
Research Article
Austin J Clin Cardiol. 2025; 11(1): 1110.
Evaluation of a One-Month Supplementation of a Nutraceutic (Hawthorn, Melissa Officinalis, Grape Seed Extract, L-Theanine and Magnesium) in Supporting Recurrent Unexplained Palpitations in Subjects with No Structural Heart Disease
Marazzi G1, Cacciotti L2, Caminiti G1,3, Arcari L2, Giamundo DM4, Misiano P5* and Pelliccia F6
1IRCCS San Raffaele, 00166 Rome, Italy
2Cardiology Department M.G. Vannini Hospital, 00177 Rome, Italy
3Department of Human Science and Promotion of Quality of Life, San Raffaele Open University, 00166 Rome, Italy
4Department of Systems Medicine, Tor Vergata University, 00133 Rome, Italy
5Dipartimento di Scienze Farmacologiche e Biomolecolari (DISFEB), Università Degli Studi di Milano, Via Pascal 36, 20133 Milan, Italy
6Dipartimento di Scienze Cardiovascolari, Universita’ Sapienza, Rome, Italy
*Corresponding author: Paola Misiano, Dipartimento di Scienze Farmacologiche e Biomolecolari (DISFEB), Università Degli Studi di Milano, Via Pascal 36, 20133 Milan, Italy Email: paola.misiano@guest.unimi.it
Received: May 28, 2025 Accepted: June 05, 2025 Published: June 10, 2025
Abstract
A benign form of palpitations is represented by recurrent palpitations not associated to cardiac diseases. Psychological and nutraceutical supports, rather than pharmacological therapy, can help in improving quality of life. Therefore, a pilot Study evaluated the effect of 30 days supplementation by a nutraceutical preparation containing Hawthorn, Melissa officinalis, Grape seed extract, L-Theanine and Magnesium in subjects with recurrent palpitations. Results obtained showed a reduction of the mean number of palpitation episodes (measured by ECG recording) after one-month supplementation vs the mean episodes registered one-month before, together with an improvement in symptomatology, evaluated by ASTA questionnaire, and no undesirable effects. The encouraging results obtained supported the use of that nutraceutical preparation in recurrent palpitations, suggesting to utilize it in clinical studies with a larger number of subjects.
Keywords: Hearth; Palpitations; Nutraceutical; Hawthorn; Grape seed
Introduction
Cardiac erethism, defined as the state of hyperexcitability of the heart, is due to the action of the sympathetic nervous system on that organ [1]. It is a benign functional condition very often linked to the psychological and emotional state (anxiety, stress), or related to a subject's level of fatigue. In the literature erethism is also known as heart palpitations, defined as the awareness of an abnormal heartbeat, a rapid or irregular pulsation of the heart [2].
Palpitations have a prevalence of 6-11% in the general population [3] and they are one of the most common symptoms in patients attending cardiology clinics (11% - 40%) [4,5].
The etiology is very multifactorial and, in some cases, cannot be determined: according to Goyal [6], 43% of palpitations have cardiac etiology, 31% psychological etiology and approximately 10% were classified as mixed (induced by drugs, caffeine, cocaine, anemia, amphetamine, mastocytosis). While some palpitations may indicate serious cardiac arrhythmias, most are benign, meaning they do not have any underlying heart disease.
Benign palpitations may be an indicator of an abnormal heart rhythm or may occur in relation to the normal heartbeat frequency; they are often associated with anxiety, stress and somatization even if they can be found in subjects without any psychological distress [4]. Generally, in the absence of associated pathologies, benign palpitations have no consequences.
Despite a good prognosis, benign palpitations cause considerable discomfort, impairment in work activity and worsening of the quality of life in affected subjects. Pharmacotherapy is mainly based of the use of mild anxiolytics and/or some beta-blockers [4].
However, in benign palpitations the heart rate is often normal, so the use of beta blockers is not very useful due to side effects and contraindications for some patients (e.g. those suffering from asthma or diabetes).
Therefore, there is no therapy in the strict sense, other than improving lifestyle (suspending stimulating agents like coffee, alcohol, medicines, drugs), avoiding stressful situations, promoting relaxation techniques and, sometimes, providing psychological support [1].
Consequently, the support of natural substances, with nonpharmacological action and with a relaxing and cardioprotective functions, can be advantageous and several examples are known [7- 11].
Beneficial effects on cardiovascular system were displayed by Hawthorn and Grape seed extract [8,12]; Melissa officinalis showed relaxant properties [7], while magnesium contributed to the normal functioning of the central nervous and muscular systems; antioxidant, relaxant and protective properties are displayed by L-Theanine form Camellia sinensis [13,14]. Combining those properties in a unique nutraceutic should possibly be of benefit in subjects with benign palpitations.
So, the aim of the present work was to evaluate the potential support of the one-month supplementation of a nutraceutic (Hawthorn, Melissa officinalis, Grape seed extract, L-Theanine and Magnesium) in subjects with unexplained palpitations with no structural heart disease.
Methods
Clinical Study
A polycentric pilot Study was performed.
Inclusion criteria were: subjects (males or females) in the 18-70 range of age, able to communicate, to make themselves understood and meet the requirements of the study, showing recurrent unexplained palpitations with no structural heart disease, without significant cardiac arrhythmias.
Exclusion criteria were: acute systemic disease; significant organic pathology, alcohol/drug abuse (also before the study); malignant neoplasm (up to 5 years before the study) or other diseases; current use of drugs or natural substances to improve sleep/ relaxation (i.e. anxiolytic, sedative, hypnotic, sympathomimetics); known intolerance to substances utilized during the study; women of childbearing potential that do not use contraceptives, women in pregnancy or breastfeeding.
Primary endpoint was to evaluate the number of palpitation episodes detected after 30 days supplementation period vs the frequency measured on 30 days before.
Secondary endpoints were to evaluate the effect of supplementation on symptoms related to palpitations (ASTA symptom) [15], on quality of life (ASTA HRQoL i.e. ASTA Health-Related Quality of Life) [16] and on safety of the supplement.
Procedures
Subjects were visited 30 days before starting the Study (T-30), the first day of the Study (T0), after 7 days (T7) and one month supplementation (T30).
At time T-30 subjects had to record all the palpitation episodes occurred, on their smartwatch, so the frequency of palpitations was monitored 30 days before supplementation and during 30 days of supplementation, when palpitation occurred: the smartwatch app was consequently activated and Electrocardiogram (ECG) was recorded to exclude potential arrythmias originated by the palpitations.
At time -30, 7, 30 days every subject completed also ASTA Symptom and ASTA HRQoL questionnaires. ASTA was developed and validated in Sweden, in patients with different forms of arrhythmias. It has been translated into several languages, and validated in English [15]. ASTA evaluates both the burden of symptoms strictly related to the arrhythmia (ASTA Symptom) and the health-related quality of life (ASTA-HRQoL) [16].
ASTA Symptom includes 9 questions and ASTA HRQoL includes 13 questions; all questions have 4 answer options, with a relative score from 0 to 3.
The scores (0-100), calculated as a percentage of the total maximum score value, show a worse situation when values are high with a more pronounced burden of symptoms related to palpitations and a more negatively influenced quality of life (HRQoL).
Supplement
Battinorm™ 500 mg sachets (Scharper, SpA), containing dry extracts of Hawthorn (250 mg), Grape seed extract from Vitis vinifera L. (Enovita™, purchased from Indena SpA) (75 mg), dry extract of Melissa officinalis (100 mg), L-Theanine from Camellia sinensis (50 mg) and Magnesium (150 mg) were administered (twice or three, if necessary) every day for 30 days.
Statistical Analysis
Data obtained from ASTA questionnaire were initially tested for normality using one-way ANOVA for repeated measures. As they were not normally distributed, data were then analyzed by Tukey’s test.
For ECG measures, data from T-30 to T-1 were compared with data obtained during supplementation (T0-T30) by Wilcoxon test.
Results
15 subjects (27-58 age range, 9 females, 6 males) were enrolled; the Study Flow diagram was displayed in Figure 1. Two sachets were taken every day, and the third sachet/day was taken from 2 to 17 times/month.
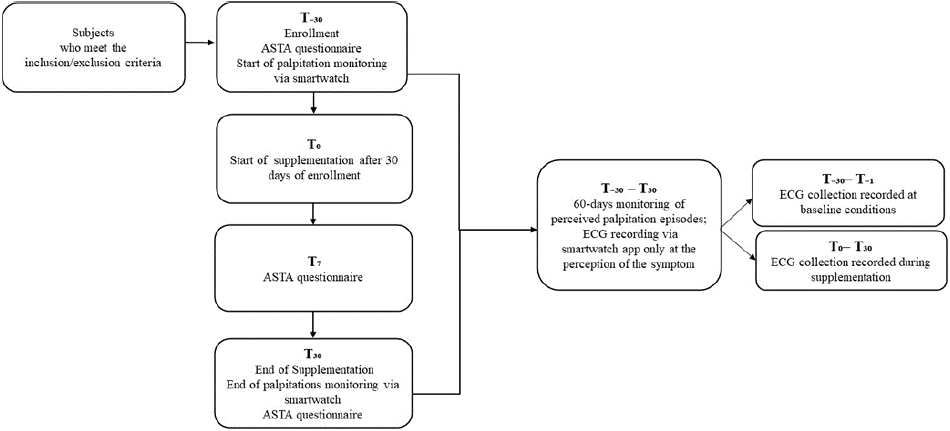
Figure 1: Study Flow diagram.
As shown in Figure 2 the frequency of palpitations (measured as number of ECG registered) after 30 days supplementation is significantly lower than the frequency of palpitations 30 days before supplementation. By normalizing ECG data on days number (data not shown), it was possible to observe that after 7 days supplementation an ECG reduction was not observed.
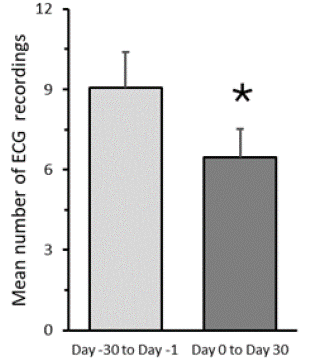
Figure 2: Effect of 30-day Battinorm™ supplementation on number of
palpitation episodes recorded by ECG.
Data are the mean ± SD of n=15 subjects.
*P<0.0001 compared to data collected from the same subjects over
the 30- day period proceeding the start of Battinorm™ supplementation
(Wilcoxon test).
ASTA symptom and ASTA HRQoL scores (Figure 3A, 3B ) are significantly reduced at the end of supplementation, showing that symptomatology related to palpitations is ameliorated.
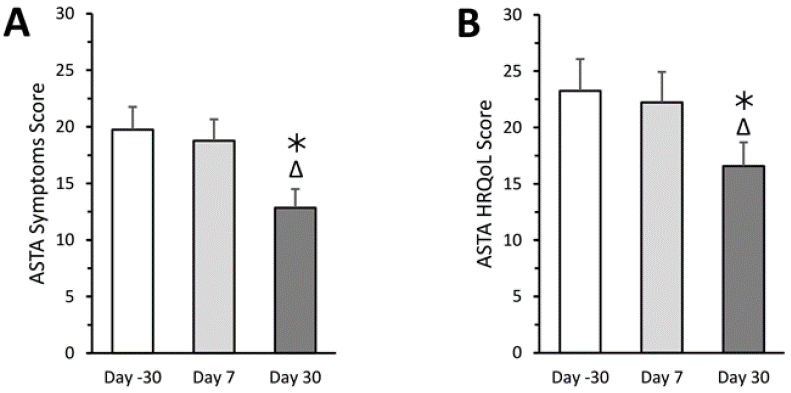
Figure 3A and 3B: Effect of 7- and 30-day supplementation on ASTA Symptoms score (panel A) and ASTA HRQoL score (panel B).
One-way ANOVA with repeated treatment:
Data are the mean ± SD of n=15 subjects. *: P<0.0001 compared to data collected at Day -30 (Tukey’s test); Δ: P<0.0001 compared to data collected at Day 7 (Tukey’s test).
No undesirable effects were described during supplementation, so supplementation was well tolerated.
Discussion
Mixed nutraceutical ingredients are produced to support several conditions, such as aging [17], hypertension [18], ocular disease [19], dyslipidemia [20,21] and chronic allergy and rhinitis [22,23].
The combination of properties displayed by different nutraceutics in a single formulation would possibly have additive beneficial effects.
Recurrent benign palpitations are a diffuse phenomenon [3] and, if neglected, it can worse the quality of life. In addition to change lifestyle by avoiding stressful conditions (not always possible) or unhealthy habits, the supplementation with nutraceutics could help.
In the present Pilot study, the potential benefits of a mixture of several nutraceutical preparations, i.e. Hawthorn, Melissa officinalis, Grape seed extract, L-Theanine and Magnesium were evaluated in subjects with recurrent palpitations, in absence of structural hearth diseases. After 30 days supplementation of Battinorm™, a reduction in mean palpitations number in respect to the previous 30-day period was observed, together with a reduction in the ASTA scores, indicating an amelioration of the quality of life. The validated ASTA questionnaires utilized in the present study represents a useful tool in patients with arrhythmia [16]. No undesirable effects were shown during or after the supplementation.
The beneficial effects produced by supplementation can be explained by the different properties displayed by the supplementation components.
Therefore, a regulation of cardiovascular function and arterial pressure already suggested in other studies for Hatworth [9] would have contributed to the regular functionality of the cardiovascular system in the supplemented subjects. Cardioprotective effects were also displayed by Melissa officinalis in a rat model of experimental autoimmune myocarditis [24]; a relief of heart palpitations was shown in a randomized, placebo double blind clinical study after 14-days Melissa officinalis [7], a medicinal plant utilized since ancient times for its numerous properties [25].
The reduction of blood pressure and perceived stress by Grape seed extracts was previously described [8]; due to its high content in polyphenols, Grape seed extract showed high antioxidant properties, improved endothelial function and promoted microcirculation [26- 28].
Properties of another component of the supplement, i.e. L-Theanine from Camellia sinensis folium, reviewed by Saeed [10] can contribute to the improvement in wellness observed in the present study. The presence of magnesium would possibly contribute to the normal functioning of the nervous and muscular systems, having also a protective role in cardiovascular disease [11,29,30].
The results of the present Pilot study are promising, supporting the use of the supplement in subjects with recurrent palpitations without structural hearth disease. Studies will be extended to a larger number of subjects to obtain more confident data.
Acknowledgement
We would like to express our gratitude to Dr. Mauro A.M Carai for statistical analysis.
References
- Piette C, Mélon P, Lancellotti P. [Cardiac erethism]. Rev Med Liege. 2023; 78: 342-344.
- Govender I, Nashed KK, Rangiah S, Okeke S, Maphasha OM. Palpitations: Evaluation and management by primary care practitioners. S Afr Fam Pract. 2004. 2022; 64: e1-e8.
- Weinstock C, Wagner H, Snuckel M, Katz M. Evidence-Based Approach to Palpitations. Med Clin North Am. 2021; 105: 93-106.
- Alijaniha F, Noorbala A, Afsharypuor S, Naseri M, Fallahi F, Mosaddegh M, et al. Relationship Between Palpitation and Mental Health. Iran Red Crescent Med J. 2016; 18: e22615.
- Kandiah JW, Blumberger DM, Rabkin SW. The Fundamental Basis of Palpitations: A Neurocardiology Approach. Curr Cardiol Rev. 2022; 18: e090921196306.
- Goyal A, Robinson K, Katta S, Sanchack K. Palpitation (Archived). In StatPearls [Internet]. 2025.
- Alijaniha F, Naseri M, Afsharypuor S, Fallahi F, Noorbala A, Mosaddegh M, et al. Heart palpitation relief with Melissa officinalis leaf extract: double blind, randomized, placebo-controlled trial of efficacy and safety. J Ethnopharmacol. 2015; 164: 378-384.
- Schön C, Allegrini P, Engelhart-Jentzsch K, Riva A, Petrangolini G. Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients. 2021; 13.
- Zeng W, Chu TTW, Fok BSP, Ho WKK, Chan JCN, Tomlinson B. Effects of Hawthorn Fruit Extract Drink in Chinese Patients With Mild Hypertension and/or Hyperlipidaemia: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Dose Response. 2024; 22: 15593258241303136.
- Saeed M, Naveed M, Arif M, Kakar MU, Manzoor R, Abd El-Hack ME, et al. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans-A comprehensive review. Biomed Pharmacother. 2017; 95: 1260-1275.
- Baker WL. Treating arrhythmias with adjunctive magnesium: identifying future research directions. Eur Heart J Cardiovasc Pharmacother. 2017; 3: 108-117.
- Walker AF, Marakis G, Morris AP, Robinson PA. Promising hypotensive effect of hawthorn extract: a randomized double-blind pilot study of mild, essential hypertension. Phytother Res. 2002; 16: 48-54.
- Liang Y, Liu C, Xiang L, Zheng X. Health benefits of theanine in green tea: A review. Trop Journ Pharm Res. 2015; 14: 1943-1949.
- Hidese S, Ogawa S, Ota M, Ishida I, Yasukawa Z, Ozeki M, et al. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients. 2019; 11.
- Walfridsson U, Walfridsson H, Middeldorp ME, Sanders P, Årestedt K. Validation of the English version of the arrhythmia-specific questionnaire in tachycardia and arrhythmia (ASTA): a Rasch evaluation study. J Patient Rep Outcomes. 2022; 6: 90.
- Walfridsson U, Arestedt K, Stromberg A. Development and validation of a new Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia (ASTA) with focus on symptom burden. Health Qual Life Outcomes. 2012; 10: 44.
- Zappelli L, Daniele S, Vergassola M, Ceccarelli L, Chelucci E, et al. A specific combination of nutraceutical Ingredients exerts cytoprotective effects in human cholinergic neurons. Pharma Nutrition. 2022; 22.
- Carrizzo A, Moltedo O, Damato A, Martinello K, Di Pietro P, Oliveti M, et al. New Nutraceutical Combination Reduces Blood Pressure and Improves Exercise Capacity in Hypertensive Patients Via a Nitric Oxide-Dependent Mechanism. J Am Heart Assoc. 2020; 9: e014923.
- Puddu A, Nicolò M, Maggi DC. Combination of Saffron (Crocus sativus), Elderberry (Sambucus nigra L.) and Melilotus officinalis Protects ARPE-19 Cells from Oxidative Stress. Int J Mol Sci. 2025; 26.
- Villano I, La Marra M, Allocca S, Ilardi CR, Polito R, Porro C, et al. The Role of Nutraceutical Supplements, Monacolin K and Astaxanthin, and Diet in Blood Cholesterol Homeostasis in Patients with Myopathy. Biomolecules. 2022; 12.
- Derosa G, Colletti A, Maffioli P, D’Angelo A, Lupi A, Zito GB, et al. Lipidlowering nutraceuticals update on scientific evidence. J Cardiovasc Med (Hagerstown). 2020; 21: 845-859.
- Tosca MA, Ferrecchi C, Meleca V, Naso M, Ciprandi G. A multicomponent food supplement with quercetin Phytosome®, zinc and vitamin C may be favorable in managing children with seasonal allergic rhinitis: a pilot study. Minerva Pediatr (Torino). 2025.
- Gamba P. Evaluation of Quercetin Phytosome and Zinc in Supporting Subjects with Chronic Allergic and Non-Allergic Rhinitis. Austin J Otolaryngology. 2024; 10.
- Draginic N, Jakovljevic V, Andjic M, Jeremic J, Srejovic I, Rankovic M, et al. Melissa officinalis L. as a Nutritional Strategy for Cardioprotection. 2021; 12: 661778.
- Shakeri A, Sahebkar A, Javadi B. Melissa officinalis L. - A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2016; 188: 204-228.
- Belcaro G, Ledda A, Hu S, Cesarone MR, Feragalli B, Dugall M. Grape seed procyanidins in pre- and mild hypertension: a registry study. Evid Based Complement Alternat Med. 2013; 2013: 313142.
- Pinna C, Morazzoni P, Sala A. Proanthocyanidins from Vitis vinifera inhibit oxidative stress-induced vascular impairment in pulmonary arteries from diabetic rats. Phytomedicine. 2017; 25: 39-44.
- Hasbal-Celikok G, Kara M, Sánchez M, Owsianik C, Gómez-Serranillos P, Yilmaz-Ozden T, et al. In Vitro Mechanistic Studies of a Standardized Sustainable Grape Seed Extract for Potential Application as a Mood- Modulating and Cognition-Enhancing Supplement. Nutrients. 2024; 16.
- Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem. 2002; 238: 163-179.
- Rosique-Esteban N, Guasch-Ferré M, Hernández-Alonso P, Salas-Salvadó J. Dietary Magnesium and Cardiovascular Disease: A Review with Emphasis in Epidemiological Studies. Nutrients. 2018; 10.
Citation: Marazzi G, Cacciotti L, Caminiti G, Arcari L, Giamundo DM, et al. Evaluation of a One-Month Supplementation of a Nutraceutic (Hawthorn, Melissa Officinalis, Grape Seed Extract, L-Theanine and Magnesium) In Supporting Recurrent Unexplained Palpitations in Subjects with No Structural Heart Disease. Austin J Clin Cardiol. 2025; 11(1): 1110.