
Research Article
Austin J Clin Cardiol. 2025; 11(1): 1109.
Triglycerides: An Overlooked Contributor to Cardiac Allograft Vasculopathy
Cochrane AB*, Topor M, Kennedy JL and Psotka M
Inova Heart and Vascular Institute, USA
*Corresponding author: Adam B. Cochrane, Pharm.D., MPH, BCTXP, Inova Schar Heart and Vascular Institute, Inova Fairfax Hospital, 3300 Gallows Road, Falls Church, VA 22042, USA Email: Adam.cochrane@inova.org
Received: March 06, 2025 Accepted: March 28, 2025 Published: April 01, 2025
Abstract
Background: Cardiac allograft vasculopathy (CAV) is a leading cause of mortality beyond the first year after transplant. Statins have been used for the prevention of CAV, and neither potency of statins nor attainment of certain LDL levels have advanced the prevention of CAV. Triglycerides have not been analyzed for their significance in the development of CAV.
Methods: This retrospective single-center cohort study analyzed the association between triglycerides with the development of CAV. Coronary angiography is performed at 1-year after transplant, with the development of CAV on angiography being the endpoint of this study.
Results: Two hundred thirty patients were analyzed over 1,263 patient years of follow-up, with 17.4% of patients who developed CAV. LDL levels were not associated with CAV development. Patients who developed CAV had higher mean triglycerides at CAV diagnosis, and at 1 year prior to CAV development. The use of omega-3 fatty acid supplementation was associated with a decreased risk of CAV.
Conclusions: Triglycerides and treatment thereof should be examined going forward to reduce the risk of CAV.
Introduction
Cardiac allograft vasculopathy (CAV) is the leading cause of mortality beyond the first year after heart transplant [1-4]. While risk factors for CAV include recipient sex, body mass index (BMI), ischemic time, diabetes mellitus, cytomegalovirus (CMV) status, previous rejection, and lipid concentrations, only some of these are modifiable [5,6].
Lipids have been an area of attention in CAV prevention and treatment since pravastatin was proven to lower low-density lipoprotein (LDL) and triglyceride levels, increase high-density lipoprotein (HDL) levels, and improve 1-year survival [7]. Triglycerides are atherogenic and have long been associated with cardiovascular disease, and triglycerides have increased in the population over the past 2 decades [8].
While optimal LDL levels to prevent CAV have been examined and statins are a standard of care for heart transplant recipients, the influence of triglycerides in CAV development remains under investigated. This study sought to analyze the association of triglycerides with development of CAV.
Materials and Methods
This was a retrospective single-center cohort analysis to determine the impact of triglycerides on development of CAV. Institutional IRB approval was given for this retrospective analysis. Data was deidentified for statistical analysis.
Inova Fairfax heart transplant patients follow an immunosuppression protocol consisting of a calcineurin inhibitor, anti-proliferative agent, and corticosteroid which is generally weaned off at 6 months. The immunosuppression protocol for this patient group had a change in calcineurin inhibitor from cyclosporine to tacrolimus in 2006, while mycophenolate mofetil’s use was ubiquitous shortly after approval so is the antiproliferative that was used for all these patients.
Patients are started on a statin for CAV prevention prior to hospital discharge, pravastatin for statin-naïve patients or continuation of a statin that the patient was admitted on. Statins were incorporated into the protocol for CAV prevention in the mid-1990s and are continued unless there is patient intolerance. Statin dose or agent are changed based on lipid profile to achieve an LDL <100 mg/dL. Additional lipid modifying agents are added as needed and per physician discretion, with referral to a lipidologist for refractory patients. High potency statins were classified as: rosuvastatin 10 mg or higher, atorvastatin 40 mg or higher, simvastatin 80 mg.
Coronary angiography is performed without intracoronary ultrasound at 1 year after transplant and annually for the first three years, except: a) if clinical suspicion for early CAV or coronary event, in which case it may be performed prior to 1 year, or b) depressed renal function and risk-benefit analysis with patients lead to deferral and altered method of CAV assessment. Angiograms beyond the third year are completed for clinical suspicion or patient symptoms. CAV was defined according to the International Society for Heart and Lung Transplantation grading system [9].
Population
Heart transplant recipients aged 18 years or older who received heart transplant between 1994 and 2021 were included if they followed at our center for at least 1 year after transplant.
Outcome
The primary endpoint was development of CAV on coronary angiography. The primary predictor was the triglyceride level prior to coronary angiography. Triglycerides captured within one year prior to CAV diagnosis or within one year prior to the most recent coronary angiogram (if no CAV development) were used for analysis.
Statistical Analysis
Population mean and standard deviations and median with interquartile range (IQR) values were compared using either a twosample t-test with unequal variances or the two-sample Wilcoxon rank-sum, respectively. One-way analysis of variance (ANOVA) was used to compare attribute proportions across quartiles of patients. A multivariable Cox proportional hazards model was created for the association between time from transplant to development of CAV and triglyceride levels in the prior year and reported as hazard ratios (HR) with 95% confidence intervals (CI). Covariates included patient age, sex, LDL level, statin potency, and omega-3 fatty acid use. A p-value <0.05 was considered significant. Sensitivity analyses were performed by further adjusting for year of transplantation and separately by excluding patients transplanted prior to the general use of tacrolimus.
Results
Two hundred thirty patients transplanted from 1993 to 2021 met criteria and were included in this analysis. There was no difference in mean age at transplant, sex, or indication for transplant between patients that developed CAV and those that did not (Table 1). Seventeen patients were transplanted prior to the general use of tacrolimus. Over a total of 1,263 patient-years of follow-up, 40 (17.4%) of 230 patients developed CAV at a median of 5.1 years (IQR 2.3, 8.0) after transplant. At 1 year after transplant, 2.8% were diagnosed with CAV, while 12.4% were diagnosed with CAV at 5 years. When most recently measured prior to CAV diagnosis or normal coronary angiography, LDL was 81.9 +/- 29.1 vs. 91.1 +/- 41.2, respectively (p=0.19). LDL 1 year prior to CAV diagnosis was 87.6 +/- 42.3 vs 88.6 +/- 37.4 (p=0.89), for patients remaining free of CAV and those who developed CAV, respectively (Table 2).
Grading
Severity
Definition
CAV0
Nonsignificant
No detectable angiographic lesion
CAV1
Mild
Angiographic LM <50% or primary vessel with max lesion <70% or branch stenosis <70%
CAV2
Moderate
Angiographic LM <50% and single primary vessel >70% or isolated branch stenosis in 2 systems >70%
CAV3
Severe
Angiographic LM >50% or >2 primary vessels or isolated branch stenosis in all 3 systems >70%or CAV1 or CAV1 with allograft dysfunction (LVEF <45%) or evidence of significant restrictive pathophysiology.
Table 1: CAV Grading and definition [2].
CAV-no
CAV-yes
p
Age (years)
51.3
49.7
P = 0.60
Sex (female)
31%
30%
P = 0.895
Indication for transplant (NICM)
64%
55%
P = 0.27
Race
p = 0.05
White
53%
42.50%
Black
30%
20%
Hispanic
4%
12.50%
Table 2: Baseline Demographic Table.
There was no statistical difference in the use of high potency statins 47% vs. 62% (p=0.16) in patients that did not develop CAV compared to those that did. Neither PCSK-9 inhibitor use or cholesterol absorption inhibitor, ezetimibe, use were different between patients that developed CAV and those that did not (p = 0.13 and p = 0.08, respectively). Nor were there differences in the use of omega-3 fatty acid agents (12% vs. 19%, p=0.33) in patients with or without CAV. Patients who developed CAV had higher mean triglyceride levels at the time of diagnosis 161 +/- 14 vs. 134 +/- 111 (p=0.008) compared to patients without CAV (Figure 1). When examining triglyceride levels 1 year prior to CAV diagnosis, the CAV group had higher triglyceride levels than the non-CAV group (144 +/- 72 vs. 131 +/- 101, p=0.06). Ten percent of patients in the lowest quartile of triglycerides developed CAV, while 32% in the highest quartile developed CAV (p=0.01 by ANOVA). CAV-1 was found in 72.5% of the population, while CAV-3 was found in 7.5% of the population (Table 3).
No CAV
CAV present
High potency statin use
47%
62%
Low potency statin use
53%
38%
Ezetimibe use
6%
15%
PCSK9 use
4%
10%
Table 3: Medication use at time of CAV diagnosis, or last measurement.
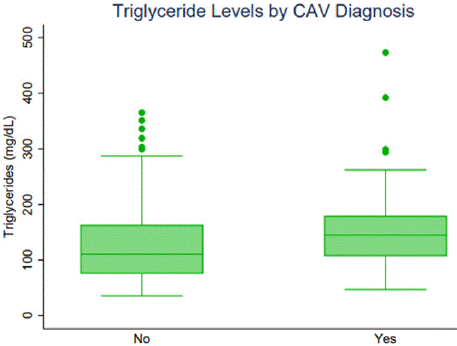
Figure 1: Triglycerides by CAV status.
In univariable Cox proportional hazard models age, triglycerides, statin potency, omega-3 fatty acid use, ischemic cardiomyopathy, and diabetes mellitus were all significant predictors for development of CAV. When entered into a multivariable model, ischemic cardiomyopathy and statin potency were no longer significantly associated with CAV development (Table 4).
Variables used in univariate analyses
Low density lipoprotein (LDL)
Triglycerides
LDL 1 year before diagnosis
Triglycerides 1 year before diagnosis
Statin potency
Indication for heart transplant
Diabetes Mellitus presence
Table 4: Variables used in univariate analyses.
In the final parsimonious model, for each 10-point increase in triglycerides, the hazard ratio for development of CAV was 1.02 (95%CI 1.00-1.04), p=0.038), increasing age (HR 1.13 for every 5 years older, 95% CI 1.02-1.24, p=0.020) and diabetes mellitus (HR 2.29, 95% CI 1.18-4.44, p =0.014) remained associated with the development of CAV. The use of omega-3 fatty acid supplementation was associated with a decreased risk of CAV (HR=0.24, 95% CI 0.09-0.66, p=0.006). Sensitivity analysis incorporating year of transplantation into the multivariable model did not change the point effect estimate for each 10-point increase in triglycerides (HR 1.02, 95% CI 1.00-1.04), nor did the exclusion of the 17 patients transplanted prior to the use of tacrolimus (HR 1.02, 95% CI 1.00-1.05). Of the patients who did not receive omega-3 fatty acid supplementation, 35 (19%) developed CAV, while only 5 (12%) of the patients who received omega-3 fatty acid supplementation developed CAV.
Discussion
Triglycerides have received little attention in the CAV literature. Kobashigawa et al., showed that pravastatin versus placebo treated patients had significantly lower mean LDL, lower mean triglyceride levels, and higher mean HDL, which together resulted in significantly improved 1-year survival; on angiography or autopsy there was significantly more coronary vasculopathy in the control group versus the pravastatin treated group.7 Agarwal et al reported that statin use was related to a reduction in long-term mortality (HR = 0.21, 95% CI 0.07-0.61), but did not report the effects the statin had on separate components of the lipid panel [10]. Ellimuttil et al., showed in their cohort, where 20% of patients developed CAV, that treatment with a statin trended toward improved CAV-free survival. Despite this difference there was no difference in LDL levels between CAV and CAV-free patients. In fact, the median LDL level did not differ between groups when stratified by tertiles [11].
Statin intensity has been studied regarding CAV. Golbus et al. reported that greater statin intensity was beneficial after heart transplantation, with a reduced statin intensity contributing to their endpoint of time to heart failure hospitalization, myocardial infarction, or revascularization [12].
Harris et al. reported their experience with CAV when targeting LDL levels. Of the 194 patients they assessed, 157 patients (81%) reached an LDL < 100 mg/dL, with 41 of those patients reaching LDL <70 mg/dL. Median triglyceride and median HDL were similar between LDL groups. Median LDL level <100 mg/d: significantly reduced the risk of developing CAV (15.9 % vs. 32.4%). The LDL <100 mg/dL cohort also had a delayed time to CAV versus the LDL>100 mg/dL group [13].
Aside from Kobashigawa and Harris studies, triglycerides have not been discussed in other studies analyzing causative associations with CAV development.
Triglycerides have an epidemiologic correlation with coronary artery disease. They are transported through the body by lipoprotein particles, namely VLDL and chylomicrons. Omega-3 fatty acid supplementation slows intestinal lipid absorption and reduces VLDL production resulting in increased triglyceride clearance by fatty acid oxidation, while also decreasing inflammation and thrombosis [14,15].
The American Heart Association recommends 4 Gm/day of omega-3 fatty acids (EPA and DHA) which have been shown to decrease triglyceride levels [8]. The 4 Gm/day dose of marine omega-3 fatty acids can provide a decrease in triglycerides of 25-30%, while also providing a slight increase in HDL and LDL.
Icosapent ethyl is a highly purified EPA ethyl ester which has been shown to decrease triglycerides by 33% in a study of patients with hypertriglyceridemia. When compared to placebo, icosapent ethyl, significantly reduced the composite endpoint of cardiovascular death, non-fatal MI, non-fatal stroke, coronary revascularization, and unstable angina [16].
REDUCE-It which studied icosapent ethyl versus mineral oil placebo, had the majority patients enrolled on a moderate or high intensity statin with both active and placebo groups having LDL <80 mg/dL at study initiation. At 1-year there was an 18.3% reduction in triglyceride in the icosapent ethyl group while there was a 2.2% increase in the placebo group. Over this time period there was also a 3.1% increase in LDL cholesterol in the icosapent ethyl group and a 10.2% increase in LDL in the placebo group [17].
Our practice is to add on an omega-3 fatty acid product (OTC if insurance will not cover a prescription agent) if a patient’s triglycerides are above a normal value while on statin therapy. The REDUCE-IT study shows there is an advantage when adding an omega-3 fatty acid product for additional triglyceride lowering effect. The current study shows an association between lower triglyceride levels, which may be accomplished by adding an omega-3 fatty acid agent, and a reduced incidence of CAV in the following year.
There is scant literature of antihypertensives, namely diltiazem, being studied for prevention of CAV [18]. The literature was published before the statin literature and has not been incorporated into standard CAV preventative protocols. Aspirin has also been used for prevention of CAV, but the data is mixed with some studies showing a benefit, others showing a benefit only in limited patients, while some show no benefit [19,20].
This is a retrospective analysis that is subject to limitations of its nature of retrospective study design, with a change in calcineurin inhibitor during the studied time-period. There were patients that were not able to start an omega-3 fatty acid product due to finances or choice not to take the agent. The use of diltiazem is very low in our transplant population though was not recorded. Aspirin use was not recorded, but aspirin 81 mg has been a consistent part of our CAV protocol. Angiogram screening beyond 5 years is not standard, so there may be some asymptomatic CAV that is not captured in this analysis. Similarly, if patient’s kidney function was not adequate for an angiogram there may be asymptomatic CAV that was not captured. CAV is the largest cause of patient death beyond 1-year after transplant and a contributor to the need for retransplant. Statins are ubiquitous in the care of heart transplant patients due to proven benefits. This study suggests the importance of including triglyceride consideration in the care of heart transplant patients as another tool that may decrease the incidence of CAV.
References
- Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands RSB. Allograft vasculopathy: The achilles’ heel of heart transplantation. Journal of the American College of Cardiology. 2016; 68: 80-91.
- Mehra MR, MD, Crespo-Leiro MG, MD, Dipchand A, MD, et al. International society for heart and lung transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy—2010. The Journal of heart and lung transplantation. 2010; 29: 717-727.
- Bonnet G, Coutance G, Van Keer J, et al. Trajectories of cardiac allograft vasculopathy after heart transplantation and association with mortality: A population-based study. European heart journal. 2020; 41.
- Van Keer JM, Van Aelst LNL, Rega F, et al. Long-term outcome of cardiac allograft vasculopathy: Importance of the international society for heart and lung transplantation angiographic grading scale. The Journal of heart and lung transplantation. 2019; 38: 1189-1196.
- Moayedi Y, Fan CPS, Tremblay-Gravel M, et al. Risk factors for early development of cardiac allograft vasculopathy by intravascular ultrasound. Clinical transplantation. 2020; 34: e14098-n/a.
- Valantine H. Cardiac allograft vasculopathy after heart transplantation: Risk factors and management. The Journal of heart and lung transplantation. 2004; 23: S187-S193.
- Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. The New England Journal of Medicine. 1995; 333: 621-627.
- MILLER M, STONE NJ, LENNIE TA, et al. Triglycerides and cardiovascular disease: A scientific statement from the American heart association. Circulation (New York, N.Y.). 2011; 123: 2292-2333.
- Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy -2010. The Journal of heart and lung transplantation. 2010; 29: 717-727.
- Agarwal S, Parashar A, Kapadia SR, et al. Long-term mortality after cardiac allograft vasculopathy: Implications of percutaneous intervention. JACC. Heart failure. 2014; 2: 281-288.
- Ellimuttil TM, Harrison K, Rollins AT, et al. Effect of statin intensity on the progression of cardiac allograft vasculopathy. Cardiac failure review. 2021; 7: e15.
- Golbus JR, Adie S, Yosef M, Murthy VL, Aaronson KD, Konerman MC. Statin intensity and risk for cardiovascular events after heart transplantation. ESC Heart Failure. 2020; 7: 2074-2081.
- Harris J, Teuteberg J, Shullo M. Optimal low-density lipoprotein concentration for cardiac allograft vasculopathy prevention. Clinical transplantation. 2018; 32: e13248-n/a.
- Bornfeldt KE. Triglyceride lowering by omega-3 fatty acids: A mechanism mediated by N-acyl taurines. The Journal of clinical investigation. 2021; 131.
- Shearer GC, Savinova OV, Harris WS. Fish oil — how does it reduce plasma triglycerides? Biochimica et biophysica acta. 2012; 1821: 843-851.
- Bays HE, Braeckman RA, Ballantyne CM, et al. Icosapent ethyl, a pure EPA omega-3 fatty acid: Effects on lipoprotein particle concentration and size in patients with very high triglyceride levels (the MARINE study). 2012; 6: 565- 572.
- Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. The New England journal of medicine. 2019; 380: 11-22.
- Schroeder JS, Gao SZ, Alderman EL, et al. A preliminary study of diltiazem in the prevention of coronary artery disease in heart-transplant recipients. New England Journal of Medicine. 1993; 328: 164-170.
- Hollis IB, Reed BN, Moranville MP. Medication management of cardiac allograft vasculopathy after heart transplantation. Pharmacotherapy. 2015; 35: 489-501.
- Benes LB, Riley TL, Murks CM, et al. The role of aspirin in the prevention of coronary allograft vasculopathy in cardiac transplant patients. Journal of Cardiac Failure. 2015; 21: S111-S112.