
Case Report
J Cardiovasc Disord. 2025; 11(1): 1057.
Intraoperative Cardiac Arrest: Diagnostic Considerations and Management: A Case Report with Literature Review
Ouzzaouit S¹*, Lamghari Y¹, Laklalech S¹, Britel D¹, Lakhal Z¹, Benyass A¹, Mezzour Y², Jidal M², Andaloussi²; Bensghir² and Doghmi N²
¹Cardiology Department of Mohamed V Military Teaching Hospital in Rabat, Morocco
²Medical Intensive Care Department of Mohamed V Military Teaching Hospital in Rabat, Morocco
*Corresponding author: Sarra Ouzzaouit, Department of Cardiology, Mohammed V Military Hospital, Mohammed V University in Rabat, Morocco Tel: +212666818886; Email: sarra.ouzzaouit001@gmail.com
Received: May 12, 2025 Accepted:June 13, 2025 Published: June 16, 2025
Abstract
The incidence of cardiac arrest in the operating room remains fortunately rare; however, when it does occur, the prognosis is generally more favorable compared to out-of-hospital settings. The most frequently implicated causes include hypoxia, hypovolemia, anaphylaxis, local anesthetic systemic toxicity, hyperkalemia, pulmonary embolism, pneumothorax, and cardiac tamponade. Certain etiologies, such as malignant hyperthermia, local anesthetic toxicity, and peri partum cardiac arrest, necessitate specific and often urgent therapeutic interventions.
With the growing use of invasive and interventional procedures, the risk of intra-hospital cardiac arrest outside the traditional operating room environment has increased. The incorporation of cognitive aids such as checklists and emergency protocols has shown to enhance the quality and efficiency of resuscitation efforts. Furthermore, the use of mechanical chest compression devices and the expanding availability of extracorporeal life support (ECLS) have contributed to advancements in the management and potential outcomes of cardiac arrest in the perioperative setting.
Keywords: Cardiac arrest; Cardiopulmonary resuscitation; Anticoagulation; Pulmonary embolism
Introduction
Cardiac arrest is widely recognized as a major public health issue, for which comprehensive cardiopulmonary resuscitation guidelines have been extensively developed. However, cardiac arrest occurring in the operating room represents a distinct and relatively rare clinical entity. For various reasons, its diagnosis can be challenging in this specific context. False alarms are not uncommon in the OR and may even outnumber actual critical events, often due to technical issues such as equipment malfunction or disconnection of monitoring sensors.
Patient-related comorbidities, including morbid obesity, cardiovascular disease, and intraoperative hypothermia, further complicate the reliability of monitoring and the early recognition of true cardiac arrest during surgery.
In this paper, we present a case of intraoperative cardiac arrest, and use this clinical observation as a basis to analyze the event and its implications. We also discuss, through a review of the current literature, the incidence, pathophysiological mechanisms, and the recommended management algorithm of IOCA a life-threatening and dramatic situation that demands rapid and coordinated multidisciplinary response.
Case Presentation
A 48-year-old male was admitted to the hospital following a road traffic accident. He presented with isolated fractures of the tibial plateau and the patella, without any associated injuries. During the six-day preoperative hospitalization period, a thorough preanesthetic evaluation was conducted. The patient was young, with no comorbidities, no history of drug allergies, no substance use, and no current or prior medication intake. Clinical examination revealed no predictors of difficult intubation or ventilation. Cardiopulmonary auscultation was unremarkable. Blood pressure, heart rate, and oxygen saturation were within normal limits on room air. Peripheral pulses were present and symmetrical in the immobilized limb, which was maintained in a posterior plaster splint. The patient was classified as ASAI.
On the day of surgery, the patient was brought to the operating room. After placement of a peripheral intravenous line and initiation of oxygen therapy, standard hemodynamic monitoring (heart rate, peripheral oxygen saturation, and non-invasive blood pressure) showed parameters within normal ranges. Spinal anesthesia was performed in the left lateral decubitus position at the L3–L4 interspace using hyperbaric bupivacaine 5 mg/ml, with a total volume of 2.5 ml (equivalent to 12.5 mg), combined with 25 micrograms of fentanyl. The intrathecal injection was administered slowly over two minutes. Post-spinal anesthesia, the patient remained hemodynamically, respiratory, and neurologically stable, with no signs of poor tolerance.
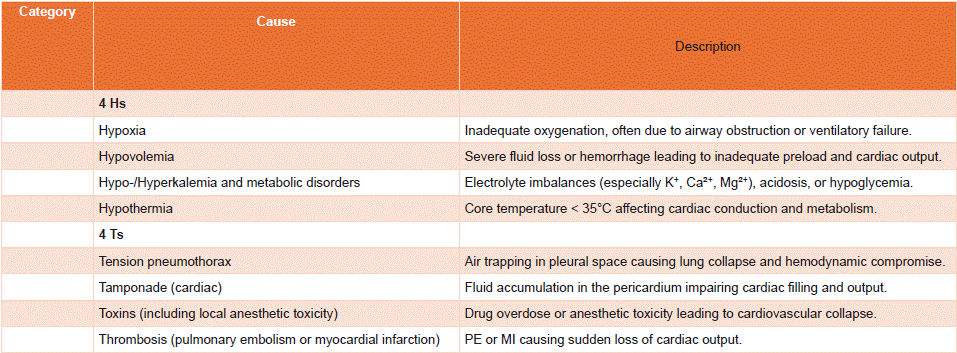
Figure 1: Main Etiologies of Cardiac Arrest and Key Aspects of Management.
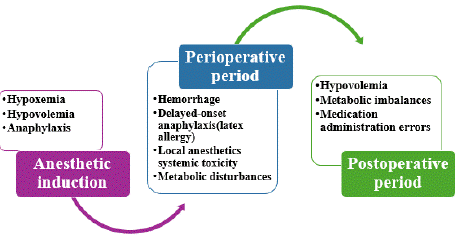
Figure 2: Etiologies of Perioperative Cardiac Arrest According to the Phase
of Patient Management.
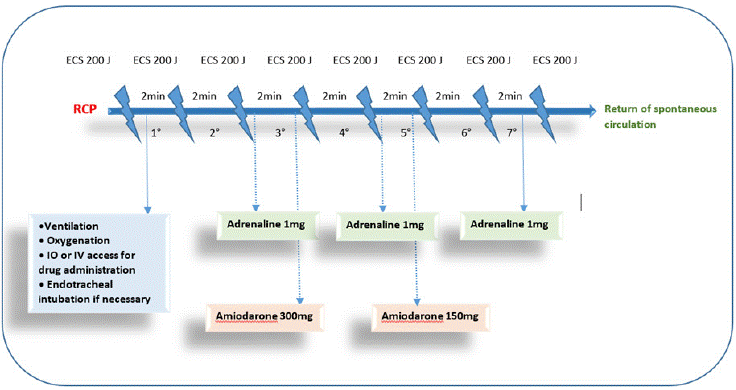
Figure 3: Shockable rhythm management algorithm based on the SFAR [14].
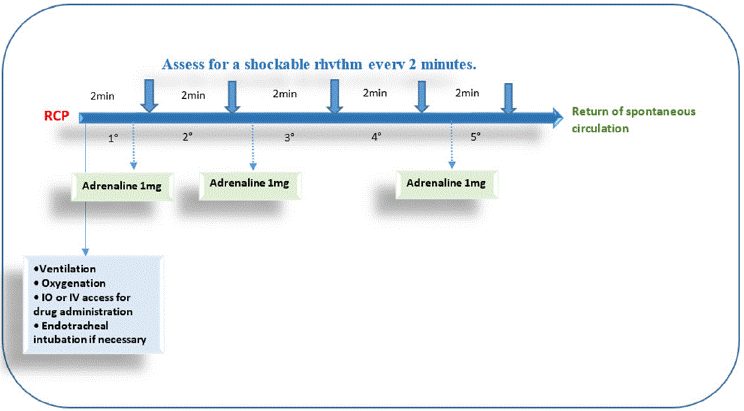
Figure 4: No Shockable rhythm management algorithm based on the SFAR [14].
A pneumatic tourniquet of appropriate size was applied to the operative limb. The initial inflation pressure was set at 220 mmHg and progressively increased to 300 mmHg to ensure a bloodless surgical field. During the procedure, the patient experienced two episodes of hypotension, with blood pressure readings around 80/44 mmHg. These episodes were successfully managed with fluid resuscitation and intravenous administration of 3 mg ephedrine.
At the end of the surgical procedure, after 2 hours and 15 minutes of tourniquet inflation, deflation was performed. The surgery had been non-hemorrhagic, with minimal blood loss throughout the intervention.
Within two minutes of deflation, the patient suddenly lost consciousness, developed profound bradycardia followed by asystole, and non-invasive blood pressure became unmeasurable.
Immediate cardiopulmonary resuscitation was initiated, including external chest compressions, intravenous administration of 1 mg adrenaline every 2 to 3 minutes, and endotracheal intubation for airway protection. Despite 30 minutes of advanced life support, the patient remained in refractory cardiac arrest and was ultimately declared deceased.
Discussion
Intraoperative cardiac arrest is a rare but devastating event, characterized by distinct features compared to cardiac arrest occurring in other clinical settings. Unlike arrests in out-of-hospital or general inpatient environments, intraoperative cardiac arrest is usually anticipated and rapidly identified due to continuous physiological monitoring in the operating room. However, the infrequent occurrence of such events can affect the rapidity and consistency of diagnosis and management by anesthesia teams. Perioperative cardiac arrest rarely occurs without warning signs, which may sometimes be subtle. Sudden and unexplained changes in vital signs should prompt anesthesiologists to consider impending cardiac collapse and communicate concerns early to the surgical team.
Data from three large registries, covering approximately 1.9 million anesthetic procedures, report an incidence ranging from 5.6 to 7.4 cases per 10,000 procedures [1–3]. More recent findings from a U.S. administrative database estimate the incidence at 5.7 per 10,000 procedures [4].
Registry-based analyses highlight several independent risk factors, including advanced age, particularly in infants under one year and elderly patients, male sex, and higher ASA physical status classification (ASA III or IV). Additional patient-specific risk factors include a history of cardiomyopathy or congestive heart failure, chronic kidney disease with creatinine clearance below 30 mL/min, and vascular diseases [4–6].
Although there is limited literature directly comparing the risk attributable to different anesthetic techniques, general anesthesia appears to carry a higher risk profile. For example, an American study reported that 64% of anesthesia-related perioperative cardiac arrests were associated with airway management complications leading to severe hypoxemia [1].
The risk of cardiac arrest is greatest with general anesthesia and appears to decline with spinal anesthesia, and even further with regional nerve blocks.
Among surgical factors, emergency surgeries constitute a significant risk factor. A study by Aloweidi et al. reported an odds ratio of 4.0 (95% CI: 1.6–10.1, p = 0.004) for perioperative cardiac arrest in emergent cases [7]. Cardiac, thoracic, and vascular surgeries are also associated with increased risk, as are prolonged surgical durations and procedures performed after-hours or during on-call periods [4–7].
Perioperative cardiac arrest is associated with significant morbidity and mortality. The overall survival rate to hospital discharge is approximately 31% [2], although outcomes can vary depending on the underlying cause of the arrest and the initial cardiac rhythm observed during resuscitation efforts. Non-shockable rhythms are associated with poorer outcomes, with a survival rate of around 26%, while shockable rhythms such as ventricular tachycardia or ventricular fibrillation are linked to a more favorable prognosis, with a survival rate of 42%. Neurological status is the most crucial determinant of long-term outcomes.
Studies have shown that 64% of survivors had no neurological sequelae, defined as a Cerebral Performance Category (CPC) score of 1 [2]. These results are favorable when compared to in-hospital cardiac arrest registries, where the survival rate to hospital discharge is generally lower, around 25% [8].
During cardiac arrest management, the diagnostic evaluation of the underlying cause must be initiated simultaneously with therapeutic interventions, particularly cardiopulmonary resuscitation. Identifying a reversible cause is fundamental and can guide specific therapeutic actions. A useful mnemonic for recalling the principal reversible causes is the "4 Hs and 4 Ts." summarized in Table 1.
In the operating room setting, certain etiologies should be considered a priority. About one-third of perioperative cardiac arrests are related to surgical procedures, while the remaining twothirds are associated with anesthesia [9]. Anesthesiologists may also encounter cardiac arrest in obstetric units and cardiac catheterization laboratories, where specific causes may predominate.
Anesthesia-related causes of perioperative cardiac arrest include airway management difficulties leading to hypoxic cardiac arrest, which is typically rapidly reversible with prompt reoxygenation and ventilation; grade IV anaphylaxis, where the arrest mechanism is predominantly hypovolemic secondary to profound vasoplegia; local anesthetic systemic toxicity (LAST) requiring intravenous lipid emulsion therapy (e.g., Intralipid®); high spinal anesthesia causing profound sympathetic blockade, bradycardia, and cardiovascular collapse; and malignant hyperthermia where cardiac arrest is triggered by severe metabolic disturbances, notably hyperkalemia and severe acidosis. Overdose or medication administration errors, such as inadvertent boluses of vasoactive agents or neuromuscular blocking agents, should also be considered.
Surgical causes include vagal stimulation during procedures like laparoscopic insufflation, uncontrolled hemorrhage, and nonthrombotic embolic mechanisms such as fat embolism, gas embolism, or cement embolism. Anaphylactic mechanisms such as latex or cement allergy should also be considered. The incidence of maternal cardiac arrest remains low in obstetric settings, estimated at 1 in 36,000 pregnancies [11]. In a UK series, 24% of maternal CA cases were related to obstetric anesthesia, particularly the hemodynamic impact of epidural analgesia [11]. A Chinese series identified three primary causes of maternal CA: hemorrhagic shock, amniotic fluid embolism, and severe preeclampsia/eclampsia [10].
In catheterization laboratories, cardiac arrest risk is mainly associated with patient comorbidities and the procedural risk level.
The highest-risk procedures are valvular replacement surgeries (e.g., TAVI), which carry a 4.3% risk of CA, mitral repairs, and atrial appendage closures [12]. Another primary cause of CA during procedures is thrombectomy in the acute or distant phase of myocardial infarction, often associated with arrhythmias resulting from pre-existing or procedure-induced myocardial ischemia (e.g., dilation, coronary dissection, distal thrombosis). Some events will lead to a pulseless rhythm, such as rupture of the free wall of the left or right ventricle in the pericardium or coronary rupture. In patients at high coronary risk or with an unknown coronary status, continuous ST-segment monitoring allows for early detection of myocardial ischemia.
Timing of cardiac arrest during perioperative care provides important diagnostic clues: during induction, arrests are mostly related to anesthesia; during the intraoperative phase, arrests often stem from surgical causes; and in the postoperative phase, hemorrhagic and metabolic causes predominate.
Management of cardiac arrest follows standardized guidelines, particularly those from the European Resuscitation Council, most recently updated in 2021 [13]. In conscious patients, sudden loss of consciousness and absence of respiratory effort should trigger basic life support measures (BLS), even without pulse confirmation. In anesthetized patients, diagnosis is based primarily on monitoring parameters such as ECG changes (e.g., asystole, ventricular fibrillation, or ventricular tachycardia), collapse of arterial pressure waveforms, disappearance of end-tidal CO2, or absence of pulse oximetry waveform.
Essential initial steps are summarized in the various algorithms provided by the French Society of Anesthesia and Intensive Care Medicine (SFAR) [14] including alerting the surgeon and operating team, recording the time of the event, calling for help and defibrillator, initiating high-quality chest compressions, and identifying whether the rhythm is shockable (ventricular fibrillation, pulseless ventricular tachycardia) or non-shockable (asystole, pulseless electrical activity).
Specific management should target the presumed etiology according to the 2021 European Resuscitation Council (ERC) guidelines [15]. In cases of hypoxia it mandates immediate effective ventilation with the highest oxygen concentration. In the operating room, hypoxemia often results from airway management difficulties or massive aspiration during induction. In “cannot intubate/cannot ventilate” scenarios, cognitive aids can be valuable.
Anaphylaxis must be suspected when shock or bronchospasm occurs, with or without skin signs. Management involves avoiding the triggering agent, if possible, rapid intravenous administration of adrenaline, volume expansion, and steroids. Glucagon is recommended for patients on beta-blockers, and sugammadex may be considered in cases of suspected rocuronium-induced anaphylaxis. If cardiac arrest occurs, standard resuscitation protocols should be followed.
Metabolic disturbances, particularly hyperkalemia, are another cause. Management focuses on rapid intracellular shifting of potassium using calcium, glucose-insulin solutions, or beta-2 agonist aerosols, while definitive potassium removal by diuresis or dialysis may take longer.
Thrombosis, mainly due to embolic events such as fibrinous, gaseous, fat, or cement emboli, can also lead to cardiac arrest. Myocardial infarction is a less frequent cause intraoperatively. Surgical or endovascular thrombectomy is preferred for fibrino-cruoric embolism; gaseous embolism management follows SFAR guidelines; myocardial infarction requires rapid collaboration with cardiology for reperfusion strategies. Emergency echocardiography helps guide diagnosis, despite technical limitations during CPR.
Tension pneumothorax must be suspected in any patient with sudden hemodynamic collapse. Diagnosis relies on clinical examination or point-of-care ultrasound (POCUS), especially in patients with prior pulmonary vulnerability. Immediate decompression via needle aspiration or open thoracostomy is critical, with thoracic decompression taking priority during resuscitation.
Cardiac tamponade, similarly, requires immediate pericardial decompression through resuscitative thoracotomy or ultrasoundguided pericardiocentesis.
Several intraoperative-specific emergencies must also be recognized. Local anesthetic systemic toxicity (LAST) presents with neurological or cardiovascular disturbances, often ventricular arrhythmias. Management combines standard resuscitation with reduced-dose adrenaline, use of amiodarone instead of lidocaine, and lipid emulsion therapy (Intralipid® or Medialipid®).
Malignant hyperthermia, a life-threatening pharmacogenetic disorder triggered by succinylcholine or volatile anesthetics, is characterized by hypercapnia, rigidity, and hyperthermia, leading to hyperkalemia and severe metabolic acidosis. Management requires immediate standard resuscitation along with specific treatment for malignant hyperthermia.
Extracorporeal CPR (E-CPR) is an emergency treatment that requires a cardiocirculatory support technique, such as ECMO or cardiopulmonary bypass, for certain patients or in specific contexts (e.g., massive pulmonary embolism, coronary syndrome, profound hypothermia). The use of E-CPR may be considered in almost all causes of cardiac arrest occurring in the operating room, except in cases of true profound hypovolemia from hemorrhagic shock or preexisting severe organ failure. The decision to use this exceptional treatment will primarily depend on the expertise of the center where the cardiac arrest occurs and the availability of the local medical technology.
Implementation in a Specialized Center
In centers with teams capable of implanting circulatory support devices, it is possible to alert the entire team (cardiopulmonary technician, specialized anesthesiologist, cardiothoracic or vascular surgeon) as soon as the cardiac arrest occurs. A rapid multidisciplinary discussion will help decide whether to implement E-CPR in addition to standard CPR measures, considering factors like patient age, comorbidities, mechanism of arrest, and duration of resuscitation.
In Centers without E-CPR Technology
In centers lacking the medical-technical capacity to implant circulatory support devices, a mobile circulatory support team may be called. This team should be notified promptly after the cardiac arrest occurs, especially if initial resuscitation measures fail. The discussion will then include factors such as transport time and total expected low-flow duration. Additionally, the use of automated external chest compression devices should be considered while waiting for the team to maintain the quality of CPR.
In our case, cardiac arrest occurred shortly after tourniquet deflation a rare complication predominantly reported in lower limb surgeries [16]. A literature review reveals very few cases of cardiac arrest post-tourniquet deflation, particularly involving the upper limb, and one such case was attributed to coronary artery spasm [16].
In our patient, the exact mechanism remains uncertain, but massive pulmonary embolism is the most probable cause. Trauma, prolonged immobilization, and surgery are well-known risk factors for deep vein thrombosis, which may subsequently embolize. Immobilization beyond three days significantly increases the risk of DVT, particularly in lower limb fractures. While thromboembolism may occur spontaneously, mechanical factors such as Esmarch bandages or tourniquet deflation are suspected to dislodge pre-existing thrombi due to sudden shifts in venous flow dynamics. Several case reports describe massive pulmonary embolism following the use of tourniquets or Esmarch bandages in both trauma and elective knee arthroplasty surgeries [17].
The most critical factor appears to be the preoperative immobilization period without adequate anticoagulation. The type of anesthesia likely does not influence embolization risk once a thrombus has formed. In nearly all documented cases, cardiovascular collapse occurred within five minutes of tourniquet release [18] consistent with our observation. Preoperative anticoagulation should be considered routine in patients with prolonged immobility. Although diagnostic testing for DVT can be invasive, costly, and of limited sensitivity, it should be considered in patients immobilized for more than three days who are scheduled for surgery involving tourniquet application.
Conclusion
In conclusion, cardiac arrest during the perioperative period remains a rare complication of anesthesia. In such cases, it is essential to consider specific causes of cardiac arrest (hypovolemia, gas embolism, drug toxicity, anaphylaxis), as the management of these conditions requires adaptation of traditional cardiac arrest algorithms.
The management of cardiac arrest is not solely dependent on individual capabilities, but rather on a trained team response. Establishing an institutional safety culture is crucial for improving care. This involves the use of cognitive aids tailored to the facility, multidisciplinary cooperation with resuscitation and surgical teams, as well as ongoing team training through continuous education and simulation exercises.
Consent
The authors confirm that written consent for the submission and publication of this case, including images, has been obtained from the patients in line with the Committee on Publication Ethics (COPE) guidance.
Author Contribution
MB: Study concept, Data collection, Data analysis, writing the paper.
RL: Study concept, Data collection, Data analysis.
RF: Study concept, Data analysis, writing the paper.
NM: Supervision and data validation
IA: Supervision and data validation
AB: Supervision and data validation
All authors reviewed the final manuscript.
Not commissioned, externally peer-reviewed.
References
- Ellis SJ, Newland MC, Simonson JA, et al. Anesthesiarelated cardiac arrest. Anesthesiology. 2014; 120: 829–838.
- Ramachandran SK, Mhyre J, Kheterpal S, et al. Predictors of survival from perioperative cardiopulmonary arrests: a retrospective analysis of 2524 events from the get with the guidelines-resuscitation registry. Anesthesiology. 2013; 119: 1322–1339.
- Nunnally ME, O’Connor MF, Kordylewski H, Westlake B, Dutton RP. The incidence and risk factors for perioperative cardiac arrest observed in the national anesthesia clinical outcomes registry. Anesth Analg. 2015; 120: 364–370.
- Fielding-Singh V, Willingham MD, Fischer MA, Grogan T, Benharash P, Neelankavil JP. A population-based analysis of intraoperative cardiac arrest in the United States. Anesth Analg. 2020; 130: Je7–34.
- Braz LG, Módolo NSP, do Nascimento P, et al. Perioperative cardiac arrest: a study of 53,718 anaesthetics over 9 yr from a Brazilian teaching hospital. Br J Anaesth. 2006; 96: 569–575.
- Hohn A, Machatschek JN, Franklin J, Padosch SA. Incidence and risk factors of anaesthesia-related perioperative cardiac arrest: a 6-year observational study from a tertiary care university hospital. Eur J Anaesthesiol. 2018; 35: 266–272.
- Aloweidi A, Alghanem S, Bsisu I, et al. Perioperative cardiac arrest: a 3-year prospective study from a Tertiary Care University Hospital. Drug Health Patient Saf. 2022; 14: 1–8.
- Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: a review. JAMA. 2019; 321: 1200–1210.
- RA Armstrong, TM Cook, AD Kane, E Kursumovic, JP Nolan, FC Oglesby, et al. Peri-operative cardiac arrest: management and outcomes of patients analysed in the 7th National Audit Project of the Royal College of Anaesthetists.
- Huang TQ, Chen DJ, Liu HS, Dai MH, Huang DJ. [Analysis of the cause and clinical characteristics of maternal cardiac arrest]. Zhonghua Fu Chan Ke Za Zhi. 2011; 46: 742-747.
- Beckett V, Knight M, Sharpe P. The Caps study: incidence, management and outcomes of cardiac arrest in pregnancy in the UK: a prospective, descriptive study. BJOG. 2017; 124: 1374–1381.
- Yadav K, Truong HT. Cardiac arrest in the catheterization laboratory. Curr Cardiol Rev. 2018; 14: 115–120.
- Lott C, Truhlár A, Alfonzo A, et al. European Resuscitation Council Guidelines 2021: cardiac arrest in special circumstances. Resuscitation. 2021; 161: 152–219.
- Romain Jouffroy, Sarah Berger, Pierre Carli, Benoît Vivien. Prise en charge de l’arrêt cardiaque en 2018. 2018.
- Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation. 2021; 161: 115-151.
- [Houng W.R. Cardiac arrest after tourniquet deflation in tibial plateau fracture surgery in a healthy man. Formosan J. Musculoskelet. Disord. 2012; 3: 34–38. 1], [Kumari I. Cardiac arrest after simultaneous release of bilateral tourniquets in lower limb orthopaedic surgery: a case report. Anaesth. Pain Intensive Care. 2013; 17: 285–288. 2].
- Lu CW, Chen YS, Wang MJ. Massive pulmonary embolism after application of an Esmarch bandage. Anesth Analg. 2004; 98: 1187-1189.
- Gupta KK, Singh A. Cardiac arrest following tourniquet release: Needs attention! Saudi J Anaesth. 2015; 9: 489-490.