
Research Article
Austin J Cancer Clin Res. 2022; 9(1): 1101.
Montelukast Performs Synergistic Effect with Carfilzomib in Multiple Myeloma by Inhibiting the Deubiquitinase UCHL1
Yu W, Wei W, Peng R, Chen H, Zhou N, Wu L, Shi H, Chen X, Wang D, Wei J, Zhao W and Zhou F*
Zhabei Central Hospital, Jing’an District, Shanghai, China
*Corresponding author: Fan Zhou, Zhabei Central Hospital, Jing’an District, Shanghai, No.619, Zhonghua Xin Road, China
Received: March 02, 2022; Accepted: March 25, 2022; Published: April 01, 2022
Abstract
Immunomodulators play an important role in combination chemotherapy of multiple myeloma. Besides, it has been reported that leukotriene-D4 receptors play an important role in carcinogenesis. The current study was carried out to assess the possible antitumor effects of montelukast (both an immunomodulator and a CysLT1R antagonist) both in single and in combination with carfilzomib in multiple myeloma cells and the potential mechanisms. We performed our experiment using cell apoptosis assay, cell cycle assay, western blot analysis, mitochondrial transmembrane potential, and chemical proteomics based on protein activity. The results showed that montelukast inhibits cell proliferation and leads to cell apoptosis in multiple myeloma cell lines. Then we performed combination of montelukast and carfilzomib on multiple myeloma cells to explore whether they are synergistic. We demonstrated that the combination treatment leads to stronger effect of cell apoptosis. Furthermore, we demonstrated the combination effect is performed independent of the leukotriene-D4 receptors inhibition. Given that protesome inhibitor leads to accumulation of ubiquitin by inhibiting the degradation of these proteins, we tested ubiquitin levels of different groups. We found that montelukast induces ubiquitin accumulation by inhibiting Ubiquitin-Specific Protease UCHL1. All of these lead to the activity of indoplasmic reticulum stress and therefore result in cell apoptosis. This brings us a new idea in the treatment of multiple myeloma. Since montelukast is cheap and widely used in clinic, if it is proved to be effective in the treatment of multiple myeloma, it will greatly reduce the economic pressure of patients and bring new hope to patients.
Keywords: Montelukast; Carfilzomib; Multiple myeloma; Cell apoptosis; UCHL1
Abbreviations
UPS: Ubiquitin-Proteasome System; MM: Multiple Myeloma; MNK: Montelukast; CFZ: Carfilzomib; CI: Combination Index; PNK: Pranlukast; ZFK: Zafirlukast
Introduction
Multiple myeloma (MM) is a clonal plasma cell neoplasm involving the bone marrow and extramedullary sites, manifested by anemia, hypercalcemia, impairment of renal function and bone destruction [1]. Over the past decades, the introduction of novel agents has improved clinical outcomes of MM patients. However, nearly all patients will relapse and require subsequent therapy finally [2,3]. Proteasome inhibitors are commonly used in the treatment of multiple myeloma. Of it, bortezomib is recommended as the frontline and effective in newly diagnosed MM. It significantly prolongs patient survival [4,5]. However, several deficiencies which limit its clinical application. Firstly, treatment is often limited by dose-limiting side effects, most due to peripheral neuropathy. Besides, prolonged continuous treatment often induces drug resistance. Moreover, bortezomib is usually not effective in refractory or relapsed setting of MM patients. Carfilzomib is the second generation of proteasome inhibitor. It is commonly used in relapsed cases with prior resistant to bortezomib [6-8]. But the outcome is also not so satisfactory. Consequently, a novel agent that could increase proteasome inhibitors sensitivity and overcome resistance would be clinically meaningful.
It is well known that proteasome inhibitors perform their effect by inhibiting proteasomes which play critical roles in the ubiquitinproteasome system (UPS). The UPS plays an important role in different cellular processes by targeted destruction of proteins, such as cell cycle progression, receptor down-regulation, gene transcription and apoptosis [9-11]. It has been an important target in the treatment of malignancies [12-14]. This process includes two specific and sequential steps: ubiquitination and proteasomal degradation. Ubiquitin is a small modifier molecule that labels proteins in a highly specific manner. Ubiquitination is a stepwise cascade of enzymatic reactions and requires the ubiquitin activating enzyme, ubiquitin conjugating enzyme and the ubiquitin ligase [15,16]. This ubiquitination process can be reversed by deubiquitinating enzymes. The balance between ubiquitination and deubiquitination activities regulates the level and activity of the protein substrates and thus cell homeostasis [17]. Proteins with a poly-ubiquitination will be degraded by the 26S proteasome. The 26S proteasome also known as the “proteasome” is a large (more than 2000kDA) multi-protein complex present in the nucleus and cytoplasm of all eukaryotic cells. It is composed of one 20S core particle and two 19S regulatory particles. The 19S regulatory particle binds a polyubiquitin chain and cleaves it from the substrate and recycles the ubiquitin. The substrate is then denaturated/unfolded and subsequently degraded into small peptides [18,19].
Immunomodulator is another important component of combination chemotherapy for multiple myeloma. As the earliest and most widely used immunomodulator in the clinic, montelukast (mnk), a selective reversible cys-leukotriene-1 receptor (LTD4 receptor) antagonist, is commonly used in the treatment of asthma. Leukotrienes (LTs) are biologically active fatty acids derived from the oxidative metabolism of arachidonic acid via a 5-lipooxygenase (5-LOX) pathway (Wenzel, 1998). 5-LOX activity leads to the formation of unstable LTA4, which can be converted into either LTB4 or several cysteinyl LT (CysLT; e.g. LTC4, LTD4, and LTE4), the latter are components of a slow-reacting substance of anaphylaxis. It is reported that 5-LOX is expressed by a wide variety of tumor cells and tissues. Therefore, LTs maybe potential targets in the treatment of malignancies [20-22]. Montelukast selectively block the cysteinyl leukotriene 1 receptor (CysLT1R). Based on these reports, we investigated whether montelukast perform antitumor effect in multiple myeloma. Furthermore, we explored the combination effect of montelukast and proteasome inhibitor carfilzomib. Finally, we searched for the potential mechanisms of these effects.
Methods
Cell culture and viability assay
The MM cell lines RPMI-8226, NCI-H929, and MM1S were routinely cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Gibco-BRL, Gaithersburg, MD, USA) in a humidified atmosphere with 5% CO2 at 37°C.
Reagents
Montelukast and carfilzomib was purchased from Selleckchem (Houston, TX, USA). The antibodies used were as follows: anti-poly (ADP-ribose) polymerase-1 (PARP-1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-caspase-9, anti-caspase-3, anti-β-actin, ubiquitin, OTUB1, UCHL1, UCHL3 and RPS27A (Cell Signaling Technology, Beverly, MA, USA).
Cell apoptosis assay
Cell apoptosis was measured using a fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) following the manufacturer’s instructions. After treatment with montelukast, carfilzomib respectively or in combination for 48 hours, cells were harvested, washed twice with ice-cold binding buffer, and resuspended with 100μl buffer. Annexin V-FITC (5μl) was then added to the suspended cells and mixed gently. This preparation was incubated for 5 minutes in the dark. Following incubation, 5μl propidium iodide was added and mixed gently. Incubation was continued for another 3 minutes. Finally, cells were analyzed by flow cytometry.
Cell cycle assay
After treatment, cells were harvested, washed twice with phosphate-buffered saline (PBS), and fixed with 75% cold ethanol at 4°C overnight. The next day, cells were incubated with RNase (100mg/ ml) for 30 minutes at 37°C. Cells were stained with propidium iodide (PI; Sigma) (250mg/ml) and incubated for another 15 minutes in the dark. Cells were then analyzed by flow cytometry.
Western blot analysis
Cells were harvested and lysed with lysis buffer. After quantification, protein extracts were equally loaded to 6%-15% sodium dodecyl sulfate–polyacrylamide gels, electrophoresed, and transferred to nitrocellulose membrane (Amersham Bioscience, Little Chalfont, UK). When completed, the membrane was blocked with 5% milk in PBS for 30 minutes at a temperature of 25ºC. The membranes were washed three times with Tris-buffered saline containing 0.05% Tween-20 and incubated with antibodies overnight at 4°C. The next day, the membranes were washed three times and horseradish peroxidase (HRP)-linked secondary antibody was added. The mixture was allowed to stand for 1-hour at a temperature of 25ºC. Signals were detected by chemiluminescence phototope-HRP kit (Merck Millipore, USA) according to manufacturer’s instructions. HRP-linked anti-β-actin antibody was used as the loading control.
Mitochondrial transmembrane potential
After treatment, cells were harvested, washed twice with phosphate-buffered saline (PBS). Then remove the supernatant and resuspend cells with 200μl PBS. Rhodamine123 was added to the cells to be tested so that the final concentration was 10μg/ml. Incubate for 30 minutes at a temperature of 37°C. Centrifugal at 2000 rpm for 10 minutes and remove unbound dyes. Centrifugal and wash cells with PBS (2000rpm for 10 minutes). Remove the supernatant and resuspend cells with 100μl PBS. Adding 5μl PI dyestuff into the cell suspension and mix them well. Stand for 15 minutes in the dark at room temperature. Adding 300μl PBS buffer and collecting the fluorescence signal of FL1-H/FL2-H excited at 488nm by flow cytometer.
Chemical proteomics based on protein activity
Cells treated were harvested and washed by PBS. Then entrifugal at 2000 rpm for 5 minutes and remove the supernatant. Make up non denatured cracking solution and resuspend cells well with appropriate solution. Incubate for 60 minutes at a temperature of 4°C to crack cell proteins. Centrifugal at 12000 rpm for 10 minutes and remove the supernatant into a new EP tube. Quantitative the proteins. Take 50μg proteins and incubate with different chemicals for 2 hours at room temperature. And then add 1μM HA-Ub-CI/Br/VME probe into the mixture. Incubate for 15 minutes at room temperature and add in 5x SDS the next step. Cooking the protein at a temperature of 98°C for 5 minutes. Finally, detecting proteins using HA antibody by western blot.
Results
To explore the effect of mnk in MM cells, we treated H929 and RPMI-8226 cell lines with mnk in different concentration and tested cell growth inhibition 48 hours later (Figure 1A). We found that mnk inhibits cell growth in a concentration-dependent manner. MM cell growth was completely inhibited treated with mkn in 80-100μmol/L. And then, we detected cell apoptosis-related proteins in MM cells that have been treated in the same way. As showed in Figure 1B, PARP-1, caspase-3 and caspase-9 were obviously cleaved in highconcentration group. It was suggested that mnk induces MM cell apoptosis in high concentration.
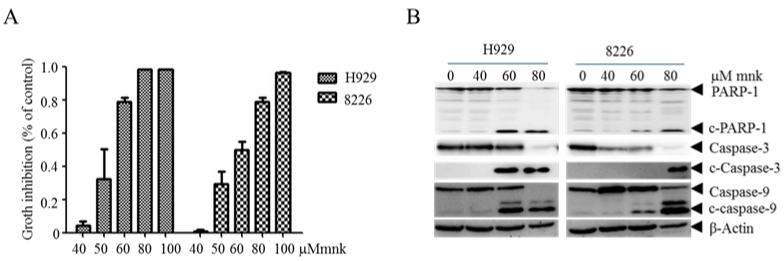
Figure 1: Montelukast inhibits cell growth and induces apoptosis in multiple myeloma cells.
A) H929 and RPMI-8226 cells were treated with mnk in different concentration of 40μmol/L, 50μmol/L, 60μmol/L, 80μmol/L, 100μmol/L respectively, and incubate it
with CCK8 after 48 hours. Then tested cell growth and calculated cell growth inhibition rate. B) Treated MM cell lines in the same way and collected cells 48 hours
later. Cracking cells and then detecting cleaves of apoptosis-related proteins by Western Blot. Each experiment was repeated three times.
As combination treatment is primarily recommended, we treated MM cell lines with mnk in combination with carfilzomib in different concentration. 48 hours later, we tested cell growth and calculated the combination index of each group (Table 1). It shows that almost all the combination index is less than 1, which means these two medicines are synergistic in MM cell lines. To approve it, we collected and cracked cells treated with mnk (5μmol/L) and cfz (10nmol/L) both in single and in combination respectively for 48 hours. Then we detected cell apoptosis rate and apoptosis-related proteins. In Figure 2A, we can see that cell apoptosis rate is significantly elevated in the combination group, especially in MM1s cells. In addition, the cleavage of PARP-1, caspase-3 and caspase-9 is obviously more in combination group than others in Figure 2B and 2C. All these results indicate that mnk in combination with cfz have a stronger cytotoxic effect.
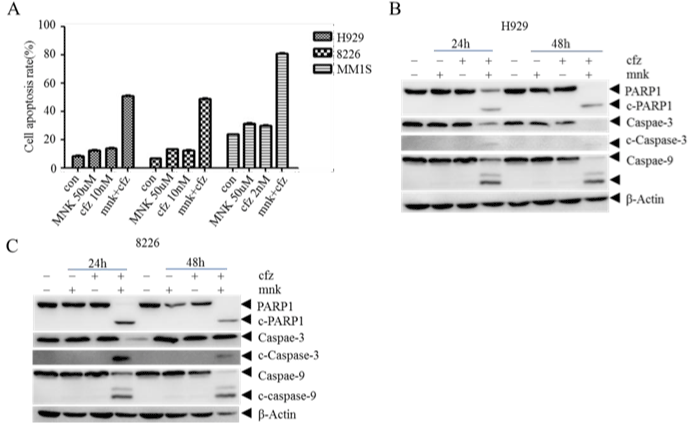
Figure 2: Montelukast in combination with carfilzomib induce advanced cell apoptosis in MM cells.
A) H929, RPMI-8226 and MM1s cells were treated with mnk in 50μmol/L and cfz in 10nmol/L both in single and in combination respectively for 48 hours. And then
collect these cells and detect cell apoptosis rate by Annexin V/PI using flow cytometry. B, C) Collecting and cracking H929 and RPMI-8226 cells treated the same
way to detect apoptosis-related proteins PARP-1,caspase-3 and caspase-9. Each experiment was repeated three times.
A
B
H929
8226
Dose mnk
Dose cfz
Effect
CI
Dose mnk
Dose cfz
Effect
CI
50
5
0.35209
1.08808
50
5
0.53559
0.99568
50
10
0.60446
0.89688
50
10
0.71275
0.96574
50
15
0.73649
0.83109
50
15
0.79109
1.01225
50
20
0.77047
0.90281
60
5
0.71224
0.81574
60
5
0.62507
0.74238
60
10
0.81925
0.82153
60
10
0.79554
0.63958
60
15
0.84895
0.92312
60
15
0.84123
0.66541
60
20
0.88134
0.65938
Table 1: The combination index of montelukast and carfilzomib in multiple myeloma.
It is well known that there are three distinct pathways of cell apoptosis, including exogenous cytokine pathway, mitochondrial pathway and endoplasmic reticulum stress pathway. Which one is performed in the combination group? To answer this question, we tested mitochondrial transmembrane potential of cells treated with mnk and cfz in the same way as before. In Figure 3A, we can see that there is no obvious difference in mitochondrial transmembrane potential in four groups. On the other hand, we detected antiapoptotic protein bcl-2 and mcl-1 which should diminish, and pro-apoptotic protein bax which should increase in mitochondrial pathway apoptosis (Figure 3B and 3C). It showed that, when cells were treated with mnk in combination with cfz, bcl-2 and bax were diminished at the same time while mcl-1 changed irregularly. So the synergistic effect of mnk and cfz is not performed by mitochondrial pathway.
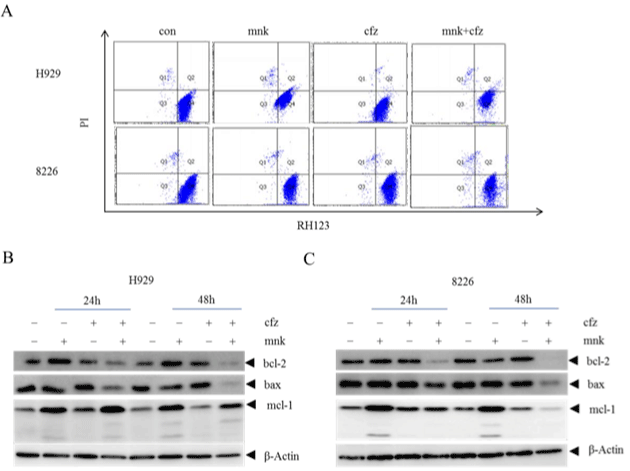
Figure 3: The combination treatment has no influence in the mitochondrial transmembrane potential.
A) H929 and RPMI-8226 cells were treated with mnk and cfz in the same way as before. Collecting cells and staining them with Rhodamine 123 after treated 24
hours. Then test mitochondrial transmembrane potential of these cells by flow cytometry. B, C) Collected cells treated with mnk and cfz in the same way after 24
hours and 48 hours respectively. Cracking and detecting changes in protein levels of bcl-2, mcl-1 and bax by Western Blot. Each experiment was repeated three
times.
To further explore the mechanism of cell apoptosis, we detected endoplasmic reticulum stress pathway related proteins (Figure 4A and 4B). As showed in the diagram, protein levels of CHOP, p-eIF2 α , GRP78 and GADD34 were significantly increased. This suggested that the endoplasmic reticulum system was highly activated in the combination group, which led to apoptosis.
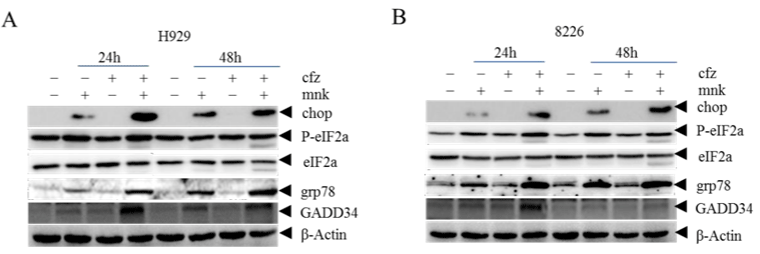
Figure 4: The combination treatment induces more cell apoptosis by activating ER stress.
H929 cells (A) and RPMI-8226 cells (B) Cells treated with mnk and cfz were collected and cracked in the same way as before. Then detected endoplasmic reticulum
stress pathway related proteins CHOP, p-eIF2, GRP78 and GADD34 by Western Blot. Each experiment was repeated three times.
As carfilzomib is the second generation proteasome inhibitor, it causes the accumulation of ubiquitin. So we tested ubiquitin levels of different groups (Figure 5A and 5B). We found that in the combination group, ubiquitin level was significantly increased both in H929 and RPMI-8226 cells. This result reveals that mnk can also lead to the accumulation of ubiquitin. But how it works? We all know that montelukast is a LTD4 receptor inhibitor. To make sure whether it works by inhibiting LTD4 receptor, we calculated the combination index of pranlukast and zafirlukast with carfilzomib in multiple myeloma.
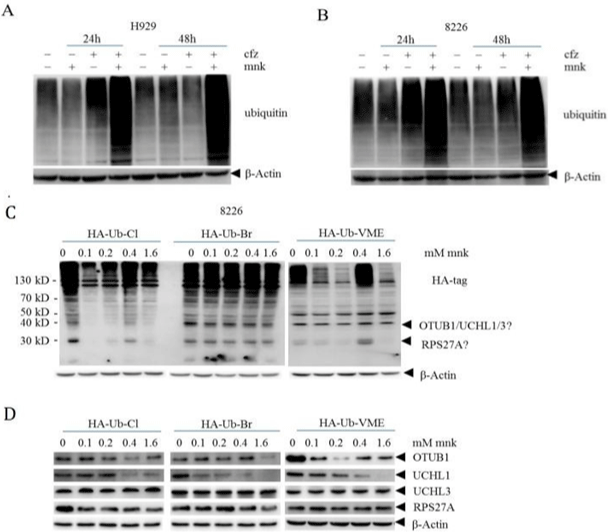
Figure 5: Montelukast in combination with carfilzomib induces advanced accumulation of ubiquitination by inhibiting the deubiquitinase UCHL1.
We got cell lysis proteins of H929 (A) and RPMI-8226 (B) in the same way as before. Then, we detected the levels of ubiquitinated proteins in different experimental
groups by Western blot. C) 8226 cells in good growth condition were collected, and the cells were lysed with non denaturing lysate for protein quantification. An
appropriate amount of protein was incubated with different concentrations of mnk for 2 hours, and then HA labeled ubiquitin probe was added for incubation. Finally,
detect the changes of protein with Ha antibody using Western blot. D) The protein changes after the above treatment were detected by OTUB1, UCHL1, UCHL3
and RPS27A antibodies to the corresponding proteins. Each experiment was repeated 3 times.
We handled H929 and 8226 cells using pranlukast or zafirlukast with carfilzomib in different concentrations for 48 hours and calculated CI index of each group. As showed in Table 2, almost all CI indexes are greater than 1. Which told us that pranlukast and zafirlukast have no synergistic effect with carfilzomib in MM? It is proved that the synergistic effect of mnk and cfz has no relationship with its LTD4 receptor inhibition effect.
Pranlukast + cfz
Zafirlukast + cfz
A
B
Dose mnk
Dose cfz
Effect
CI
Dose mnk
Dose cfz
Effect
CI
H929
40
5
0.25428
1.03885
40
5
0.28704
1.42844
40
10
0.7286
0.97329
40
10
0.75206
1.21902
50
5
0.21832
1.19951
50
5
0.39609
1.40457
50
10
0.74058
0.96861
50
10
0.78292
1.25683
60
5
0.32534
1.00931
60
5
0.56996
1.27586
60
10
0.7988
0.89092
60
10
0.80967
1.28997
C
D
8226
Dose mnk
Dose cfz
Effect
CI
Dose mnk
Dose cfz
Effect
CI
40
5
0.21489
1.62039
40
5
0.36723
1.38202
40
10
0.52253
1.52249
40
10
0.61814
0.96815
50
5
0.21097
1.8477
50
5
0.44696
1.16291
50
10
0.53298
1.66116
50
10
0.782
0.62033
60
5
0.31352
1.77873
60
5
0.5289
0.95956
60
10
0.56172
1.75806
60
10
0.81346
0.56955
Table 2: The combination index for pranlukast or zafirlukast with carfilzomib in multiple myeloma.
Then we adopted a chemical proteomics method based on enzyme activity to explore the possible target of mnk. The results were shown in Figure 5C. We can see that the bands changed obviously at the position where the molecular weight of the protein was about 40KD and 30KD. As the molecular weight of the probe is 12KD, the molecular weight of the proteins corresponding to the two bands is about 30KD and 20KD. By comparing the molecular weight of deubiquitinase family proteins with the two bands, we guessed that the possible target of mnk include OTUB1, UCHL1, UCHL3 and RPS27A. Subsequently, we tested these proteins by western bolt. As showed in Figure 5D, there was almost no change in proteins UCHL3 and RPS27A. Besides, the changes of protein OTUB1 in different group were inconsistent. While the protein levels of UCHL1 decreased gradually with the increase of drug concentration in all three groups. Which indicated that mnk may perform its effect by targeting UCHL1 in MM cells.
Discussion
This project aims to explore the effect of the oldest immunomodulator montelukast in multiple myeloma. As carfilzomib is more commonly used in recurrent or refractory MM, we chose cafizomib for the combination treatment. In our experiment, we found that mnk induces cell apoptosis in MM and has synergistic effect with proteasome inhibitor carfilzomib. The combination treatment of mnk and cfz induces more cell apoptosis and more cleavage of apoptosis related proteins. To explore the underlined mechanism, we firstly tested mitochondrial transmembrane potential and anti-apoptotic proteins. The results show that mnk in combination with cafizomib induces cell apoptosis by activating endoplasmic reticulum stress pathway. In the following research, we reach the conclusion that mnk inhibits the deubiquitinase UCHL1,which induces the advanced accumulation of ubiquitin and therefore actives the endoplasmic reticulum stress pathway. And finally leads to cell apoptosis.
Several researchers have highlighted that the capacity of deubiquitination enzymes to act as oncogenes and tumor suppressors [23-26]. As a member of the deubiquitinase family, UCHL1 is frequently over-expressed in mature B-cell malignancies, including MM [27,28]. Besides, UCHL1 is found to be a biomarker of aggressive multiple myeloma required for disease progression [23]. It may impact tumorigenesis by promoting AKT phosphorylation and regulating cyclin dependent kinases [29], β-catenin [30], HIF-1 [31], NOXA [32], and tubulin polymerization [33]. Therefore, UCHL1 is a potential target in the treatment of MM.
It is well known that carfilzomib induces MM cell apoptosis by inhibiting proteasomes, which plays critical roles in the UPS. Furthermore, we found that montelukast also destructs the UPS by inhibiting the deubiquitinase UCHL1. Therefore, when we combined carfilzomib and montelukast in multiple myeloma, it will lead to more tumor cell apoptosis. We have proved it in MM cell lines. However, we need to do more validation in primary multiple myeloma cells and in clinical treatment. This requires us to do more work in the future.
At present, most new drugs for the treatment of multiple myeloma are very expensive. This brings huge economic burden on patients and a lot of patients cannot get better treatment because of economic reasons. Since montelukast is cheap and widely used in clinic, if it is proved to be effective in the treatment of multiple myeloma, it will greatly reduce the economic pressure of patients and bring new hope to patients.
Acknowledgement
We are grateful to the Jing’an district health bureau scientific research fund (Grant No.2019QN05) for their financial support.
References
- Medical Masterclass contributors, Firth J. Haematology: multiple myeloma. Clin Med (Lond). 2019; 19: 58-60.
- Chari A, Martinez-Lopez J, Mateos MV, Bladé J, Benboubker L, Oriol A, et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood. 2019; 134: 421-431.
- Orlowski RZ, Lonial S. Integration of novel agents into the care of patients with multiple myeloma [published correction appears in Clin Cancer Res. 2017; 23: 2605]. Clin Cancer Res. 2016; 22: 5443-5452.
- Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Primers. 2017; 3: 17046.
- Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015; 385: 2197- 2208.
- Groen K, van de Donk N, Stege C, Zweegman S, Nijhof IS. Carfilzomib for relapsed and refractory multiple myeloma. Cancer Manag Res. 2019; 11: 2663-2675.
- Leleu X, Martin TG, Einsele H, Lyons RM, Durie BGM, Iskander KS, et al. Role of proteasome inhibitors in relapsed and/or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2018; 19: 9-22.
- Guerrero-Garcia TA, Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Mitsiades C, et al. The power of proteasome inhibition in multiple myeloma. Expert Rev Proteomics. 2018; 15: 1033-1052.
- Park J, Cho J, Song EJ. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch Pharm Res. 2020; 43: 1144-1161.
- Cvek B, Dvorak Z. The ubiquitin-proteasome system (UPS) and the mechanism of action of bortezomib. Curr Pharm Des. 2011; 17: 1483-1499.
- Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009; 458: 438-444.
- Sahasrabuddhe AA, Elenitoba-Johnson KS. Role of the ubiquitin proteasome system in hematologic malignancies. Immunol Rev. 2015; 263: 224-239.
- Deshaies RJ. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014; 12: 94.
- Dou QP, Zonder JA. Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr Cancer Drug Targets. 2014; 14: 517-536.
- Çetin G, Klafack S, Studencka-Turski M, Krüger E, Ebstein F. The Ubiquitin- Proteasome System in Immune Cells. Biomolecules. 2021; 11: 60.
- Pickart CM, Eddins MJ. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2004; 1695: 55-72.
- Crawford LJ, Irvine AE. Targeting the ubiquitin proteasome system in haematological malignancies. Blood Rev. 2013; 27: 297-304.
- Cao B, Mao X. The ubiquitin-proteasomal system is critical for multiple myeloma: implications in drug discovery. Am J Blood Res. 2011; 1: 46-56.
- Tanaka K, Mizushima T, Saeki Y. The proteasome: Molecular machinery and pathophysiological roles. Biol. Chem. 2012; 393: 217-234.
- Tsai MJ, Chang WA, Chuang CH, Wu KL, Cheng CH, Sheu CC, et al. Cysteinyl Leukotriene Pathway and Cancer. Int J Mol Sci. 2021; 23: 120.
- Peters-Golden M, Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot. Essent. Fat. Acids. 2003; 69: 99-109.
- Haeggstrom JZ, Kull F, Rudberg PC, Tholander F, Thunnissen MM. Leukotriene A4 hydrolase. Prostaglandins Other Lipid Mediat. 2002; 68-69: 495-510.
- Hussain S, Bedekovics T, Chesi M, Bergsagel PL, Galardy PJ. UCHL1 is a biomarker of aggressive multiple myeloma required for disease progression. Oncotarget. 2015; 6: 40704-40718.
- Hussain S, Zhang Y, Galardy PJ. DUBs and cancer: The role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 2009; 8.
- Zhang Y, Foreman O, Wigle DA, Kosari F, Vasmatzis G, Salisbury JL, et al. USP44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J Clin Invest. 2012; 122: 4362-4374.
- Hussian S, et al. The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia. 2010; 24: 1641-1655.
- Otsuki T, Yata K, Takata-Tomokuni A, Hyodoh F, Miura Y, Sakaguchi H, et al. Expression of protein gene product 9.5 (PGP9.5)/ubiquitin-C-terminal hydrolase 1 (UCHL-1) in human myeloma cells. Br J Haematol. 2004; 127: 292-298.
- Ovaa H, Kessler BM, Rolen U, Galardy PJ, Ploegh HL, Masucci MG. Activitybased ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc Natl Acad Sci USA. 2004; 101: 2253-2258.
- Kabuta T, Mitsui T, Takahashi M, Fujiwara Y, Kabuta C, Konya C, et al. Ubiquitin C-terminal hydrolase L1 (UCH-L1) acts as a novel potentiator of cyclin-dependent kinases to enhance cell proliferation independently of its hydrolase activity. J Biol Chem. 2013; 288: 12615-12626.
- Bheda A, Yue W, Gullapalli A, Whitehurst C, Liu R, Pagano JS, et al. Positive reciprocal regulation of ubiquitin C-terminal hydrolase L1 and beta-catenin/ TCF signaling. PLoS ONE. 2009; 4: e5955.
- Goto Y, Zeng L, Yeom CJ, Zhu Y, Morinibu A, Shinomiya K, et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1alpha. Nat Commun. 2015; 6: 6153.
- Brinkmann K, Zigrino P, Witt A, Schell M, Ackermann L, Broxtermann P, et al. Ubiquitin C-terminal hydrolase-L1 potentiates cancer chemosensitivity by stabilizing NOXA. Cell Rep. 2013; 3: 881-891.
- Bheda A, Gullapalli A, Caplow M, Pagano JS, Shackelford J. Ubiquitin editing enzyme UCH L1 and microtubule dynamics: implication in mitosis. Cell Cycle. 2010; 9: 980-994.