
Case Report
Austin J Clin Med. 2025; 10(1): 1055.
A Retrospective Study on the Prevalence and In Vitro Antifungal Susceptibility among Isolates from Invasive Candidiasis in Saudi Arabia
Al-Amri A1, Al-Dabbagh M1, Al-Hababi R2, AlJishi Y3*, Alraddadi B4, Alzomor O2, Atta M3, Hassanien A5, Qutub M4, Naglaa Mohamed6 and Utt E7
1King Abdulaziz Medical City, Jeddah, Saudi Arabia; King Abdullah International Medical Research Centre, Jeddah, Saudi Arabia; King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
2King Saud Medical City, Riyadh, Saudi Arabia
3King Fahad Specialist Hospital, Dammam, Saudi Arabia
4King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
5Pfizer, Jeddah, Saudi Arabia
6Pfizer Inc., New York, New York, USA
7Pfizer Inc., Groton, Connecticut, USA
*Corresponding author: AlJishi Y, King Fahad Specialist Hospital-Dammam, Al Muraikebat Area, Ammar Bin Thabet Street, Dammam 32253, Saudi Arabia Tel: +966504824956; Email: yamama.jishi@gmail.com
Received: May 12, 2025 Accepted: June 05, 2025 Published: June 09, 2025
Abstract
Background: Invasive candidiasis (IC) is a progressive and potentially fatal infection. Five Candida species (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis and C. krusei) typically account for most IC cases, but their relative frequencies can vary to institutional level.
Methods: This retrospective observational study aimed to analyze the species distribution and antifungal susceptibility of Candida isolates from confirmed IC cases, determined according to the local criteria, at four participating hospitals in three major regions of Saudi Arabia. The study reviewed laboratory records for all IC cases, confirmed by positive culture of a Candida species as part of routine specimen analysis. Data included isolate identification, determined using standard methodologies, and antifungal susceptibility, according to published breakpoints.
Results: Among a total of 1,095 isolates, C. albicans was the most common (28.8%), followed by C. parapsilosis (17.5%), C. tropicalis (17.2%), C. glabrata (15.1%) and C. auris (8.9%). C. albicans showed high susceptibility of > 87%. C. albicans, C. parapsilosis and C. tropicalis exhibited high susceptibility to caspofungin, micafungin and amphotericin B (= 98.5%). C. glabrata showed high susceptibility of = 86.4% to voriconazole, micafungin, flucytosine, and amphotericin B. The susceptibility of C. dubliniensis to all agents was 51.9%– 75.0%. The rate of multidrug resistance among C. auris isolates was 33.7%.
Conclusions: These results provide real-world insights into the distribution of Candida species, and antifungal susceptibility, resistance patterns, and resistance phenotypes. This information is valuable for understanding the local epidemiology of IC and guiding appropriate treatment strategies to aid in improving patient care.
Keywords: Invasive candidiasis; Infection; Candida species; Saudi Arabia; Antifungal resistance; Antifungal susceptibility.
Abbreviations
CDC: Centers for Disease Control and Prevention; CLSI: Clinical and Laboratory Standards Institute; IC: Invasive Candidiasis; ICU: Intensive Care Unit; KAMC-J: King Abdulaziz Medical City, Jeddah; KFSH-D: King Fahad Specialist Hospital, Dammam; KFSH&RC-J: King Faisal Specialist Hospital and Research Center, Jeddah; KSMC-R: King Saud Medical City Hospital, Riyadh; MIC: Minimum Inhibitory Concentration; MDR: Multidrug-resistant; R: Resistant; S: Susceptible; XDR: Extensively Drug-resistant; Y: Years.
Introduction
Invasive candidiasis (IC) is a severe and potentially life-threatening infection that includes candidemia (bloodstream infections) and deep-seated tissue candidiasis (following dissemination to other body sites) [1]. Studies using global patient data have reported mortality rates for nosocomial candidemia and IC that exceed 30% [2-5]. IC is most commonly observed among patients in the intensive care unit (ICU) and only around 10% of IC cases occur in the community [6].
Candidiasis is often opportunistic and reportedly, five Candida species account for more than 90% of diagnosed cases: C. albicans, C. glabrata, C. parapsilosis, C. tropicalis and C. krusei [7,8]. Their relative frequencies will vary by geographical location, even within the same region and country, and may vary according to clinical setting and practice [7].
Globally, more than 700,000 cases of IC are reported annually [6] and in Saudi Arabia, reported IC rates were 1.55 and 1.65 cases per 1,000 hospital discharges or admission, and 2.6 cases per 100 ICU admissions [9–11]. The rate of candidemia in Saudi Arabia ranged between 0.2 and 0.76 cases per 1,000 hospital discharges [12]. The causative pathogen in the majority of candidemia cases is C. albicans, although its incidence is declining globally [8]. By contrast, the isolation of C. glabrata, which is associated with increasing patient age, is increasing [7,8].
C. glabrata has been designated as a “critical threat” fungal pathogen by the World Health Organization [13], along with C. auris, a highly transmissible emerging pathogen which is increasingly being reported and now identified in 47 countries [14]. Among a global collection of C. auris isolates, 23% were multidrug-resistant (MDR), 80% were resistant to fluconazole, a commonly used antifungal agent, and resistance was also demonstrated to other first-line agents among the echinocandins and polyenes [15].
The objective of this retrospective study was to determine the most common Candida species causing IC and their antifungal susceptibility to help to improve patient care.
Methods
Study Design
This retrospective observational study aimed to analyze the species distribution and antifungal susceptibility of isolates from confirmed IC cases at four participating hospitals from three of the major regions in Saudi Arabia.
Data Collection
The study included all clinical isolates of Candida species that were determined by local criteria to cause IC. Data were collected from the laboratory records of adult and pediatric patients from the following hospitals in Saudi Arabia: King Abdulaziz Medical City, Jeddah (KAMC-J); King Fahad Specialist Hospital, Dammam (KFSH-D); King Faisal Specialist Hospital and Research Center, Jeddah (KFSH&RC-J); and King Saud Medical City Hospital, Riyadh (KSMC-R). The records were reviewed for all cases of IC confirmed by positive culture of a Candida species, as a part of routine specimen analysis. Demographic information on the submitted isolates from confirmed IC cases are presented in Supplementary Table 1.
Data were collected for qualifying isolates of Candida species that were tested between January 2017 and December 2022. The collected data included species identification, minimum inhibitory concentration (MIC) data or categorical antifungal susceptibility, source of isolate, patient age and sex, and ward type. The isolates were obtained from both adult and pediatric patients and were limited to confirmed IC.
Isolation and Identification of Candida Species
The routine isolation and identification of Candida species was conducted using local standard methodologies. Isolates obtained from direct plating of specimens on solid media, or from positive blood culture bottles, were identified using classical morphological and biochemical tests, or automated systems, such as Vitek® MS or Vitek® 2 (bioMerieux, Marcy-l’Etoile, France).
Antifungal Susceptibility Testing
The identified isolates were tested locally for their susceptibility to a range of antifungal agents using the Vitek® 2 or Sensititre™ YeastOne™ systems (Thermo Fisher Scientific, Waltham, MA USA). KAMC-J provided MIC data, and KFSH-D, KFSHRC-J and KSMC-R provided categorical antifungal susceptibility data. The MIC results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) breakpoints available at the time of testing [16]. For C. auris, which lacks established CLSI breakpoints, the study applied tentative MIC breakpoints proposed by the United States Centers for Disease Control and Prevention (CDC) [17]. These included MIC thresholds of resistance for fluconazole (= 32 μg/mL), amphotericin B (= 2 μg/mL), caspofungin (= 2 μg/mL), and micafungin (= 4 μg/mL). In the absence of CDC tentative breakpoints for other triazoles, the CLSI epidemiological cutoff value for voriconazole against C. glabrata (= 0.25 μg/mL) was used [18].
Ethical Approval
Local institutional review board approval was obtained for each participating hospital before initiating data collection. Patient consent was not required.
Data Analysis
Statistical Analysis: The data collected from the four participating hospitals were collated and analyzed using SAS version 9.4 (Cary, NC, USA) by Micron Research Ltd (Ely, Cambridgeshire, UK) to determine the local epidemiology of IC.
Species Distribution: The distribution of Candida species was determined overall and by hospital. The proportions of each species identified were calculated to provide the prevalence of different Candida species in confirmed IC cases.
Antifungal Susceptibility: The susceptibility of Candida isolates to commonly used antifungal agents (fluconazole, voriconazole, caspofungin, micafungin, amphotericin B, and flucytosine) at the four participating hospitals was analyzed. Susceptibility data were calculated overall, and stratified by patient age groups and by year, for C. albicans, C. auris, C. dubliniensis, C. glabrata, C. parapsilosis, and C. tropicalis. Three hospital laboratories (KFSH-D, KFSH&RC-J, and KSMC-R) submitted categorical susceptibility data. KAMC-J submitted MIC data, which were interpreted as described above (see ‘Antifungal susceptibility testing’) to determine susceptibility categories.
Resistance Patterns: The percentages of isolates from each species that were azole-resistant, echinocandin-resistant, MDR, and extensively drug-resistant (XDR) were calculated. MDR was defined as nonsusceptibility of an isolate to at least one agent in two or more drug classes, while XDR was defined as nonsusceptibility to at least one agent in three or more drug classes, except for C. auris, when MDR and XDR were based on numbers of resistant isolates (not non-susceptible). This analysis provided insights into the resistance patterns of different Candida species in the local IC population.
Cross-resistance: The analysis also determined cross-resistance between antifungal agents for all species. The Spearman Rank correlation test was used to assess the correlation between MIC values of different antifungal agents. A correlation coefficient (r) of = 0.6 indicated cross-resistance and was used to identify potential patterns of cross-resistance between antifungal agents.
Results
Distribution of Isolates
A total of 1,095 Candida isolates were included in the study between 2017 and 2022. Supplementary Table 1 presents the collected demographic data on source of isolate, patient age and sex, and ward type. Overall, the most common species identified was C. albicans (28.8%), followed by similar numbers of C. parapsilosis (17.5%) and C. tropicalis (17.2%), and C. glabrata (15.1%) (Figure 1). C. auris comprised 8.9% of isolates, surpassing C. krusei and C. dubliniensis (each 3.1%) (Figure 1).
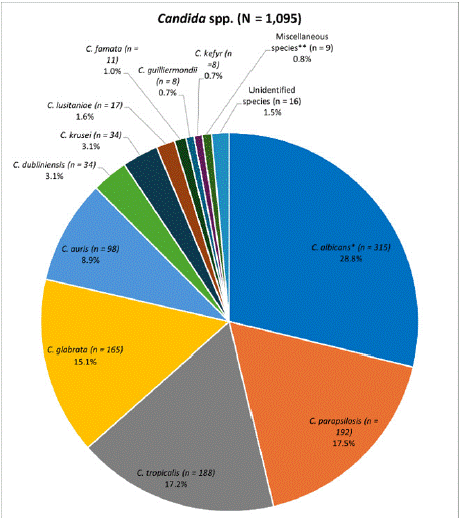
Figure 1: Overall distribution of all Candida species collected from
confirmed invasive candidiasis cases between 2017 and 2022 from four
hospitals in Saudi Arabia.
*Includes one isolate from KFSH&RC-J identified as C. albicans/C. glabrata.
**Comprises C. ciferrii (n = 3), C. utilis (n = 2), C. haemulonii (n = 1), C.
lipolytica (n = 1), C. orthopsilosis (n = 1) and C. rugosa (n = 1).
The number of Candida isolates collected by each hospital and the years of data collection were: KAMC-J, 201 (2020–2022); KFSH-D, 233 (2017–2021); KFSH&RC-J, 79 (2018–2022); and KSMC-R, 582 (2017–2021). The distribution of species varied across the four hospitals, with different rates of each species observed (Figure 2). KAMC-J had the lowest rate of C. albicans (17.4%) and C. tropicalis (10.4%) isolates and the highest rate of C. auris (22.4%). By contrast, KFSH-D had the highest rate of C. albicans (33.9%) but no C. auris isolates. KFSH&RC-J had the highest rates of C. tropicalis (32.9%) and C. glabrata (24.1%), and the lowest rate of C. parapsilosis (7.6%). KSMC-R had the highest rate of C. parapsilosis (22.0%) and lowest C. glabrata rate (11.2%).
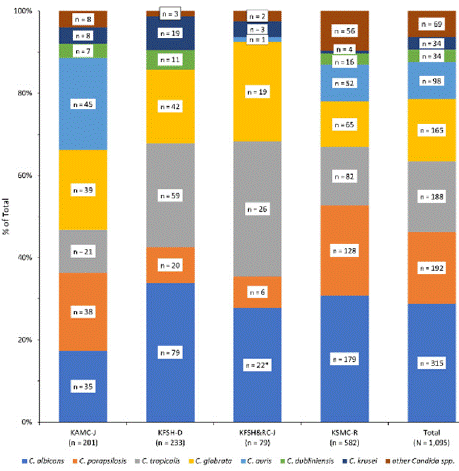
Figure 2: Distribution of Candida species collected from confirmed invasive
candidiasis cases between 2017 and 2022 from four hospitals in Saudi
Arabia, by hospital.
Abbreviations: KAMC-J, King Abdulaziz Medical City, Jeddah; KFSH-D, King
Fahad Specialist Hospital, Dammam; KFSH&RC-J, King Faisal Specialist
Hospital and Research Center, Jeddah; and KSMC-R, King Saud Medical
City Hospital, Riyadh.
*Includes one isolate from KFSH&RC-J identified as C. albicans/C.
glabrata.
Antifungal Susceptibility
Overall, C. albicans showed high susceptibility rates of > 87% to all agents (Table 1). C. parapsilosis and C. tropicalis also exhibited high susceptibility to caspofungin, micafungin and amphotericin B (= 98.5%), with rates of 83.0%–91.7% to the remaining agents. Comparing the agents, the highest susceptibility of C. glabrata was to amphotericin B (99.1%), followed by voriconazole, micafungin and flucytosine (86.4%–89.2%), with lower susceptibility to caspofungin (74.0%) and fluconazole (47.1%). Approximately 89% of C. auris was resistant to fluconazole but less than 14% of isolates were resistant to the other agents.
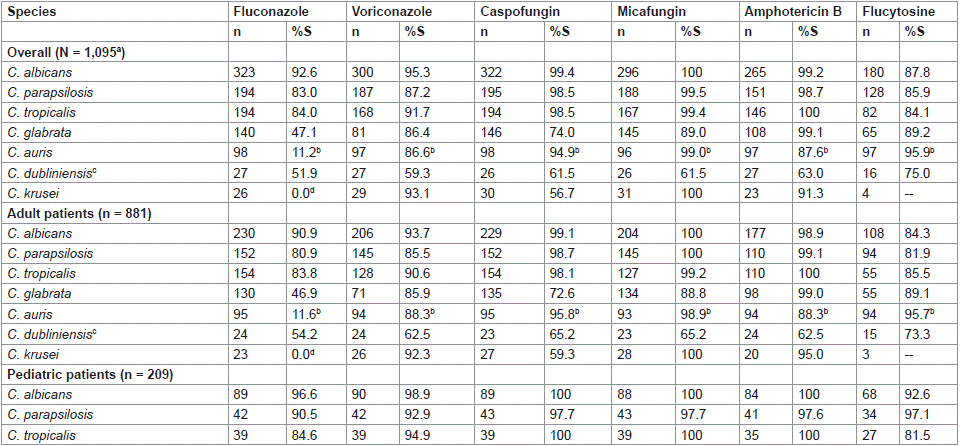
Table 1: Rates of antifungal susceptibility among 7 main Candida species collected from confirmed invasive candidiasis cases, overall and from adult (= 18 years)
and pediatric (0–17 years) patients, between 2017 and 2022 from four hospitals in Saudi Arabia.
C. krusei is intrinsically resistant to fluconazole but susceptible to voriconazole, micafungin, and amphotericin B (91.3%–100%), with lower susceptibility to caspofungin (56.7%) (Table 1). C. dubliniensis showed lower susceptibility to all agents (51.9%–75.0%).
The pattern of antifungal susceptibility of isolates from adult patients was similar to the overall collection (Table 1). For the pediatric patients, data were available for three Candida species (C. albicans, C. parapsilosis and C. tropicalis) (Table 1). More than 90% of C. albicans and C. parapsilosis from pediatric patients were susceptible to all six tested agents, whereas C. tropicalis showed lower susceptibility to fluconazole (84.6%) and flucytosine (81.5%), and higher susceptibility to the other four agents (94.9%–100%).
Susceptibility Trends
There was a decreasing trend in susceptibility to the azoles over the study period, and to flucytosine between 2017 and 2020 (Figure 3). Micafungin and amphotericin B remained active over the years; however, C. glabrata susceptibility to micafungin decreased during the study period. Moreover, comparing the species, C. glabrata showed the most pronounced changes, remaining >90% susceptible only to amphotericin B throughout the study period.
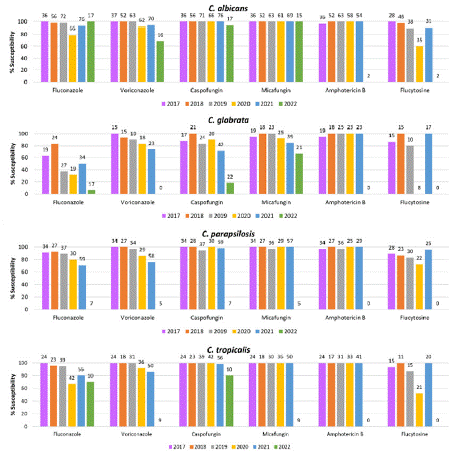
Figure 3: Rates of antifungal susceptibility of 4 main Candida species
collected from confirmed invasive candidiasis cases between 2017 and
2022 from four hospitals in Saudi Arabia, by year.
Total number of isolates tested shown above each column.
% S values not shown for species with < 10 isolates.
Resistance Phenotypes
Azole resistance was low (< 15%) for all named species except C. auris, C. krusei and C. famata (> 53%) (Table 2). Echinocandin resistance rates were also low, with the highest rate observed for C. krusei (8.8%). MDR rates were highest for C. auris, C. dubliniensis, C. krusei, and C. famata (29.4%–36.4%), followed by C. glabrata and C. lusitaniae (12.5%–17.5%). For species with = 10 isolates, less than 4.7% of the remaining species were MDR. XDR isolates were most common for C. dubliniensis (26.5%), with lower rates for the other species with = 10 isolates (= 6.3%).
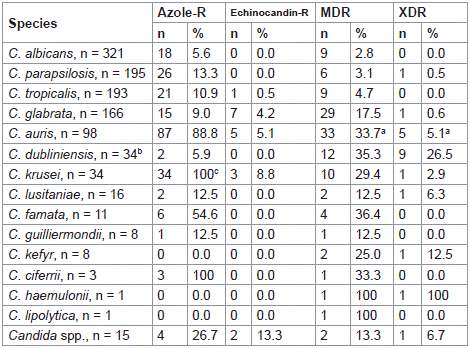
Table 2: Rates of resistance phenotypes among Candida species collected
from confirmed invasive candidiasis cases between 2017 and 2022 from four
hospitals in Saudi Arabia.
Cross-resistance
Positive correlations (r = 0.6) were observed between several pairs of antifungal agents for different species, indicating crossresistance. Examples of positively correlated pairs for C. albicans were voriconazole and caspofungin (r = 0.73), voriconazole and micafungin (r = 0.82), caspofungin and micafungin (r = 0.82), caspofungin and flucytosine (r = 0.69), and micafungin and flucytosine (r = 0.63). Positive correlations were also observed for C. auris (fluconazole and voriconazole [r = 0.65]), for C. glabrata (micafungin and flucytosine [r = 0.79]), and for C. tropicalis (caspofungin and micafungin [r = 0.73]; and micafungin and flucytosine [r = 0.65]).
Discussion
This study of Candida isolates from four hospitals in three of the major regions of Saudi Arabia aimed to investigate the prevalence of different Candida species causing IC between 2017 and 2022, and the susceptibilities of isolates to a panel of antifungal agents.
Overall, C. albicans accounted for approximately 30% of the isolates, followed by C. parapsilosis, C. tropicalis and C. glabrata, which occurred at similar rates (15.1%–17.5%). These four most common species are consistent with previous hospital studies conducted in Saudi Arabia; however, the reported order and frequencies of each species varied between the hospitals [9,11,19–25,27,28]. For example, the overall prevalence of C. albicans at KSMC-R (30.8%) was slightly lower than other data from Riyadh (38.3%–50.3%) [9,11,25,26], but higher in KFSH-D (33.9%) compared with published data from Dammam (18.8%) [27]. C. glabrata was found to be the most prevalent species in two studies [27,28], and over a 10-year period in Saudi Arabia, C. glabrata, as causative pathogen of IC, showed a 10-fold increase (P < 0.001) while C. albicans remained fairly stable [9]. In our study, C. glabrata exceeded C. parapsilosis at all hospitals except KSMC-R.
A notable finding in the current study was the emergence of C. auris, which accounted for 8.9% of the total isolates, and replaced C. krusei as one of the five most commonly reported species causing candidiasis [7,8]. C. dubliniensis, which showed the lowest susceptibility of all the species presented, was identified at low levels (< 5%) at three of the four hospitals.
Regarding antifungal susceptibility, fluconazole showed the lowest activity against Candida species, while micafungin demonstrated the highest activity, followed by caspofungin and amphotericin B. C. albicans exhibited the highest rates of susceptibility among the seven main Candida species identified, among which, C. dubliniensis tended to display the lowest antifungal susceptibility. Echinocandin resistance remained uncommon among all species. According to the clinical practice guidelines for the management of IC in the Middle East region, echinocandins are preferred for the treatment of proven and suspected Candida infections, particularly for C. glabrata or C. krusei, and in critically ill patients or those with previous exposure to azoles [20].
C. dubliniensis showed a low rate of azole resistance (5.9%) and no echinocandin resistance, while multidrug resistance rates above 29% were observed in C. auris, C. dubliniensis, C. krusei and C. famata. The rates of multidrug resistance remained low (< 5%) among C. albicans, C. parapsilosis, and C. tropicalis. Of concern was that 33.7% of C. auris isolates were MDR and 88.8% were azole-resistant. The management of C. auris poses a challenge and there are currently no specific guidelines for managing this species in our region [20]. The US CDC recommends the use of echinocandins, although antifungal resistance to these agents is increasing [29].
Cross-resistance between antifungal agents in the current study was observed most frequently for the echinocandins in C. albicans, as has been discussed in the literature but not by other studies from Saudi Arabia [27,28,30], although previous echinocandin exposure was associated with antifungal resistance (P = 0.006) in a candidemia study from Jeddah where C. glabrata was the dominant species [28]. In the current study, C. tropicalis exhibited cross-resistance between both echinocandins and flucytosine, while cross-resistance in C. auris was limited to the azoles.
This was a valuable study, with real-world findings on the distribution of Candida species, their antifungal susceptibility, trends over time, resistance phenotypes, and cross-resistance patterns. It provided important data, despite certain limitations, such as the retrospective study design, small number of participating hospitals, and varied total isolate numbers collected by each hospital. Further research could include the molecular characterization of Candida isolates to provide information on resistance mechanisms, and the continued monitoring of epidemiology to determine factors associated with species distribution and antifungal susceptibility.
In conclusion, this study highlighted the wide variation in the distribution of Candida species causing IC in Saudi Arabia and the rates of resistance phenotypes. Our findings emphasize the importance of considering hospital-specific epidemiology when managing this condition.
Transparency Declarations
Ashraf Hassanien, Naglaa Mohamed are current employees of Pfizer and Eric Utt is a former Pfizer employee. The remaining authors have none to declare.
Author Contributions
Abdulfattah Al-Amri, Mona Al-Dabbagh, Raed Al-Hababi, Yamama Aljishi, Basem Alraddadi, Omar Alzomor, Manal Atta and Mohammed Qutub participated in data collection. All authors were involved in data interpretation, drafting and reviewing the manuscript, and approved the final manuscript.
Funding Statement
Pfizer Saudi Arabia funded this study and were involved in the study design. In addition, Pfizer Saudi Arabia funded medical writing/ editorial support, provided by Wendy Wilkinson and Neera Hobson of Micron Research Ltd (Ely, UK), and also funded data management services provided by Micron Research Ltd.
References
- Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015; 373: 1445.
- Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012; 54: 1110.
- Doi AM, Pignatari AC, Edmond MB, Marra AR, Camargo LF, Siqueira RA, et al. Epidemiology and microbiologic characterization of nosocomial candidemia from a Brazilian national surveillance program. PLoS One. 2016; 11: e0146909.
- Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild MJGT, Bohlius J, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019; 25: 1200.
- Kobayashi T, Marra AR, Schweizer ML, Ten Eyck P, Wu C, Alzunitan M, et al. Impact of infectious disease ID consultation in patients with candidemia: a retrospective study, systematic literature review, and meta-analysis. Open Forum Infect Dis. 2020; 7: ofaa270.
- Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel). 2017; 3: 57.
- Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010; 14: e954.
- Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty years of the SENTRY Antifungal Surveillance Program: Results for Candida species from 1997-2016. Open Forum Infect Dis. 2019; 6: S79.
- Omrani AS, Makkawy EA, Baig K, Baredhwan AA, Almuthree SA, Elkhizzi NA, et al. Ten-year review of invasive Candida infections in a tertiary care center in Saudi Arabia. Saudi Med J. 2014; 35: 821.
- Moghnieh R, Alothman AF, Althaqafi AO, Matar MJ, Alenazi TH, Farahat F, et al. Epidemiology and outcome of invasive fungal infections and methicillinresistant Staphylococcus aureus (MRSA) pneumonia and complicated skin and soft tissue infections (cSSTI) in Lebanon and Saudi Arabia. J Infect Public Health. 2017; 10: 849.
- Al-Dorzi HM, Sakkijha H, Khan R, Aldabbagh T, Toledo A, Ntinika P, et al. Invasive candidiasis in critically ill patients: A prospective cohort study in two tertiary care centers. J Intensive Care Med. 2020; 35: 542.
- Al-Tawfiq JA. Distribution and epidemiology of Candida species causing fungemia at a Saudi Arabian hospital, 1996-2004. Int J Infect Dis. 2007; 11: 239.
- World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization; 2022.
- Ragusa P, Prinzivalli A, Pizzini S, Libero G, Lo Moro G, Brescia V, et al. Candida auris: A bibliometric analysis of an emerging global health threat. J Infect Public Health. 2023; 16: 1696.
- Chow NA, Muñoz JF, Gade L, Berkow EL, Li X, Welsh RM, et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio. 2020; 11: e03364-19.
- Clinical and Laboratory Standards Institute. Performance standards for antifungal susceptibility testing of yeasts. 3rd ed. CLSI supplement M27M44S. Clinical and Laboratory Standards Institute; 2022.
- Centers for Disease Control and Prevention. Antifungal susceptibility testing for C. auris. 2024.
- Clinical and Laboratory Standards Institute. Epidemiological cutoff values for antifungal susceptibility testing. 2nd ed. CLSI supplement M59. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2018.
- Al Thaqafi AH, Farahat FM, Al Harbi MI, Al Amri AF, Perfect JR. Predictors and outcomes of Candida bloodstream infection: eight-year surveillance, western Saudi Arabia. Int J Infect Dis. 2014; 21: 5.
- Alothman AF, Al-Musawi T, Al-Abdely HM, Salman JA, Almaslamani M, Yared N, et al. Clinical practice guidelines for the management of invasive Candida infections in adults in the Middle East region: Expert panel recommendations. J Infect Public Health. 2014; 7: 6.
- Alkharashi N, Aljohani S, Layqah L, Masuadi E, Baharoon W, Al-Jahdali H, et al. Candida bloodstream infection: Changing pattern of occurrence and antifungal susceptibility over 10 years in a tertiary care Saudi hospital. Can J Infect Dis Med Microbiol. 2019; 2019: 2015692.
- Al-Musawi TS, Alkhalifa WA, Alasaker NA, Rahman JU, Alnimr AM. A sevenyear surveillance of Candida bloodstream infection at a university hospital in KSA. J Taibah Univ Med Sci. 2020; 16: 184.
- Muzaheed, Alshehri BA, Rabaan AA, El-Masry OS, Acharya S, Alzahrani FM, et al. A 20-year retrospective clinical analysis of Candida infections in tertiary centre: Single-center experience. J Infect Public Health. 2022; 15: 69.
- Alhatmi H, Almansour S, Abanamy R, Akbar A, Abalkhail M, Alharbi A, et al. Clinical characteristics and outcome of candidemia: Experience from a tertiary referral center in Saudi Arabia. Saudi J Med Med Sci. 2022; 10: 125.
- Al-Hedaithy SS. The yeast species causing fungemia at a university hospital in Riyadh, Saudi Arabia, during a 10-year period. Mycoses. 2003; 46: 293.
- Almoosa Z, Ahmed GY, Omran A, AlSarheed A, Alturki A, Alaqeel A, et al. Invasive candidiasis in pediatric patients at King Fahad Medical City in Central Saudi Arabia. Saudi Med J. 2017; 38: 1118.
- Alkhalifa W, Alhawaj H, Alamri A, Alturki F, Alshahrani M, Alnimr A. Clinical and microbiological characteristics of candidemia cases in Saudi Arabia. Infect Drug Resist. 2023; 16: 4489.
- Aldardeer NF, Albar H, Al-Attas M, Eldali A, Qutub M, Hassanien A, Alraddadi B. Antifungal resistance in patients with Candidaemia: a retrospective cohort study. BMC Infect Dis. 2020; 20: 55.
- Centers for Disease Control and Prevention. Clinical treatment of C. auris infections. 2024.
- Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019; 25: 792.