
Research Article
Austin J Clin Immunol. 2022; 8(1):1045.
M1- and M2-Polarized Macrophages Modified Inflammatory Environment and Affects Malignant Behaviors of Cancer Stem-like Cells (CSCs) Derived from Human Melanoma Cells A375
Chen Z, Pu Y, Zhou K, Liu R, Cen Y and Chen J*
Department of Burns and Plastic Surgery, West China Hospital, Sichuan University, Chengdu, P.R. China
*Corresponding author: Junjie Chen, Department of Burns and Plastic Surgery, West China Hospital, Sichuan University, Chengdu 610041, P.R. China
Received: January 27, 2022; Accepted: February 23, 2022; Published: March 02, 2022
Abstract
Tumors contain a sub-population of cells characterized by self-renewal and expanding capacities which were known as cancer stem-like cells (CSCs). Sphere formation in conditioned serum-free medium supplemented with epidermal growth factor and basic fibroblast growth factor is a well-accepted manner for enriching CSCs from several kinds of tumor cells, including melanoma cells A375. The effects of polarized macrophages on malignant behaviors of melanoma cells were well studied, however, little was known about the effects of inflammatory environment modified by polarized macrophages on melanoma CSCs. In this study, tied to figure out these effects, we firstly stimulated the polarization of M1- and M2-state macrophages and confirmed the successful stimulation via detecting hallmarkers. Then the successful enrichment of A375 CSCs was confirmed by performing self-renewal capacity. Via detecting the malignant behaviors, it is observed that inflammatory environment modified by M1-polarized macrophages inhibited proliferation, blocked cell cycle phases at G1/G0, decreased invasive, tumor formatting abilities and induced cisplatin-sensitivity by promoting apoptosis. Contrarily, M2-conditioned medium desensitized A375 CSCs to cisplatin without affecting other malignant behaviors including proliferation, cell cycle, invasion and tumor formation. Meanwhile, M2- conditioned medium protected stemness of CSCs under oxidative stress. How the modified inflammatory microenvironment affects CSCs was performed by adding macrophage-specific cytokines and the results indicate that M1-polarized and M2-polarized macrophages partially affected CSCs via increased secretion of cytokines, including IL-1β, TNF-α and TGF-β. Taken together, we provide evidence that different macrophage polarization affected malignant behaviors of A375 CSCs partially via secretion of cytokines.
Keywords: Melanoma; Cancer stem-like cells (CSCs); Macrophage; Inflammatory microenvironment; Malignant behavior; Stemness
Introduction
Macrophages are innate immune cells that contribute to tissue homeostasis, comprise the host defense system and play critical regulatory roles on the initiation and propagation of several kinds of disease, including cancer [1-3]. Tissue-resident macrophages originate from circulating bone marrow-derived monocytic precursors differentiate into mature macrophages and polarize into different sub-populations that exert different phenotypes stimulated by different microenvironmental challenges [4,5]. Characterized by functional phenotypes, two main polarized macrophages which display a differential expression profile of cytokines, enzymes, and cell-surface markers are classified: the classical M1 and the alternative M2 macrophages [6]. M1 macrophages stimulated by interferon-γ and lipopolysaccharide (LPS), and characterized by the production of proinflammatory factors and upregulated cell surface marker, including TNF-α and IL-1β. Conversely, the M2 macrophages stimulated by IL-4 exert anti-inflammatory and pro-tumorigenic activities [7]. In this way, macrophages are highly response to microenvironment stimuli, and subsequently exert feedback regulation to inflammatory microenvironment.
By secreting cytokines, chemokines and growth factors, polarized macrophages contribute to the formation of microenvironment, and thus regulate the processes of tumorigenesis, tumor metastasis and therapeutic resistance [8-11]. A recent study showed that, in lung tumors, M2-polarized macrophages dominant M1-polarized macrophages [12]; contrarily, in colon carcinoma, it is reported that M1-polarized macrophages are dominant [13]. Interestingly, the co-existence of M1- and M2-polarized macrophages could exert controversial roles. Yuan and colleagues reported that, in lung cancer, M2-polarized macrophages promote cell invasion and tumor progression, whereas M1-polarized macrophages suppress proliferation, reduce angiogenesis and induce the apoptosis of lung cancer cells [14]. Hypoxia, considered as critical microenvironmental stimuli, tightly regulates physiological processes of macrophages residented in solid tumors [15]. Continued presence in hypoxic tumor microenvironment polarizes macrophages to M2-polarized phenotype with altered gene expression, such as upregulation of hypoxia-inducible factors (HIFs), vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP)-7 [15]. It is also reported that hypoxia-stimulated M2 macrophages promotes malignant behaviors of tumors via several mechanisms, including induction of angiogenesis, and modification of inflammatory microenvironment [16,17]. Taken together, macrophages-induced modification of inflammatory microenvironment is affected by multifactors and the subsequent effects on tumor are critical for evaluating the malignant of tumors.
Melanoma is considered as one of the most aggressive skin cancers, refractory to treatment due to highly chemoresistant ability [18]. Melanoma responds well to several strategies, including targeted and immune therapy; however, the resistance was presented in a remarkable proportion of patients, especially in targeted therapy [19,20]. One of the main sources of raise of resistance is the subpopulation of melanoma, which is characterized by self-renewal capacity and stemness defined as cancer stem-like cells (CSCs) [21]. Several studies have demonstrated that the subpopulation in melanoma share many characteristics with CSCs are widely presented in melanoma patients [22]. Wouters reported that, the putative melanoma CSCs were detected in several tumor cells in human melanomas, and display significant chemoresistance compared with their parental melanoma cells [23]. Moreover, Luo reported that, human melanoma CSCs induce chemoresistance via diverse mechanisms, including inducing ATP-binding-cassette (ABC) transporters, ABCB1 and ABCB5 [24].
The expression patterns of cytokines and chemokines was reported to be modified in macrophages CSCs compared to the parental cells, and subsequently contributes to angiogenesis, tumorigenesis [25,26]. Hypoxia is reported to play critical promoting roles in secretion of cytokines and chemokines of macrophages [27], including TNF-α, IL-β and VEGF [28]. However, how macrophage-modified inflammatory microenvironment affects malignant behaviors of CSCs is still largely unknown.
In this study, we monitored the secretions of cytokines and chemokines in macrophages after hypoxic treatment. Then the effects of modified inflammatory microenvironment on CSCs derived from melanoma cells were evaluated.
Material and Methods
Cell culture
Human monocyte cell lines THP-1, human melanoma cell line A375 were purchased from American Type Culture Collection (ATCC Manassas, VA, USA). THP-1 was cultured in RPMI-1640 (Life Technologies, Grand Island, NY, USA) and A375 was cultured in MEM medium (Life Technologies, Grand Island, NY, USA). All medium was supplemented with 100U/ml penicillin and 100μg/ml streptomycin, 10% fetal bovine serum (FBS, Life Technologies, Grand Island, NY, USA) and maintained in a humidified atmosphere of 5% CO2 at 37oC.
Generation of M1 and M2-polarized macrophages
Mφ-polarized macrophages were generated by culturing THP-1 cells with a final concentration of 200ng/ml phorbol-12-myristate- 13-acetate (PMA, Sigma-Aldrich, St. Louis, MO, USA) for 24 hours (h). After 24-hour stimulation, attached cells were cultured in respective media supplemented with 10% FBS, and containing LPS (100ng/ml, Sigma-Aldrich, St. Louis, MO, USA) + IFN-γ (100ng/ ml, PeproTech, Rocky Hill, NJ, USA) or IL-4 (20ng/ml, PeproTech, Rocky Hill, NJ, USA) for 24h to generate M1-polarized macrophages or M2-polarized macrophages.
Preparation of conditioned medium
After stimulation, M1-polarized and M2-polarized macrophages were cultured in fresh medium. After 24h, the supernatant was centrifuged at 2,000×g for 15min and collected to be used as conditioned medium in the following experiments.
ELISA
After 24 hour stimulation, macrophage supernatant was collected and centrifuged at 2,000×g for 15min and collected. The presence of cytokines and chemokines is using commercially available ELISA for human IL-1β, TNFα, TGF-β, CCL17, and CCL22 following the protocols supplied by the manufacturers. All commercial kits were bought from BOSTER (Wuhan, China).
Flow cytometry assay
For detecting the presence of CD86 and CD206, stimulated macrophages were resuspended with suspension buffer supplemented with 0.2% BSA, 0.01% NaN3 and stained with PE-conjugated mouse anti-human mAbs against CD86 (Cat. No.: 374205), CD206 (Cat. No.: 321105), and isotype-matched IgG (Cat. No.: 400111) for 30min at 4h. Flow cytometric analysis was followed using 3 laser Navios flow cytometers (Beckman Coulter, Brea, CA, USA). All antibodies were purchased from Biolegends (San Diego, CA).
For measuring cell cycle phases, cells were co-incubated with conditioned media for 24h and suspended with trypsin and pooled with cell culture medium containing loose cells, centrifuged at 800×g for 15min. After being suspended in ice-cold PBS, cells were fixed in 5ml ice-cold 70% ethanol overnight. Then, cells were collected and suspended in buffer containing 0.1% Triton X-100, 50μg/ml propidium iodide (PI, Sigma-Aldrich, St. Louis, MO, USA). Samples were incubated at room temperature for 30 min in darkness prior to analysis on Navios flow cytometer.
For detecting apoptosis, cisplatin was added to the final concentration of 10μg/ml in Mφ-, M1-, and M2-conditioned medium and used for cell culture for 24h. Then, cells were stained with Annexin V-FICT and PI (Sigma-Aldrich, St. Louis, MO, USA) followed by flow cytometry analysis.
Western blot
To measure relative protein levels, western blot was performed as described previously [29,30]. To extract total protein, cells resuspended in PBS supplemented with a protease inhibitors PMSF (Sigma-Aldrich, St. Louis, MO, USA) was sonicated by employing SoniConvert® Sonicator (DocSense, Chengdu, China) followed by the manufacturer’s instruction. Primary antibodies used were listed as followed: IL-1β (Cat. No.: ab8320), HLA-DR (Cat. No.: ab92511), CCL17 (Cat. No.: ab182793), CD163 (Cat. No.: ab182422), Oct4 (Cat. No.: 181557), c-Myc (Cat. No.: ab32072), β-actin (Cat. No.: ab8226); All primary antibodies were purchased from Abcam and diluted in 1:2,000. HRP-conjugated Secondary antibodies were listed as followed: Goat anti-mouse IgG antibody (Cat. No.: ab97040) and goat anti-rabbit IgG antibody (Cat. No.: ab7090) were purchased from Abcam and diluted in 1: 10,000. Proteins were detected by ECL detection reagent (Life Technologies, Grand Island, NY, USA).
Enrichment of CSCs from melanoma cells A375
Cells were seeded at 1×106 cells per 60-cm2 flask and cultured in DMEM/F-12 medium (Life Technologies, Grand Island, NY, USA), supplemented with EGF (10ng/ml), basic-FGF-2 (10ng/ml), 2% B27. Medium was half-refreshed every three days. After 10-14 days, the formed spheres were collected for further analysis.
Serial replating assay
Spheres were singled and seeded at 2000 cells per 6-wells plate and cultured in serum free medium described above for 14 days. Then the formed spheres were counted and collected for next passage.
Soft agar tumor formation
A layer of agar containing 1ml of 0.6% low-melting agar dissolved in growth media was laid into wells of a 6 well cell culture dish. A second layer of agar containing 1ml of 0.3% low-melting agar dissolved in growth medium containing 5×103 cells was placed on top of the first layer. The dish was maintained in a humidified incubator at 37oC for 14 days. Cells were then stained for 1h with 0.01% crystal violet and the dish was imaged.
CCK-8 assay
1×104 cells were seeded in 96-well plates overnight. After specific treatment, CCK-8 solution (10μl/well) was added and incubated at 37oC for 1h in a humidified incubator. The absorbance value was measured at 450nm wavelength on a Multiskan spectrum microplate reader (Thermo Electron Corporation, Waltham, MA, USA). The experiments were repeated three times.
Apoptosis assay
Cells were double-stained with annexin V-FITC/PI according to the manufacturer’s protocol (BD Biosciences, Franklin Lakes, NJ, USA). The percentage of apoptotic cells were detected by flow cytometry after staining.
Statistical analysis
SPSS16.0 (SPSS, Chicago) statistical software was employed for statistical analysis. All data was expressed as mean ± SD. One-way analysis of variance was performed for multiple group comparisons. P value<0.05 was considered as statistically significant.
Results
Stimulation and characterization of M1- and M2-polarized macrophages
Human Mφ macrophages were generated by culturing THP-1 cells with 200ng/ml PMA for 24h, and then polarized toward the M1 and M2 phenotypes by stimulation with LPS and IL-4, individually. 48h later, the cytokine/chemokine released in culture medium were measured under M1- and M2-pplarizing conditions. As shown in Figure 1A, with regard to the M1-type cytokines, the significant increases in the amount of secreted IL-1β, TNF-α, and HLA-DR were detected, and with regard to the M2-type chemokines, the significant increase in the amount of CCL17 and CCL22 were observed. For further confirming the expression patterns of cytokines and chemokines after different stimulations, semi-quantitative western blot was employed to evaluate the protein levels of IL-1β, HLA-DR, CC17 and CD163 in stimulated cells as representation. As shown in Figure 1B, these protein levels presented the consistent tendency with the ELISA results. Then the surface expression of the Mφ differentiation marker, CD86, the prototypical M1 marker, and the M2 marker, CD206, was then analyzed by flow cytometric analysis to further confirm polarization. As illustrated, LPS stimulation induced the typical M1 phenotype, characterized by strong induction of CD86 compared to unpolarized Mφ in terms of percentage of positivestained cells (Figure 1C and 1D). Conversely, IL-4 stimulated Mφ cells displayed phenotypic features of M2-polarized cells, by increasing surface expression of CD206 related to unstimulated Mφ (Figure 1C and 1D).
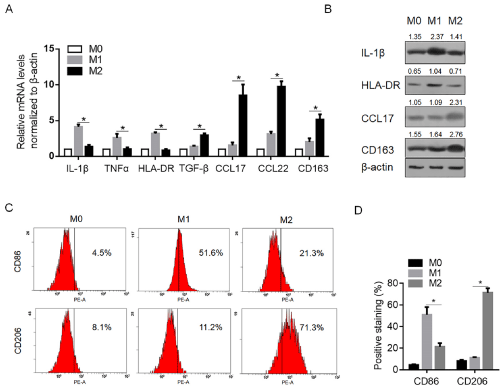
Figure 1: Establishment of M1 and M2 macrophages. A. M0 cells were established by culturing THP-1 cells with 200ng/ml PMA for 24h. Then M0 cells were
cultured with 100ng/ml LPS and 100ng/ml IFN-γ for 24h to establish M1, and were cultured with 20ng/ml IL-4 for 24h to establish M2. The mRNA levels of IL-1β,
TNF-α, HLA-DR, CCL17, CCR22, and CD163 were determined by quantitative PCR. B. Semi-quantitative western blot was performed to detect the protein levels of
IL-1β, HLA-DR, CCL17, and CD163 in M0, M1 and M2 cells. C. the positive staining of surface marker of CD86 and CD206 in M0, M1 and M2 cells were determined
by flow cytometry analysis. N=3; mean±SD.
Enrichment and characterization of CSCs from melanoma cell line A375
For enriching CSCs, the parental cell A375 was cultured in serum-free medium as described in material and methods section. After 14-day culture, obvious spheres were obtained which display different morphology relative to the parental A375 cells (Figure 2A). To characterize the stemness of enriched cells, self-renewal capacity was performed. As expected, stemness was maintained from 1 to 4 passages (Figure 2B). The expressing level of Oct4 was also semi-quantitative measured for it is considered to be tightly relative to maintenance of stemness in stem-like cells, and Oct4 display consistent higher expression levels compared to the parental cells (Figure 2C). CSCs present more malignancy compared to parental cells, and this promoted us to confirm whether the colony formation ability of CSCs is different with parental cells. As depicted in Figure 2D, CSCs displayed obvious higher colony formation ability compared to parental cells. Taken together, the enriched CSCs from A375 cells presented more malignancy and self-renewal capacity, indicated the successful enrichment of target cells.
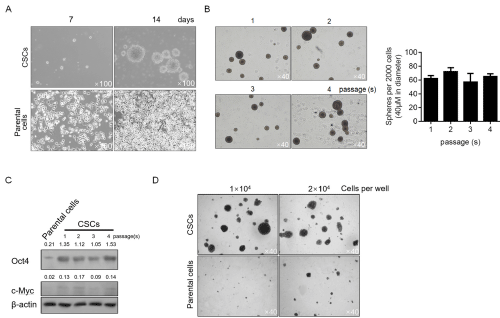
Figure 2: Characterization of cancer stem-like cells derived from melanoma A375 cells. A. After being cultured in serum-free medium, spheres were observed
at day 7 and 14. B. For identifying the self-renewal capacity of CSCs, 2000 singled cells were replating in serum-free medium for 14-day culturing, and spheres
(>40μM in diameter) were counted. C. tumor formation ability of CSCs was compared with parental cells. D. the protein levels of Oct4 and c-Myc in different
passages were detected by semi-quantitative western blot.
Different inflammatory microenvironments affect the malignant behaviors and chemosensitivity to CSCs
To determine the effects of macrophage-modified inflammatory microenvironment on malignant behaviors of CSCs, the medium conditioned by Mφ-, M1- and M2-polarized macrophages was supplemented with fresh medium at ratio of 1:1 and employed for analysis. After co-incubation with different conditioned medium for 48h, cells were fixed and stained with PI followed flow cytometric analysis for evaluating distribution of cell cycle phases. As illustrated in Figure 3A (upper panel and lower panel on the left), M1 conditioned medium (CM) significantly increased the proportion of G1/G0 phase of CSCs, meanwhile, M2 CM presented no obvious effect on CSCs. Cell viability assay was then performed and the consistent results was observed, which shows that M1 CM significantly decreased cell viability, indicated the constant proliferation inhibition (Figure 3A, low panel on the right). We then also detected the effects of CM on other malignant behaviors of CSCs, including invasion and colony formation. Consistent with the tendency to proliferating ability, M1 CM inhibited invasion and colony formation of CSCs, and M2 CM showed no detectable effects on these phenotypes (Figure 3B and 3C). Chemoresistance is one of the main characteristics of CSCs, and this promoted us to evaluate the chemosensitivity of CSCs to cisplatin (DDP). As illustrated in Figure 3D, M1 CM significantly sensitized CSCs to DDP (IC30=2.35±0.07 μg/ml; IC50=4.35±0.11 μg/ ml, P<0.05, vs. Mφ CM group). In contrast, M2 CM significantly desensitized CSCs to DDP (IC30=10.57±1.37 μg/ml; IC50=13.51±1.01 μg/ml, P<0.05, vs. Mφ CM group). To further confirm the pro- and anti-apoptotic effects of M1 and M2 CM on DDP treatment in CSCs, 10μg/ml DDP was added. Expectedly, the results showed that the proportion of Annexin V-positive stained cells and cleaved caspase-3 were increased after M1 CM treatment, and decreased after M2 CM treatment (Figure 3E).
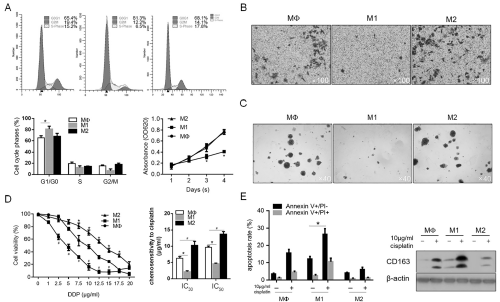
Figure 3: The effects of M1 and M2 macrophages on malignant behaviors and chemoresistance in CSCs. A. M1 and M2 macrophages regulated cell cycle
distribution and thus regulated cell proliferation. B. Transwell assay was performed to detect the effects of M1 and M2 CM on invasion in CSCs. C. soft agar colony
formation was performed to detect the tumor formation ability in vitro. D. Chemosensitivity of CSCs co-cultured with M1 and M2 CM was measured by cck-8 assay.
E. After 10μg/ml cisplatin treatment, apoptotic cell death was measured.
M2-polarized macrophages modified the inflammatory microenvironment and contributed to maintenance of CSC stemness
We then aimed to evaluate the effects of CM on CSC stemness. By performing serial replating assay, it is observed that, under original condition, M2 showed no detectable effect on self-renewal capacity of CSCs (Figure 4A). In contrast, M1 CM almost completely inhibited the formation of new spheres at passage 2, without knowing whether it is achieved via inhibiting proliferation or stemness of CSCs. It is interesting to know the effects of CM on CSCs under stresses. Oxidative stress is a widely existed stress in solid tumors, thus H2O2 induced oxidative stress was employed. Be performing sphere formation assay, it is observed that H2O2 significantly inhibited sphere formation in CSCs, which was reversed by addition of Reactive oxygen species scavenger NAC, indicated that accumulated ROS by H2O2 is necessary (Figure 4B). Under oxidative stress caused by H2O2, existence of M2 CM significantly protected CSCs from loss of stemness, which is not observed in M1 CM-treated group (Figure 4C). By measuring cell viability, it is also observed that M2 CM exerted protective effects to cell viability (Figure 4D).
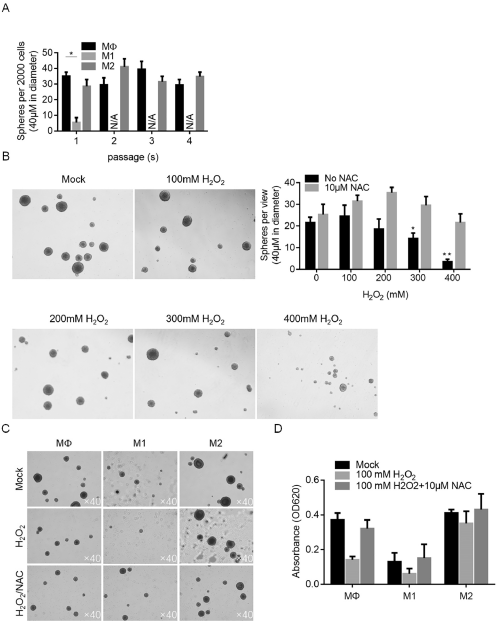
Figure 4: The effects of M1 and M2 conditioned medium on maintenance of stemness of CSCs. A. Serial replating assay was performed to detect the selfrenewal
capacity of CSCs after co-culture of M1 and M2 conditioned medium. B. Oxidative stress induced by H2O2 exposure affected self-renewal capacity. C. M2
conditioned medium exerted protective effects to self-renewal capacity of CSCs against oxidative stress induced by H2O2 exposure.
Inflammatory microenvironment modified by M1- and M2- polarized macrophages contributes partially to malignant behaviors of CSCs
To determine the effects of pro-inflammatory cytokines upregulated in M1-conditioned medium, 1ng/ml IL-1β or 10ng/ ml TNF-α was supplemented in medium and cultured for 14 days, and then the formed spheres were imaged. No detectable decrease of formed spheres was observed after addition of IL-1β or TNF-α (Figure 5A). By performing CCK-8 analysis, it is observed that IL- 1β or TNF-α no detectably inhibited proliferation in CSCs, which indicated that whether IL-1β and TNF-α affect stemness of CSCs are still uncertain. We then assessed whether TGF-β contributes to anti-oxidative stress and induce chemoresistance. As shown in Figure 5C, TGF-β obviously reversed the inhibitory effect of H2O2 on maintenance of stemness, without affecting chemoresistance of CSCs to cisplatin (Figure 5D). Taken together, inflammatory microenvironment modified by M1- and M2-polarized macrophages affected CSCs via release of cytokines without fully understood.
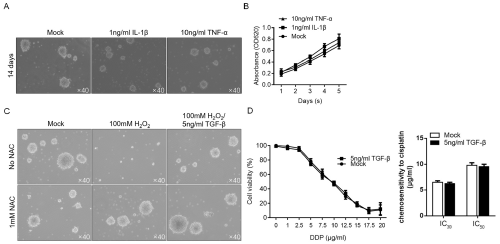
Figure 5: Differential expression patterns of cytokines partially contribute to the effects of M1- and M2-polarized macrophages. After Co-incubation of 1ng/ml IL-1β
or 10ng/ml TNF-α with singled CSCs, the sphere formation (A) and proliferation (B) were analyzed. Addition of 5ng/ml TGF-β exerted protective effects against
oxidative stress (C) without affecting chemosensitivity in CSCs (D).
Discussion
Our study showed that different polarization of macrophages can affect malignant behaviors and maintenance of stemness of cancer stem-like cells enriched from melanoma cell line A375, partially via modifying the inflammatory microenvironment, including by regulating the release of pro-inflammatory cytokines, IL-1β and TNF-α, or anti-inflammatory cytokines TGF-β. Being considered as one critical factor of tumor regulation, the regulatory effects of macrophages on tumors are widely investigated. By secreting high amounts of pro-inflammatory cytokines, such as TNF-α, interleukin (IL)-12, and IL-23, M1 macrophages promote inflammation and are found in active inflammation sites [31]. In contrast, M2-polarized macrophages stimulated by IL-4 or IL-10 are frequently activated during the resolution of inflammation via secreting anti-inflammatory cytokines, including CCL17 and CCL22 [32].
Many studies have revealed the effects of polarized macrophages on CSCs derived from different cancer cells. Ning and colleagues reported that, by co-culturing M2-polarized macrophages with ovarian cancer stem-like cells, the self-renewal capacity and malignant behaviors were obviously improved [33]. Matsukuma and colleagues revealed that polarized macrophages regulate the expression of chaperone protein calreticulin and thus inhibits the malignant behaviors of CSCs derived from pancreatic cancer cells [34]. To mimic microenvironment of tumors, polarized macrophages were co-cultured with tumors, and this results that it is difficult to determine whether the detected phenotype are caused by modified inflammatory microenvironment. For figuring out the accurate effects of inflammatory microenvironment modified by polarized macrophages, macrophage-conditioned medium was employed for further analysis. Moreover, the hallmark cytokines of M1-polarized and M2-polarized macrophages, including IL-1β, TNF-α and TGF-β, were picked separately and their effects on CSCs were tested.
IL-1β, a pleiotropic pro-inflammatory cytokine, is found to increase sphere-forming capability of colon cancer cells in serum-free medium, via upregulating stemness factor genes Bmi1 and Nestin [35]. Under chronic inflammation, TNF-α is involved in tumor development [36], metastasis [37], angiogenesis [38] and negative regulation of immune homeostasis [39]. M1-conditioned medium presented inhibitory effects on CSCs derived from melanoma cells with significant upregulated IL-1β and TNF-α. This controversial finding indicated that the tumor-promoting activities of IL-1β and TNF-α were potentially abolished by other factors, which are worth for searching in further study.
TGF-β exerts dual effect on tumor behaviors. Via suppressing tumor proliferation, inducing apoptosis, and promoting cancer cell differentiation into normal cells, TGF-β acts as a anti-tumor factor [40]. However, TGF-β also stimulates angiogenesis and immunosuppression and enhances cell mobilization once cancer cell resistance to the suppressive effects of TGF-β occurs [10]. In our results, without presenting detectable tumor inhibitory effects, TGF-β exerted protective effect against oxidative stress. It is still unknown that whether the protective effect of TGF-β is induced by the resistance to TGF-β.
In summary, our results suggest that polarized macrophages may affect CSCs derived from melanoma cells via modifying the inflammatory microenvironment. Although the roles of TGF-β in protecting CSCs from oxidative stress was observed, due to the complexity of factors released by macrophages, we failed to clearly figure out which factors are involved in these processes.
Declaration
Acknowledgements: The author would like to thanks for Mr. Tie Shi for his language editing and his suggestion of statistical analysis.
Funding: This research was supported by the National Natural Science Foundation of China (81571915) and application Basic Project of Technology Department of Sichuan Province (2016JY0153).
Availability of data and materials: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions: JJC, ZXC and YP designed the experiments. ZXC, KZ and YP performed experiments on cell sorting and related cellular experiments. RQL and YC are responsible for data collection and performed statistical analysis. All authors read and approved the final manuscript.
References
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. IMMUNITY. 2010; 32: 593-604.
- Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. ANNU REV IMMUNOL. 2015; 33: 643-675.
- Gross M, Salame TM, Jung S. Guardians of the Gut - Murine Intestinal Macrophages and Dendritic Cells. FRONT IMMUNOL. 2015; 6: 254.
- Davies LC, Rosas M, Jenkins SJ, et al. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. NAT COMMUN. 2013; 4: 1886.
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. NAT REV IMMUNOL. 2011; 11: 723-737.
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. TRENDS IMMUNOL. 2002; 23: 549-555.
- Semenza G. Signal transduction to hypoxia-inducible factor 1. BIOCHEM PHARMACOL. 2002; 64: 993-998.
- Robinson BD, Sica GL, Liu YF, et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. CLIN CANCER RES. 2009; 15: 2433-2441.
- Sun Y, Campisi J, Higano C, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. NAT MED. 2012; 18: 1359-1368.
- Principe DR, DeCant B, Mascarinas E, et al. TGFbeta Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. CANCER RES. 2016; 76: 2525-2539.
- Ma X, Aoki T, Tsuruyama T, Narumiya S. Definition of Prostaglandin E2-EP2 Signals in the Colon Tumor Microenvironment That Amplify Inflammation and Tumor Growth. CANCER RES. 2015; 75: 2822-2832.
- Zhang B, Yao G, Zhang Y, et al. M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo). 2011; 66: 1879-1886.
- Bogels M, Braster R, Nijland PG, et al. Carcinoma origin dictates differential skewing of monocyte function. ONCOIMMUNOLOGY. 2012; 1: 798-809.
- Yuan A, Hsiao YJ, Chen HY, et al. Opposite Effects of M1 and M2 Macrophage Subtypes on Lung Cancer Progression. Sci Rep. 2015; 5: 14273.
- Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor- Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS NANO. 2016; 10: 633-647.
- Casazza A, Laoui D, Wenes M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. CANCER CELL. 2013; 24: 695-709.
- Laoui D, Van Overmeire E, Di Conza G, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. CANCER RES. 2014; 74: 24-30.
- Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J AM ACAD DERMATOL. 2011; 65: S17-S25.
- Rodriguez-Cerdeira C, Carnero GM, Lopez-Barcenas A, et al. Advances in Immunotherapy for Melanoma: A Comprehensive Review. Mediators Inflamm. 2017; 2017: 3264217.
- Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. CANCER RES. 2005; 65: 9328-9337.
- Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. CANCER RES. 2005; 65: 9328-9337.
- Cho RW, Clarke MF. Recent advances in cancer stem cells. CURR OPIN GENET DEV. 2008; 18: 48-53.
- Wouters J, Stas M, Gremeaux L, et al. The human melanoma side population displays molecular and functional characteristics of enriched chemoresistance and tumorigenesis. PLOS ONE. 2013; 8: e76550.
- Luo Y, Ellis LZ, Dallaglio K, et al. Side population cells from human melanoma tumors reveal diverse mechanisms for chemoresistance. J INVEST DERMATOL. 2012; 132: 2440-2450.
- Ojha T, Pathak V, Shi Y, et al. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv Drug Deliv Rev. 2017; 119: 44-60.
- Ping YF, Bian XW. Consice review: Contribution of cancer stem cells to neovascularization. STEM CELLS. 2011; 29: 888-894.
- Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015; 3: 83-92.
- Alhawarat FM, Hammad HM and Hijjawi MS, et al. The effect of cycling hypoxia on MCF-7 cancer stem cells and the impact of their microenvironment on angiogenesis using human umbilical vein endothelial cells (HUVECs) as a model. PEERJ. 2019; 7: e5990.
- Lonardo E, Hermann PC, Mueller MT, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. CELL STEM CELL. 2011; 9: 433-446.
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. CELL. 2008; 133: 704-715.
- McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013; 110: 17253-17258.
- Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J EXP MED. 2007; 204: 1057-1069.
- Ning Y, Feng W, Cao X, et al. Genistein inhibits stemness of SKOV3 cells induced by macrophages co-cultured with ovarian cancer stem-like cells through IL-8/STAT3 axis. J Exp Clin Cancer Res. 2019; 38: 19.
- Matsukuma S, Yoshimura K, Ueno T, et al. Calreticulin is highly expressed in pancreatic cancer stem-like cells. CANCER SCI. 2016; 107: 1599-1609.
- Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. MOL CANCER. 2012; 11: 87.
- Krzystek-Korpacka M, Diakowska D, Kapturkiewicz B, Bebenek M, Gamian A. Profiles of circulating inflammatory cytokines in colorectal cancer (CRC), high cancer risk conditions, and health are distinct. Possible implications for CRC screening and surveillance. CANCER LETT. 2013; 337: 107-114.
- Ji H, Cao R, Yang Y, et al. TNFR1 mediates TNF-alpha-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. NAT COMMUN. 2014; 5: 4944.
- Zhou B, Zhuang XM, Wang YY, et al. Tumor necrosis factor alpha induces myofibroblast differentiation in human tongue cancer and promotes invasiveness and angiogenesis via secretion of stromal cell-derived factor-1. ORAL ONCOL. 2015; 51: 1095-1102.
- Zhang YH, Yan HQ, Wang F, et al. TIPE2 inhibits TNF-alpha-induced hepatocellular carcinoma cell metastasis via Erk1/2 downregulation and NFkappaB activation. INT J ONCOL. 2015; 46: 254-264.
- Shen W, Tao GQ, Zhang Y, Cai B, Sun J, Tian ZQ. TGF-beta in pancreatic cancer initiation and progression: two sides of the same coin. CELL BIOSCI. 2017; 7: 39.