them coming from the United States, which provided four studies. Italy, North America, China, Iran, and Turkey have all given three studies, followed by India with two, Afghanistan, Brazil, the Netherlands, Switzerland, North America + Europe, Germany + United Kingdom + Canada, and Afghanistan each with one.
Risk of Bias
Figures 2 and 3 illustrate the thorough risk-of-bias results and risk of each study. In overall, 12.5% of studies had a minimal risk of bias, 70.8% showed some concerns, and 16.7% had a high risk [14,19,26]. have an overall low risk of bias, but [31-34] have an overall high risk of bias [31,34] report that incomplete outcome data or selective reporting was not apparent [14,19,26,31] have clearly explained the randomization technique and concealment; nonetheless, the fact that all studies do not fully describe concealment raises concerns about the overall risk of bias.
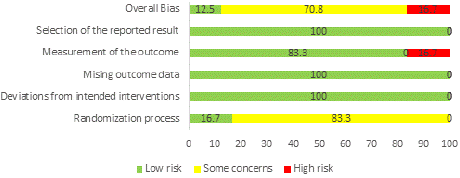
Figure 2: Overall risk of bias.
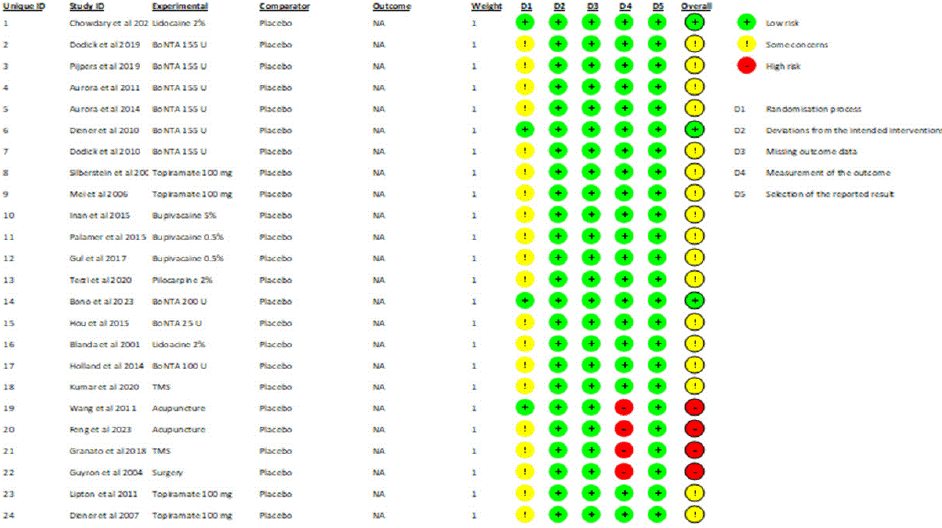
Figure 3: Risk of bias for individual studies.
Efficacy Outcomes
Mean Monthly Headache Days Reduction: In this NMA 3 RCTs included with Two treatments are Topiramate 100 mg, Bupivacaine 2%, In network plot three treatment trails estimated the treatment effect drawn from direct comparisons (Figure 4.1), 4 comparisons with indirect evidence and incontinences checked through node splitting analysis (Figure 4.2). When compared to control/placebo, the network meta-analysis results found that three drugs had a high effect on the reduction of MMHD in the follow up period from 1 month to 3 months, such as Topiramate 100 mg (MD: -5.39, 95% CI -18.9, 7.41), Bupivacaine 2% (MD: -0.80, 95% CI -19.02, 17.42) (Figure 4.2.1). Relative effectiveness illustrated by the league table (Figure 4.2.2). P-score showed top highest-ranked treatment was Topiramate 100 mg (0.73) and the lowest-ranked Bupivacaine 2% was (0.44) (Figure 4.2.3), Furthermore, the Tau-Squared test showed heterogeneity for this comparison (τ², 84.39)
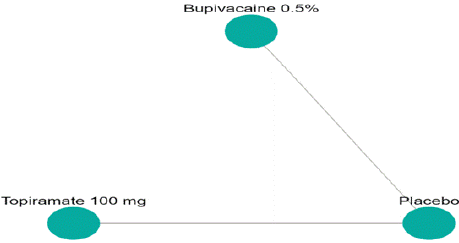
Figure 4.1: Network plot for Mean monthly headache days at post
treatment.
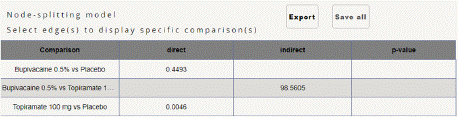
Figure 4.2: Node splitting analysis for Mean monthly headache days at
post treatment.
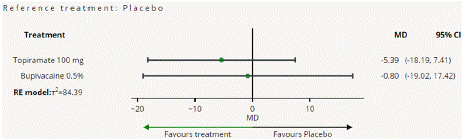
Figure 4.2.1: All treatments Vs Placebo for Mean monthly headache days
at post treatment.
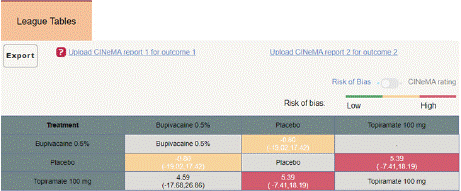
Figure 4.2.2: League table for Mean monthly headache days at post
treatment.
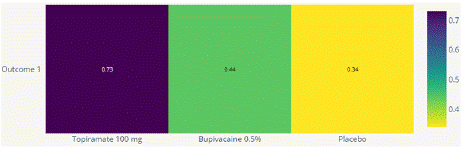
Figure 4.2.3: P scores for Mean monthly headache days at post treatment.
Mean Monthly Headache Days Reduction from Baseline to Post treatment: In this NMA 7 RCTS included with Three treatments BoNTA 155 U, BoNTA 195 U, Lidocaine 2%, and participants. Three treatment trails estimated the treatment effect drawn from direct comparisons (Figure 5.1), three comparisons with indirect evidence and incontinences checked through node splitting analysis (Figure 5.2). When compared to common comparator placebo/control the network meta-analysis results showed that two treatments have a significant effect on the reduction of MMHD in the follow up period from 1 month to 6 months, such as Lidocaine 2% (MD: 4.20, 95% CI: 1.55, 6.85), BoNTA 195 U (MD: 2.30, 95% CI: 1.95, 2.65), BoNTA 155 U (MD: 1.20, 95% CI: -0.83, 3.28) (Figure 5.2.1). Relative effectiveness illustrated by league table (Figure 5.2.2). The p-score showed highestranked treatment was Lidocaine 2% (0.96) and the bottom lowestranked treatment was Placebo (0.04). (Figure 5.2.3), Furthermore, the Tau-Squared test showed that not applicable due to few studies.
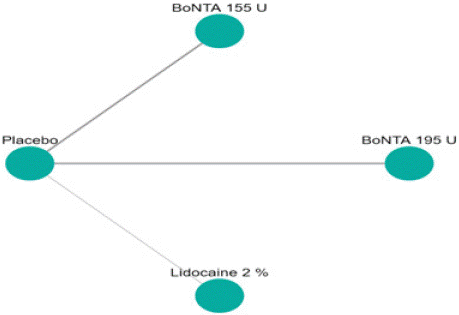
Figure 5.1: Network plot of Monthly Mean change headache days from
baseline to post treatment.
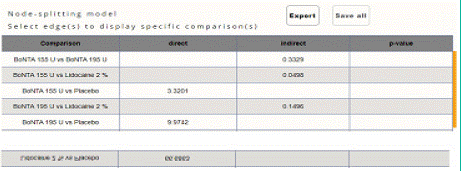
Figure 5.2: Node splitting analysis of Monthly Mean change headache days
from baseline to post treatment.
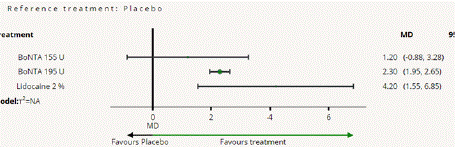
Figure 5.2.1: All treatments Vs Placebo for Monthly Mean change
headache days from baseline to post treatment.
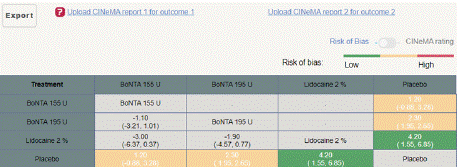
Figure 5.2.2: League table for Monthly Mean change headache days from
baseline to post treatment.

Figure 5.2.3: P score heat map for Monthly Mean change headache days
from baseline to post treatment.
Visual Analogue Scale Score Reduction: In this NMA 12 RCTS included with 9 treatments TMS, Acupuncture, BoNTA 100 U, BoNTA 200 U, BoNTA 25 U, Bupivacaine 2%, Placebo, Lidocaine 2%, Pilocarpine 0.2%. Nine comparisons estimated the treatment effect derived from direct evidence (Figure 6.1), 50 comparisons with indirect evidence and incontinences checked through node splitting analysis (Figure 6.2). Compared to common comparator (control/ placebo), the network meta-analysis results found that three drug have a significant effect on the decrease of mean Visual Analogue Scale Score in the follow up period from 1 month to 6 months, such as BoNTA 25 U (MD: 3.50, 95% CI: 1.91, 5.09), BoNTA 25 U (MD: 2.40, 95% CI: 0.65, 4.15), TMS (MD: 2.18, 95% CI: -1.79, 6.15), BoNTA 100 U (MD: 1.84, 95% CI: 0.02, 3.66), Bupivacaine 2% ( MD: 1.24, 95% CI: 0.23, 2.25), (Figure 6.2.1). Relative effectiveness illustrated by league table (Figure 6.2.2). P-score showed that high-ranked treatment was BoNTA 25 U (0.94) and the low ranked treatment was Lidocaine 2% (0.26) (Figure 6.2.3), and the Tau-Squared test showed heterogeneity for this comparison (τ², 0.75)
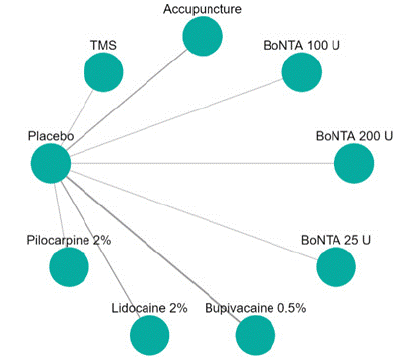
Figure 6.1: Network plot for Visual Analogue scale mean reduction.
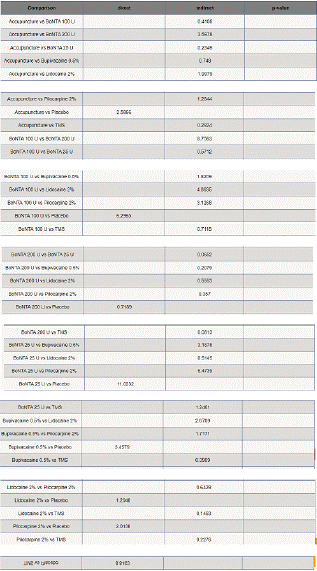
Figure 6.2: Node splitting table VAS mean reduction.
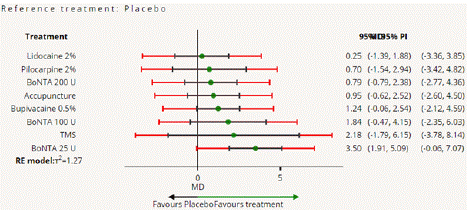
Figure 6.2.1: All treatments Vs Placebo for VAS mean reduction.
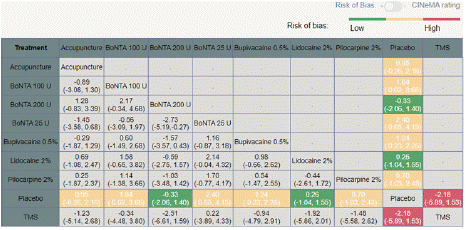
Figure 6.2.2: League table of VAS mean reduction.
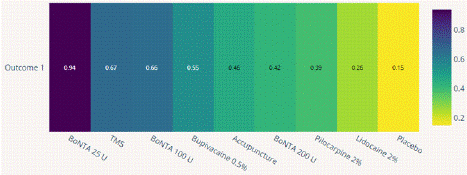
Figure 6.2.3: P Score heat map for VAS mean reduction.
Migraine Disability Assessment Score (MIDAS) Reduction: In this outcome 5 studies included with 5 interventional treatments BoNTA 200 U, Topiramate 100 mg, Lidocaine 2%, Surgery. Network plot have five treatment trails estimated the treatment effect from direct comparisons (Figure 7.1), 10 comparisons with indirect evidence and incontinences done by node splitting analysis (Figure 7.2). Compared to the control/placebo, the NMA results effect on the reduction of mean MIDAS in the follow up period from 1 month to 12 months, such as BoNTA 200 U (MD: 13.56, 95% CI: -11.68, 38.80), Lidocaine 2% (MD: -9.0, 95% CI: -32.46, 14.46), Surgery (MD: 1.59, 95% CI: -21.67, 24.85) (Figure 7.2.1). The relative effectiveness was depicted using the league table (Figure 7.2.2). P-score showed that high ranked treatment was BoNTA 200 U (0.73) and the low ranked treatment was Topiramate 100 mg (0.37) (Figure 7.2.3) Furthermore, the Tau- Squared test showed extremely large and substantial heterogeneity for this comparison (τ², 140.82.)
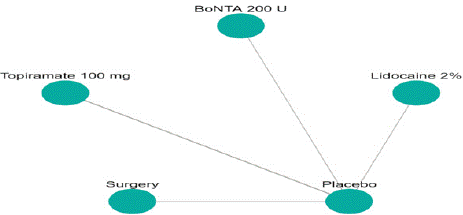
Figure 7.1: Network plot for MIDAS mean reduction.
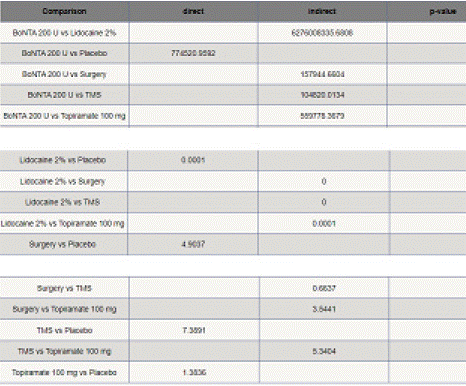
Figure 7.2: Net split table for MIDAS mean reduction.
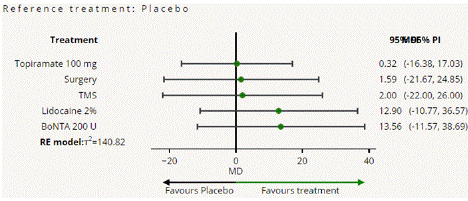
Figure 7.2.1: All treatments Vs Placebo for MIDAS mean reduction.
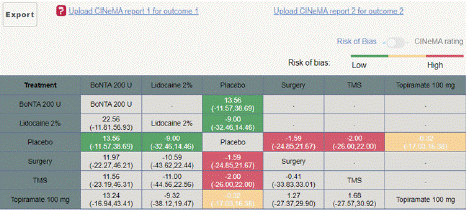
Figure 7.2.2: League table MIDAS for MIDAS mean reduction.
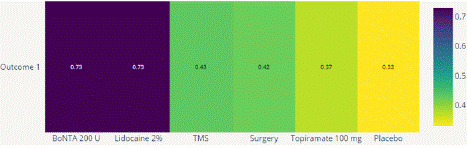
Figure 7.2.3: P Scores for MIDAS mean reduction.
Headache Impact Test (HIT) Score Reduction: In this NMA 5 RCTS included with 5 treatments BoNTA 255 U, BoNTA 195 U, Lidocaine 2%. Three treatment trails estimated the treatment effect from direct evidence (Figure 8.1), Nine comparisons with indirect evidence and incontinences checked through node splitting analysis (Figure 8.2). Compared to the control/placebo, the network meta-analysis results found that two drugs had a significant effect on the reduction of mean HIT in the follow up period from 3 month to 6 months, such as Lidocaine 2% (MD: -4.50, 95% CI: -7.98, -1.02), BoNTA 195 U (MD: -2.30, 95% CI: -2.73, -1.83) (Figure 8.2.1). Relative effectiveness illustrated by league table (Figure 8.2.2). Based on the p-score the high ranked treatment was Lidocaine 2% (0.96), and the low ranked treatment was BoNTA 155 U (0.17) (Figure 8.2.3) Furthermore, the Tau-Squared test showed not applicable due to few studies.
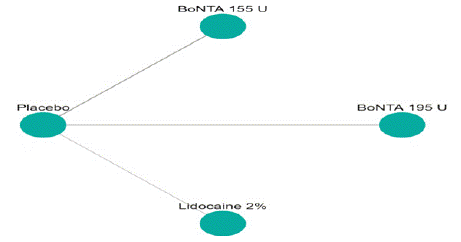
Figure 8.1: Network Plot for HIT mean reduction.
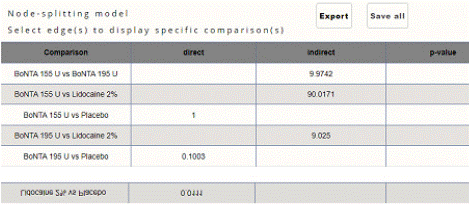
Figure 8.2: Node split analysis of HIT mean reduction.
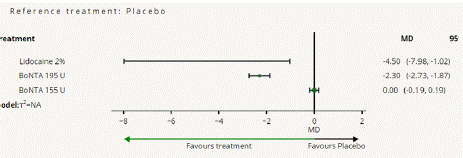
Figure 8.2.1: All treatments Vs Placebo mean reduction.
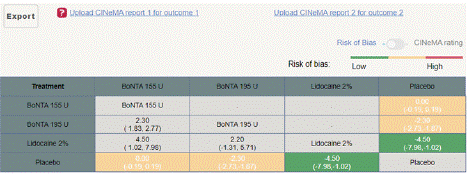
Figure 8.2.2: League table of MIDAS mean reduction.
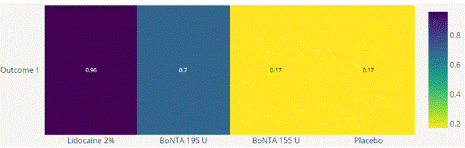
Figure 8.2.3: P Score for HIT mean reduction.
Discussion
To the best of our understanding, this has been the first systematic review and NMA of pharmacological and non-pharmacological treatments for migraine. There were twenty-four studies in all that reviewed both pharmacological (19 studies) and non-pharmacological (5 studies) techniques. This study uses 10 treatment options and the findings reveal significant variability in the effectiveness of the treatments across different outcomes. When compared to BoNTA 155 U and BoNTA 195 U, lidocaine 2% was the most effective treatment for reducing mean monthly headache days, with a P-score of 0.96. This suggests that lidocaine 2% might prove to be an excellent choice in this context, which is especially noteworthy given the growing interest in anaesthetic agents for migraine management. When compared to bupivacaine, topiramate 100 mg was found to be the most effective therapy for decreasing the mean number of headache days per month after therapy (P-score of 0.76). BoNTA 25 U was the most effective for VAS score reduction achieving the highest P-score of 0.86 compared with BoNTA 100 U, TMS, Bupivacaine 0.5%, Acupuncture, Pilocarpine 2%, Lidocaine 2%, BoNTA 200 U which aligns with its known efficacy in reducing pain severity associated with migraines. BoNTA 200 U was determined to be the best effective treatment for lowering migraine-related disability, outperforming Topiramate 100 mg, surgery, TMS, and Lidocaine 2% (P-score = 0.78). Lidocaine 2% had the highest efficacy in decreasing HIT scores, ranking first with a P-score of 0.96 when compared to BoNTA 155 U and BoNTA 195 U. Previous studies have demonstrated the effectiveness of Botulinum toxin A (BoNTA) for migraine treatment. The PREEMPT studies [18,20] showed that BoNTA 155 U and 195 U significantly decreased the monthly average number of headache days. Our findings are consistent with this evidence, since BoNTA 25 U had the highest effect on lowering VAS scores (P-score = 0.86), indicating that it is effective in reducing pain intensity. While prior studies solely looked at BoNTA's effect on headache days, our NMA takes a broader approach, evaluating its efficacy across a variety of endpoints, including pain severity (VAS), disability (MIDAS), and total headache impact (HIT). Interestingly, this research demonstrates that BoNTA 200 U outperforms other therapies in terms of MIDAS score reduction (P-score = 0.74). The most noteworthy finding from our NMA was that Lidocaine 2% was the most effective treatment in lowering mean monthly headache days (P-score of 0.96). The possible use of lidocaine in the treatment of acute and preventative migraines has been the subject of recent investigations [37], especially in situations of refractory migraines. The evidence from our research supports the wider use of lidocaine, especially as it works well to reduce headache frequency and HIT (P-score of 0.96). On the other hand, the findings of previous studies on non-pharmacological therapies such as TMS have been inconsistent. There is less evidence to support TMS's effectiveness in treating chronic migraineurs, despite certain studies' findings [38] that it can decrease the frequency and severity of headaches in episodic migraine. This variability is reflected in our data, where TMS performed lower on all of the outcome measures we looked at. This implies that although TMS might offer certain advantages, it might not be as effective or reliable in treating chronic migraine as pharmacologic treatments such as BoNTA or lidocaine. One notable difference between our study and previous studies is the direct comparison of pharmacological and nonpharmacological therapy. Prior research [39] aimed to focus on a single kind of treatment this NMA included both, offering a more complete picture of how the therapies compare. Acupuncture, a popular non-pharmacological therapy, ranked lower than expected across major outcomes, which could imply difference in study quality and patient characteristics in previous studies. The observed variation in treatment rankings across various outcome measures emphasizes the complexities of chronic migraine care and the necessity for individualized treatment regimens. While Lidocaine 2% and BoNTA formulations appear to be consistently helpful across numerous outcomes, lower scores for other therapies such as TMS and certain BoNTA dosages indicate that not all treatment modalities are equally beneficial across diverse patientreported outcomes.
Strengths
The main strength of the study is to assesses the dosedependent effects of pharmacological therapies such as BoNTA, providing additional clinical insights by reporting new findings like the effectiveness of Lidocaine 2% to decrease headache days and the complete evaluation across pharmacological and nonpharmacological therapies. An additional advantage of the study, its focuses on several aspects of the illness, potentially enhancing patient responses in multimodal model approach such as headache days, VAS, MIDAS, and HIT scores, which were frequently ignored in previous investigations.
Limitations and Future Research
This NMA has limitations that must be acknowledged. First, the heterogeneity of the included studies, particularly study design, sample size, and treatment protocols, may introduce variability that could affect the reliability of the results. The inclusion of pharmacological and non-pharmacological interventions, each with varying mechanisms of action, further adds to the complexity and potential bias of the analysis. Another limitation is the relatively small number of studies available for certain treatments such as Pilocarpine 2% and TMS, which limits the ability to draw definitive conclusions about their comparative effectiveness. Moreover, the study did not account for potential confounding factors such as comorbid conditions, medication adherence, and variations in patient demographics, all of which could influence treatment outcomes. Due to a limited information and the diversity of Treatment emergent adverse events this NMA was not used to assess the safety of the tested therapies. To improve the findings' generalizability, additional research is needed to examine their efficacy for varied patient populations. Second, longterm evaluation is also critical. As we all know, migraine sufferers may require long-term treatment, thus the efficacy and safety of drugs is critical. Given the observed diversity in treatment efficacy across several outcome measures, future research should focus on identifying patient subgroups who may benefit the most from specific treatments
Conclusion
In conclusion, this NMA provides a comprehensive comparison of various pharmacological and non-pharmacological treatments for migraine, highlighting Lidocaine 2% as the most effective in reducing headache days, while BoNTA 25 U was most effective for reducing pain severity, BoNTA 200 U for reducing disability, and BoNTA 155 U for reducing headache impact. Our results generated evidence from global literature and the findings of our results help the clinicians to frame the effective treatment regimens and also policy makers to allocate resources for effectively for the management of migraine treatment.
Codes
BoNTA: Botulinum Toxin; TMS: Transcranial Magnetic Stimulation; ROB: Risk of Bias; U: Units; mg: milligrams; CI: Confidence Interval; NMA: Network Meta-analysis; MD: Mean Difference; MMHD: Mean Monthly Headache Days; RCTs: Randomized Controlled Clinical Trails; VAS: Visual Analogue Scale; HIT: Headache Impact Test; MIDAS: Migraine Disability Assessment Score.
References
- Eikermann-Haerter K, Can A, Ayata C. Pharmacological targeting of spreading depression in migraine. Expert Rev Neurother. 2012; 12: 297-306.
- Viero FT, Rodrigues P, Trevisan G. Cognitive or daily stress association with headache and pain induction in migraine and tension-type headache patients: a systematic review. Expert Rev Neurother. 2022; 22: 257-268.
- Caponnetto V, Ornello R, Frattale I, Di Felice C, Pistoia F, Lancia L, et al. Efficacy and safety of greater occipital nerve block for the treatment of cervicogenic headache: a systematic review. Expert Rev Neurother. 2021; 21: 591-597.
- Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020; 21: 137.
- Care AJoM. Economic Impact of Migraines: AJMC; 2020.
- Eigenbrodt AK, Ashina H, Khan S, Diener HC, Mitsikostas DD, Sinclair AJ, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. 2021; 17: 501-514.
- Excellence NIoHaC. Botulinum Toxin Type A for the Prevention of Headaches in Adults with Chronic Migraine 2021.
- Maizels M, Scott B, Cohen W, Chen W. Intranasal lidocaine for treatment of migraine: a randomized, double-blind, controlled trial. Jama. 1996; 276: 319-321.
- Peled ZM. A Novel Surgical Approach to Chronic Temporal Headaches. Plast Reconstr Surg. 2016; 137: 1597-1600.
- ElHawary H, Barone N, Baradaran A, Janis JE. Efficacy and Safety of Migraine Surgery: A Systematic Review and Meta-analysis of Outcomes and Complication Rates. Ann Surg. 2022; 275: e315-e323.
- Lampl C, MaassenVanDenBrink A, Deligianni CI, Gil-Gouveia R, Jassal T, Sanchez-Del-Rio M, et al. The comparative effectiveness of migraine preventive drugs: a systematic review and network meta-analysis. J Headache Pain. 2023; 24: 56.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021; 372: n71.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019; 366: l4898.
- Chowdhury D, Tomar A, Deorari V, Duggal A, Krishnan A, Koul A. Greater occipital nerve blockade for the preventive treatment of chronic migraine: A randomized double-blind placebo-controlled study. Cephalalgia. 2023; 43: 3331024221143541.
- Dodick DW, Silberstein SD, Lipton RB, DeGryse RE, Adams AM, Diener HC. Early onset of effect of onabotulinumtoxinA for chronic migraine treatment: Analysis of PREEMPT data. Cephalalgia. 2019; 39: 945-956.
- Pijpers JA, Kies DA, Louter MA, van Zwet EW, Ferrari MD, Terwindt GM. Acute withdrawal and botulinum toxin A in chronic migraine with medication overuse: a double-blind randomized controlled trial. Brain. 2019; 142: 1203- 1214.
- Aurora SK, Dodick DW, Diener HC, DeGryse RE, Turkel CC, Lipton RB, et al. OnabotulinumtoxinA for chronic migraine: efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand. 2014; 129: 61-70.
- Aurora SK, Winner P, Freeman MC, Spierings EL, Heiring JO, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011; 51: 1358-1373.
- Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010; 30: 804-814.
- Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010; 50: 921-936.
- Silberstein S, Lipton R, Dodick D, Freitag F, Mathew N, Brandes J, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009; 49: 1153- 1162.
- Mei D, Ferraro D, Zelano G, Capuano A, Vollono C, Gabriele C, et al. Topiramate and triptans revert chronic migraine with medication overuse to episodic migraine. Clin Neuropharmacol. 2006; 29: 269-275.
- Palamar D, Uluduz D, Saip S, Erden G, Unalan H, Akarirmak U. Ultrasoundguided greater occipital nerve block: an efficient technique in chronic refractory migraine without aura? Pain Physician. 2015; 18: 153-162.
- Gul HL, Ozon AO, Karadas O, Koc G, Inan LE. The efficacy of greater occipital nerve blockade in chronic migraine: A placebo-controlled study. Acta Neurol Scand. 2017; 136: 138-144.
- Terzi T, Karakurum B, Üçler S, İnan LE, Tulunay C. Greater occipital nerve blockade in migraine, tension-type headache and cervicogenic headache. J Headache Pain. 2002; 3: 137-141.
- Bono F, Mazza MR, Magro G, Spano G, Idone G, Laterza V, et al. Regional Targeted Subcutaneous Injection of Botulinum Neurotoxin Type A in Refractory Chronic Migraine: A Randomized, Double-Blind, Placebo-Controlled Study. Toxins (Basel). 2023; 15.
- Hou M, Xie JF, Kong XP, Zhang Y, Shao YF, Wang C, et al. Acupoint injection of onabotulinumtoxin A for migraines. Toxins (Basel). 2015; 7: 4442-4454.
- Blanda M, Rench T, Gerson LW, Weigand JV. Intranasal lidocaine for the treatment of migraine headache: a randomized, controlled trial. Acad Emerg Med. 2001; 8: 337-342.
- Hollanda L, Monteiro L, Melo A. Botulinum toxin type a for cephalic cutaneous allodynia in chronic migraine: a randomized, double-blinded, placebocontrolled trial. Neurol Int. 2014; 6: 5133.
- Kumar A, Mattoo B, Bhatia R, Kumaran S, Bhatia R. Neuronavigation based 10 sessions of repetitive transcranial magnetic stimulation therapy in chronic migraine: an exploratory study. Neurol Sci. 2021; 42: 131-139.
- Wang LP, Zhang XZ, Guo J, Liu HL, Zhang Y, Liu CZ, et al. Efficacy of acupuncture for migraine prophylaxis: a single-blinded, double-dummy, randomized controlled trial. Pain. 2011; 152: 1864-1871.
- Feng XX, Huang KY, Chen L, Zhou K. Clinical efficacy of the shallow puncture and more-twirling acupuncture method in migraine treatment and its effects on serum 5-HT and β-EP levels. Technol Health Care. 2023; 31: 533-540.
- Granato A, Fantini J, Monti F, Furlanis G, Musho Ilbeh S, Semenic M, et al. Dramatic placebo effect of high frequency repetitive TMS in treatment of chronic migraine and medication overuse headache. J Clin Neurosci. 2019; 60: 96-100.
- Guyuron B, Kriegler JS, Davis J, Amini SB. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg. 2005; 115: 1-9.
- Lipton RB, Silberstein S, Dodick D, Cady R, Freitag F, Mathew N, et al. Topiramate intervention to prevent transformation of episodic migraine: the topiramate INTREPID study. Cephalalgia. 2011; 31: 18-30.
- Diener HC, Agosti R, Allais G, Bergmans P, Bussone G, Davies B, et al. Cessation versus continuation of 6-month migraine preventive therapy with topiramate (PROMPT): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2007; 6: 1054-1062.
- Inan LE, Inan N, Karadaş Ö, Gül HL, Erdemoğlu AK, Türkel Y, et al. Greater occipital nerve blockade for the treatment of chronic migraine: a randomized, multicenter, double-blind, and placebo-controlled study. Acta Neurol Scand. 2015; 132: 270-277.
- Zhong J, Lan W, Feng Y, Yu L, Xiao R, Shen Y, et al. Efficacy of repetitive transcranial magnetic stimulation on chronic migraine: A meta-analysis. Front Neurol. 2022; 13: 1050090.
- Naguit N, Laeeq S, Jakkoju R, Reghefaoui T, Zahoor H, Yook JH, et al. Is Acupuncture Safe and Effective Treatment for Migraine? A Systematic Review of Randomized Controlled Trials. Cureus. 2022; 14: e20888.