
Research Article
Austin J Cancer Clin Res. 2025; 12(1): 1118.
Chemoprevention Potentials of Selected Free Radical Scavengers in Regressing Neoplasms: A Systematic Review
Mfaume M. Mleke*, Kauke B. Zimbwe and Sartaz Begum
Oncology pharmacy section, Department of Pharmacy and Compounding, The Benjamin Mkapa Hospital, Dodoma, United Republic of Tanzania
*Corresponding author: Mfaume M. Mleke, Oncology pharmacy section, Department of Pharmacy and Compounding, The Benjamin Mkapa Hospital, P. O BOX 11088, Dodoma, United Republic of Tanzania Tel: +255745966687; Email: mlekemfaume4@gmail.com
Received: April 22, 2025 Accepted: May 07, 2025 Published: May 12, 2025
Abstract
Introduction: This systematic review was done to evaluate the potential roles of free radical scavengers(antioxidants) in cancer prevention, regressing, and enhancing conventional cancer therapies.
Study Design: Systematic review of clinical trials, randomized controlled trials and primary research studies were screened from 2010 to 22 November 2024 from PubMed and Web of science.
Methodology: A qualitative evidence synthesis was performed by thoroughly searching the PubMed and Web of Science database and the clinical trials, randomized controlled trials and primary research studies from (2010 -November 22, 2024.) were selected for the systematic review Using the following boolean search operators. Chemoprevention” AND “Antioxidant”, (Lycopene OR Carotenoids) AND (Prostate Cancer [Mesh]) AND (“Prevention”[Title/Abstract]). (Antioxidants OR “Free radical scavengers”) AND (Cancer OR Tumor) AND (“Clinical trial”[Publication Type]).
Results: High-dose intravenous vitamin C (15–65g twice weekly) showed tumor regression in pancreatic, renal, and B-cell lymphoma cases. Combination therapy (vitamin C, β-carotene, selenium, zinc) significantly slowed breast cancer progression. Lycopene and tocopherols, combined with docetaxel, inhibited prostate cancer, while CoQ10 (300 mg/day) reduced oxidative stress in liver cancer. Vitamin D3 (50,000 IU/week) prevented cervical precancer progression, and nicotinamide (500–1000 mg/day) cut non-melanoma skin cancer risk by 23%. However, vitamin E + selenium unexpectedly increased prostate cancer risk (OR: 1.32).
Conclusion: Antioxidants show promise in cancer prevention and therapy, but risks like vitamin E/selenium highlight the need for caution. Large-scale trials are essential for clinical application.
Keywords: Chemoprevention; Free radicals; Antioxidants; Malignancy; Oxidative stress
Introduction
In recent years emergency of cancer cases is increasing brought by inappropriate social life style including alcoholism, cigarette, smoking, high intake of red meat and environmental pollutions predisposing individuals to high risk of malignancy development, so the use of FR scavengers will benefit not only in clinical practice but even the community at large. Free radical scavengers use is a promising strategy for prevention, curing, and reducing the risk of several malignancies. Also, the use of FR can help restore balance and counteract the cancer cells resistance towards antineoplastic. Moreover, free radical scavenger was used in combatting antineoplastic toxicities without compromising antineoplastic efficacy. Free radicals are highly oxygen or nitrogen reactive species, naturally formed during metabolism or by exposure to environmental toxins. Endogenously these free radical scavengers they are produced in the oxidative reaction process of mitochondrial respiratory chain as byproducts of normal cellular metabolism (C. Nathan & Cunningham-Bussel, 2013) or from external sources (pollution, cigarette smoke, radiation, medication, Alcoholism) .Free radical scavengers are a group of natural, synthetic or biological agents that counteract the pathological effects or oxidative stress caused by Reactive Oxygen (ROS) and Nitrogen species (NS) once in excessive concentration and reaches a toxic threshold level in human physiologic system(reference). Pathological effects of FR include malignancies, aging, cardiovascular diseases, neurodegenerative disorders and metabolic disorders like diabetes (reference).
Reactive oxygen species (ROS) actions include both inhibition and activation of proteins, mutagenesis of DNA and activation of gene transcription. These actions promote or suppress inflammation, immunity and carcinogenesis [1,2]. The phenomenon of ROS overproduction by tumour cells has been widely confirmed [15]. In some cases, excessive ROS production has been attributed to mutations in a mitochondrial gene that encodes a component of the mitochondrial electron transport chainClick or tap here to enter text. ROS release by tumour cells might sensitize, or even self-activate, their growth factor receptors by inhibiting the associated tyrosine phosphatases [14,15]. Tumour cell ROS production might also help to explain how tumour cells alter their central carbon metabolism [15] for example, towards synthesis of nucleic acid precursors [16] and it might also contribute to immunosuppression. Moreover, the mutagenic actions of ROS that are derived from inflammatory cells can contribute to the initial stages of tumorigenesis [17].
After a tumour is established, ROS derived from radiotherapy, from chemotherapy or from the tumour cells themselves might contribute to the genomic instability of tumour cells [18] fostering drug resistance, just as ROS production that is induced in bacteria by antibiotics can cause mutations that promote antibiotic resistance. Finally, tumour cell-derived ROS can trigger the tumour stroma to produce angiogenic factors [12].
In many types of cancer, an increased oxidative stress has been observed as common feature compared with normal cells. ROS induced DNA and protein damage can contribute to cancer initiation through cellular signaling pathways which are associated with tumor cell proliferation, survival and tumor progression [19,20]. Cancer cells have higher metabolism level than normal cells; they can continuously produce and maintain higher ROS concentrations to maintain their high proliferation rate. The produced ROS can cause oxidative damage of DNA, which in turn to generate mutations in DNA that enhances both the processes of aging and carcinogenesis [20-22].
ROS can cause DNA damage, which may excite the p53 in normal cells and consequently activate stress responses as well as DNA repairing. Many studies have found the defect of p53 in cancer cells. Depletion of the p53, ROS-mediated DNA damage would accumulate owing to compromised DNA repair function. It would severely promote the genomic instability and lead to an activation of oncogenes and decrease in antioxidants, thus, increase in ROS levels, leading to more DNA damage and genetic instability.
This brings us to our review study which focuses in emphasizing the use of free radical scavengers or antioxidants both conventional and natural agents which are available in clinical settings or nonclinical settings as a potential mechanism to offset an increased risk of cancer.
Materials and Methods
Search Strategies
This systematic review was conducted in accordance with the preferred Reporting items for systematic Reviews and Meta- Analyses (PRISMA) statement guidelines [13]. The comprehensive literature search was conducted in the PubMed and Web of science databases for relevant (2010 -November 22, 2024) were selected for the systematic review adhering studies. Chemoprevention” AND “Antioxidant”, (Lycopene OR Carotenoids) AND (Prostate Cancer [Mesh]) AND ("Prevention"[Title/Abstract]). (Antioxidants OR "Free radical scavengers") AND (Cancer OR Tumor) AND ("Clinical trial"[Publication Type]).
Eligibility Criteria’s
In this study inclusion of studies that had extensive and explicit focus on examining the Potential role played by Free radical scavengers in regressing neoplasms were used. Studies published in language other than English were exclude as we do not have the capacity within the team to extract data from them. Systematic review studies were not significantly given much of attention compared to primary research. Incomplete clinical trials specifically phase 1 and phase 2 clinical trial were excluded. We did not include gray literature as we believe that the peer reviewed studies identified in our search is sufficient to respond to our research question and also outside of the scope of data were excluded.
Data Extraction and Management
Data were extracted systematically by two M.M.M and K.B.Z independent reviewers using a standardized form. Discrepancies were resolved through consensus or consultation with a third reviewer S.B. Extracted information included study characteristics (author, year, phase, sample size), Neoplasms varieties, details of the free radical scavengers interventions (Antioxidants, dosages), and key findings.
Risk of Bias Assessment
The evaluation of the risk of bias for individual studies included in this systematic-analysis was conducted using the updated RoB 2 tool for randomized trials developed by the Cochrane Collaboration [14]. This tool provides a structured approach to assessing biases related to the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result.
Each study was critically appraised across these specified domains, ensuring a thorough examination of potential biases that could influence the reliability and validity of the study findings. Discrepancies in the risk of bias assessments were resolved through discussion or by involving a third reviewer, ensuring a consensusbased approach to risk determination (Figure 1, 2).
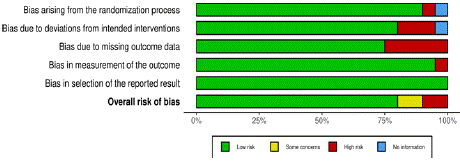
Figure 1: Shows risk of Bias assessment for several studies included for
systematic reviews.
Figure 2: Risk of bias Domains.
Results
The literature selection process is illustrated in Figure 3. The initial search yielded 110 studies, from which 20 duplicate records were removed. An additional 10 studies were excluded based on their titles and abstracts due to their r irrelevance to our research study. During the full-text review, we further excluded studies for the following reasons: 12 Systematic reviews were not included, 13 Studies were not related to chemoprevention potentials by free radical scevengers .and finally 55 studies were extensively evaluated and included in our systematic review study.
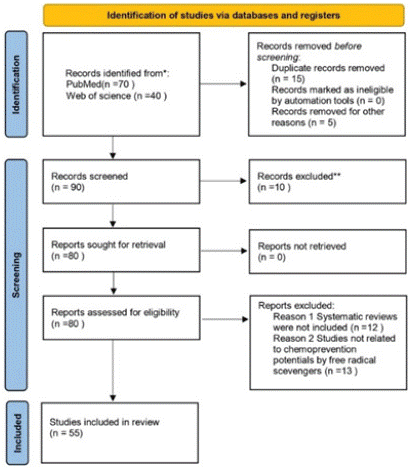
Figure 3: PRISMA flow diagram (15). For more information,
visit: http://www.prisma-statement.org/(accessed on 30 November 2024).
Discussion
Ascorbic Acid (Vitamin C)
It has been reported by numerous studies, that vitamin C plays a critical role in fighting some types of malignancies or used as chemoprevention to reduce the risk of cancer initiation, development and progression or recurrences [16-20]. Polireddy et al., 2017 conducted a controlled randomized clinical trial on the effect of high dose parenteral ascorbic acid in regressing pancreatic cancer growth and metastasis alone or in combination with gemcitabine where by 12 participants were enrolled and among this sample population it was observed that one participant after the use of ascorbic acid was liable for surgery indicating the debulking effect in tumor size under Ascorbic acid use, also of these 12 participants survival rate after the use of ascorbic acid of one year after diagnosis was 50% (6 patients) and survival rate of more than 2 years after diagnosis was 8.3%
Finally, it was observed that one participant had a remarkable tumor response to treatment regimen and this participant prior to administration was diagnosed with stage 3 pancreatic ductal carcinoma who failed FOLFIRNOX treatment and was on disease progression. After enrollment in the trial, the participant received a total of 70 doses of IVC (64 doses of 100 grams/infusion when in Phase II, and 6 doses from 25–75 g/infusion when in Phase I) and gemcitabine (715 mg/m2) for 9 cycles. Imaging showed tumor stabilization/shrinkage and improvement in appearance of margins which became more distinct. These findings shows the potentials of IVC as a promising therapeutic regimen in treating pancreatic carcinoma and also supports for patients at high risk of developing these malignancies would benefit from this antioxidants. Also, to cement these findings vitamin c has pharmacological role in inhibiting malignancy development through inhibiting alpha tubulin acetylation by depleting NAD+ in cancer cells in similar ways as Paclitaxel work hence suffocating cancer cells and also it selectively raises hydrogen peroxide levels to initiate cytotoxicity to these tumor cells in alignment to this it is well said that Vitamin C increases sensitivity of Chemotherapeutic regimens by selectively raising hydrogen peroxide damage to cancer cells compared to normal cells. Through causes shrinkage or debulking of tumor size directly causing younger and tender tumor cells to proliferate and these are highly sensitive and have high drug permeability thus enhancing anticancer effects of chemotherapeutic regimens.
Also, padayatt et al., 2006 [18] conducted a prospective study review for three cases in accordance with National Cancer Institute (NCI) Best Case Series guidelines.One case involved a patient diagnosed with pulmonary metastatic renal carcinoma who declined conventional cancer treatments and opted for high Intravenous dose of ascorbic acid therapy of 65g twice per week for a period of 10 months, then after one year follow up chest radiographic results was found to be normal confirming tumor regression. Another case involved a patient diagnosed with bladder tumor who also declined conventional cancer treatments and opted for intravenous high dose of ascorbic acid of 30g twice per week for 3 months, followed by 30 g once every 1–2 months for 4 years, interspersed with periods of 1–2 months during which he had more frequent infusions .9 years after diagnosis the patient was found to be in good health and no symptoms of recurrence or metastasis.
A third case was associated with patient diagnosed with B-cell lymphoma. The patient's oncologist recommended local radiation therapy and chemotherapy. Although she agreed to a 5-week course of local radiation therapy, the patient refused chemotherapy, electing instead to receive vitamin C intravenously. She received 15 g of vitamin C twice per week for about 2 months, 15 g once to twice per week for about 7 months, and then 15 g once every 2–3 months for about 1 year. The patient remains in normal health 10 years after the diagnosis of diffuse large B-cell lymphoma, never having received chemotherapy.from these findings it is note worth to appraise the high intravenous dose of Ascorbic acid starting from 15g to 65g given twice weekly for 3 months and then once weekly every 1 to 2 months for a maximum period of 1 year could significantly provide therapeutic effects relevance among cancer patients as from these findings.
Significantly, daily oral intake of vitamin C has been reported to have therapeutic usefulness in some forms of hematological malignancies like lymphocytic leukemia. Gamin et al., 2014 [19] Observed from their randomized controlled study which included 84 participants with chronic lymphocytic leukemia for which 44 patients only received antileukemic agents and 40 patients received antioxidants (Vitamin C) and antileukemic agents. Then it was reported that daily intake of Vitamin C a dose of 1000mg significantly reduced infections and increased patients’ prognosis and tolerance to chemotherapeutic agents in a group that received both antioxidants and antileukemic agents.
Amin et al. 2021 reported that administration of vitamin C at a dose of 1000mg/day for 8 weeks in women with endometriotic cancer led to a significant reduction in Peripheral reactive oxygen species (ROS) and hence attenuating the excess essive oxidative stress damage in endometriotic cancer. Also, some studies indicated that vitamin C can lead to endometriotic cyst volume and weight reduction. Besides, vitamin C has a significant dose-dependent effect in natural killer cells and endometriotic tissue implantation [21,22]. It seems that vitamin C and vitamin E can prevent the peroxidative process spread by their chain-breaking property. Indeed, these vitamins help tissue to balance oxidant-antioxidant levels [23]. The known role of ROS in the endometriotic cell proliferation, along with increased ROS production in endometriosis in response to inflammation [24], shows the importance of ROS reduction in endometriosis treatment. Hence, the antioxidant action of vitamins may reduce the clinical symptoms of endometriosis
These findings show a strong evidence and clinical plausibility on the use of Vitamin C as therapeutic agents when administered in high dose intravenously for tumor regression or as chemoprevention to reduce the risk for cancer in high-risk group likewise to prevent recurrences of neoplasms after treatment. Also, Vitamin C by acting as free radical scavengers maintains physiological pH body balance hence alkaline cellular microenvironment which is favorable environment for normal cellular differentiation, development and growth, But harsh environment for tumor cell survival. However, despite of these significant findings, the use of this antioxidants is negligible in most of our clinical settings, Tanzania and mostly Sub-Saharan countries this could possibly be due to some factors such as Health insurances does not cover for the costs, lack of awareness on thorough use of these antioxidants also less valuable research studies have not been done to evaluate and bring to stage this invaluable information.
Nicotinamide (Vitamin B3)
It is noteworthy that vitamin B3 (Niacinamide) has been widely used for its health benefits as antiaging, skin protective and as an immunomodulatory agent. several studies have reported potential role of this vitamin in chemoprevention of nonmelanoma skin cancers such as basal cell and squamous cell carcinoma mostly caused by ultraviolet (UV) radiation and other forms of carcinogens or cocarcinogens [25-29].
Chen et al., 2015 conducted a randomized controlled trial of nicotinamide for skin cancer chemoprevention where by the study enrolled about 386 participants who had at least two nonmelanoma skin cancers allocated at a ratio of 1:1 to nicotinamide and placebo group respectively. Thereafter, the participants were prospectively followed to determine development of new squamous cell carcinoma, and basal cell carcinomas and the number of new actinic keratoses and Nicotinamide safety. After a 12 months intervention period it was significantly observed a substantial decrease in rate of new nonskin melanomas mainly basal cell carcinoma and squamous cell carcinoma in Nicotinamide group compared to placebo group [25]. Also, similar results were obtained in one clinical lab investigative study which evaluated the potential role of Nicotinamide in fighting skin cancers induced by UV radiation with Arsenic co-carcinogen. The study ended up with substantial effectiveness of amide derivative of Vitamin B3 against nonskin melanomas development [27].
Interestingly Nicotinamide was found to have immunomodulatory effect, enhancing massive DNA repair through maintenance of NAD+ vital for ATP formation that is highly utilized in cellular damage for DNA cell repair. Thus, through this it was confirmed this bioenergetic vitamin proved to increase cutaneous immunity and cutaneous antitumor activity hence reducing nonskin melanomas induced by UV radiation and other many co-carcinogens that community is exposed in the Environments [27,29]. Also, Minocha et al. 2017 reported similar results in a randomized controlled trial with two wings, one wing was given oral intake of nicotinamide and other wing was on placebo and the results was that Nicotinamide wing had significant robust levels of CD 4, CD 8, and T regulatory cells compared to placebo wing. The study ended up with the findings that Nicotinamide had a chemo preventive effect against nascent and preexisting nonskin melanomas through immunoprotective mechanisms [26]. However, this vitamin could provide a significant result once combined with chemotherapy regimens as Chen et al., 2015 reported that after a discontinuation of Vitamin B3 use its chemoprotective effects was lost, suggesting that patients at high risk of developing new superficial basal cell carcinomas would significantly benefits Nicotinamide when concomitantly used with chemotherapeutic regimen [25].
Despite these findings and the fact that Vitamin B3 amide derivative is inexpensive and widely available over the counter vitamin that could serve a potential chemopreventive agent in highrisk population of developing nonskinmelanomas and it could easily be translatable in clinical settings, its use in this area is still minimal especially in sub-saharan countries including Tanzania. This delays in use could be due to lack of disseminated therapeutic information, few studies reported on the use of this vitamin in nonskin melanomas and fear about safety profile of this vitamin, but it had been well postulated that Nicotinamide is safe when used in pharmacological doses of 1g up to maximum dose of 3g [25].
Coenzyme Q10 (Ubiquinone)
It had been well reported that excessive free radicals’ formation triggers massive inflammation and potentiate progression or worsens the conditions of many diseases including malignancies by altering normal cellular proliferation, differentiation and growth through oxidative DNA damage and disrupting homeostatic redox balance which leads to harsh acidic cellular microenvironments that in turns expose normal cells to turn into malignancy. Coenzyme Q10 is a lipid soluble endogenous antioxidant with potential capacity to scavenge free radicals hence offsetting negative cellular toxic effects of the free radicals in body physiology.
Several studies had reported the clinical efficacy of coenzyme Q10 in attenuating oxidative stress and inflammation which directly impacts the progression of some types of cancers and many other metabolic diseases through its antioxidant mechanisms, mostly hepatocellular carcinoma [30-32].
Lee et al., 2013 Conducted a randomized placebo-controlled study with patients diagnosed with primary hepatocellular carcinoma where 41 patients were recruited one wing with 21 patients was given 300mg/day of coenzyme Q10 and 20 was on placebo. Then the patients were prospectively followed for an intervention period of about 12 weeks and the results was that intervention group on coenzyme Q10 significantly had decreased oxidative stress and inflammatory marker levels and total antioxidant capacity was highly elevated compared to placebo group suggesting that this lipid soluble antioxidant could be therapeutically used in patients with hepatocellular carcinoma after surgery to overcome oxidative stress storm hence serving as potential chemo preventive agent [30].
Also consistent results were found in one study that investigated risks of ubiquinone deficiency in patients with oral cancer where the study enrolled 194 patients with oral cancers stratified in four cancer stages to evaluate if they had deficiency of this lipid soluble antioxidants, consequently the study found a significant decrease in this endogenous antioxidants which adversely affected disease prognosis as lack of this micronutrient triggers excessive free radicals accumulation which is bad for malignancy status [32]. Therefore these findings indicate, supplementation of Coenzyme Q10 to cancer patients would significantly reduce level of inflammation and oxidative stress that manifests and affects overall patients recovery Also consistent results were found in one study that investigated risks of ubiquinone deficiency in patients with oral cancer where the study enrolled 194 patients with oral cancers stratified in four cancer stages to evaluate if they had deficiency of this lipid soluble antioxidants, consequently the study found a significant decrease in this endogenous antioxidants which adversely affected disease prognosis as lack of this micronutrient triggers excessive free radicals accumulation which is bad for malignancy status [32]. Therefore these findings indicate, supplementation of Coenzyme Q10 to cancer patients would significantly reduce level of inflammation and oxidative stress that manifests and affects overall patients recovery
Moreover, given known pharmacological effects of Coenzyme Q10 as reported by several studies (33,34)which found not only that Coenzyme Q10 acts as antioxidant but also have many other therapeutic functions including anti-inflammatory effects by modulating the expression of cyclooxygenase-2 and nuclear factor -κB (NF-κB) in liver tissues. This brings us to our study with focus on chemoprevention by the use of free radical scavengers in fighting cancer, despite these invaluable findings and the easiness that coenzyme Q10 could readily be translatable into clinical practice as a complementary treatment plan but yet its use in this area in most of the Sub-Saharan countries including Tanzania is insignificant. This could be brought by several reasons such as no disseminated therapeutic information to trigger its use in this scope, lack of health insurance coverage and availability as whole. But given the high morbidity and mortality rate of this chronic disease, clinically therapeutic benefits would highly outweigh these impeding factors.
Branched Chain Amino Acids (Leucine, Isoleucine and Valine)
Numerous studies have highlighted the significant therapeutic potential of branched-chain amino acids, particularly in the context of malignancies, predominantly hepatocellular carcinoma [35-39]. In 2011, Yoshi and colleagues conducted a prospective controlled clinical study involving 93 hepatocellular carcinoma patients receiving treatment. Their research aimed to evaluate the effects of daily intake of branched-chain amino acids (Livact 12g/day) on patients with HCC and insulin resistance. After a 60-month follow-up period, the study observed a significant reduction in the recurrence of hepatocellular carcinoma in patients with insulin resistance, along with positive changes in factors like VEGF and sVEGFR2. This suggests that BCAA could be a promising strategy for inhibiting angiogenesis, which plays a crucial role in providing nutrients for tumor growth, especially in cases of recurrent HCC following curative treatment. due to high chances of intrahepatic metastasis amplified by angiogenesis which is potentially vital for nutrients supply to for tumor growth [35,39]. Furthermore, in another retrospective study conducted by Kakazu and colleagues in 2013, the importance of supplementing with branched-chain amino acids in patients with hepatocellular carcinoma, whether newly diagnosed or experiencing recurrence, was investigated. This study specifically examined the relationship between BCAA supplementation and changes in albumin levels in patients with compensated liver cirrhosis. A total of 89 patients were enrolled, with 29 in the intervention group receiving BCAA and 60 in the control group. The results were notably significant, as the group that received branched-chain amino acids experienced increased albumin levels, suggesting their potential benefit in reducing the risk of ascites and hepatic encephalopathy among patients with hepatocellular carcinoma [36].
More over Recent research findings indicate that branchedchain amino acids (BCAA) can mitigate the onset of hepatocellular carcinoma (HCC) in individuals with both obesity and hepatitis C virus infection and cormobid diseases like Diabetes by improving insulin resistance [38]. Furthermore, this research revealed that BCAA possesses valuable properties such as anti-fibrotic, anti-angiogenic, and anti-apoptotic effects. These attributes are crucial in halting the advancement and even reversing the growth and development of tumors. These findings offer a basis for considering BCAA treatment for HCC patients with liver cirrhosis as a means of anti-fibrotic therapy and chemoprevention. Additionally, the reduction in ammonia levels resulting from BCAA treatment could be harnessed for managing Hepatic Encephalopathy [38].
Despite the potential significant therapeutic benefits outlined in the aforementioned studies, the utilization of branched-chain amino acids (BCAA) in most healthcare facilities within Sub-Saharan countries, including Tanzania, remains extremely limited. This is the case despite their established safety and vital role as chemopreventive agents for high-risk groups, including obese individuals, diabetic patients, and chronic alcoholics. They also serve as replacement therapy and play a crucial role in managing active diseases like Hepatocellular carcinoma and hepatic cirrhosis, protecting patients from complications such as ascites and hepatic encephalopathy. Moreover, BCAA can readily find application in clinical settings, given their extensive use in various therapeutic contexts. Several factors may contribute to this situation, including a lack of widely disseminated therapeutic information to promote its use in this context, limited health insurance coverage, and availability constraints. However, considering the significant morbidity and mortality rates associated with chronic diseases, the clinical therapeutic benefits of branched-chain amino acids would far outweigh these hindrances.
Ursodeoxycolic Acid
Ursodeoxycholic acid (UDCA) is a secondary bile acid with a wellestablished role in the treatment of certain hepatobiliary disorders. In recent years, research has explored the potential chemopreventive properties of UDCA in various types of cancer. In this context randomized controlled studies were used to synthesize evidence on the effectiveness of ursodeoxycholic acid as valuable chemoprevention agent and we further went on the clinical laboratory investigative studies to obtain possible mechanisms that ursodeoxycholic acid plays so as to insinuate its paramount importance in chemoprevention.
Several studies have highlighted the potential mechanisms through which ursodeoxycholic acids plays in chemoprevention in various types of malignancies including Colorectal Cancer, Hepatocellular Carcinoma, Esophageal Cancer, Pancreatic Cancer, Breast Cancer, Prostate Cancer. Kim et al., 2017 [40] reported form their study that ursodeoxycholic acid reduces colon cancer cells proliferation through antioxidant effects by scavenging reactive oxygen species. Moreover, it was found that ursodeoxycholic acid inhibited G1/S and G2/M transition phases in colon cancer cells and also it significantly increased expression of cell cycle inhibitor such as p27 and p21.
Also, as it is well known that massive and uncontrolled inflammatory conditions could predispose to tumor initiation, development or progression. Ward et al., 2017 likewise reported from their clinical laboratory study that ursodeoxycholic acid and its active metabolite which is more potent in actions could serve as potential antii-inflammatory agent hence could potentially serve as a chemopreventive agent against colon cancer (70).Also, Huo x et al., 2011 reported that this hydrophilic bile acid blocks the harmful effects of deoxycholic hydrophilic bile acid that usually induces DNA damage while inducing apoptotic resistance. Despite the potential significant therapeutic benefits outlined in the aforementioned studies, the utilization of branched-chain amino acids (BCAA) in most healthcare facilities within Sub-Saharan countries, including Tanzania, remains extremely limited. This is the case despite their established safety and vital role as chemopreventive agents for highrisk groups, including obese individuals, diabetic patients, and chronic alcoholics. They also serve as replacement therapy and play a crucial role in managing active diseases like Hepatocellular carcinoma and hepatic cirrhosis, protecting patients from complications such as ascites and hepatic encephalopathy. Moreover, BCAA can readily find application in clinical settings, given their extensive use in various therapeutic contexts. Several factors may contribute to this situation, including a lack of widely disseminated therapeutic information to promote its use in this context, limited health insurance coverage, and availability constraints. However, considering the significant morbidity and mortality rates associated with chronic diseases, the clinical therapeutic benefits of branched-chain amino acids would far outweigh these hindrances.
Vitamin D3
Vitamin D3 has undergone extensive research to evaluate its potential in cancer prevention, with a specific focus on colorectal cancer. Vitamin D3's role in regulating cell growth and inhibiting abnormal cell development makes it an intriguing subject of study. Research and clinical trials have explored its possible advantages in a range of cancers, including skin melanoma, cervical cancer, prostate cancer, and breast cancer. Several primary researches and randomized clinical trials were taken to synthesize the evidence behind this wonderful therapeutic benefit of Vitamin D3 in numerous malignancies, including skin melanoma cervical, prostate and breast cancer and furthermore some studies have indicated its potential role in reducing severity of pelvic pai once combined with Omega-3-Fatty [43,44].
One notable study conducted by Johansson et al. in 2021 centered on the chemopreventive properties of Vitamin D3 in melanoma patients. This randomized trial involved 104 study participants were randomized to receive an oral solution every 50 days, containing 100,000 IU of vitamin D3 (an average of 2000IU/day) [43]. The parameters that were used as a primary end points was the Breslow value, whereby the patients that were diagnosed of melanoma skin cancer with Breslow value of less than 3 highly benefited from vitamin D supplementation as serum 1 alpha,25 (OH) Vitamin D3 was found to raise and thus suggesting that relapse could be highly reduced among these patients .Contrary to the patients with Breslow value of greater than 3 benefited less from Vitamin D supplementation as it was found that serum levels of Vitamin D3 supplementation was not significant and this might be due to the scientific reasons that cancer decreased expression rate of vitamin D related proteins associated with cancer development and control, as well as other triggered changes in RNA expression of vitamin D targeted genes contributing to vitamin D status [43]. The mechanism behind these findings could be related to immunomodulatory effects of vitamin D hence serving as evaluable agent in fighting against skin melanoma. Thus, from these findings we postulate that supplementation of vitamin D in patients receiving immunotherapy would highly benefit the low-risk group of carcinomas, here referred as those patients with Breslow value of less than 3mm while those with Breslow value greater than would benefit less.
Additionally, a study by [44,45] reported findings on the longterm Vitamin D supplementation in cervical intraepithelial neoplasia in their randomized, double blind controlled trial, Whereby 58 patients diagnosed with cervical intraepithelial neoplasia were recruited and then randomized and the intervention group (29 patients) were administered with 50,000 IU of vitamin D3 and then prospectively followed for a period of six months and comparison were made to the placebo group (29patients). The study reported that Vitamin D supplementation every two weeks at a dosage of 50000 IU significantly reduced progression of cervical intraepithelial neoplasia, the study concluded that these significant therapeutic effects was achieved through Vitamin D antioxidant effects by raising GSH levels, anti-inflammatory through regulation of inflammatory biomarkers, and Overall TAC. These findings are in consistence to the aforementioned researches [43,44]. Despite the potential therapeutic benefits of Vitamin D in cancer, its utilization remains limited, particularly in countries like Tanzania. Factors such as the absence of health insurance coverage and insufficient research may contribute to the underutilization of this promising avenue of treatment."
One notable study conducted by Johansson et al. in 2021 centered on the chemopreventive properties of Vitamin D3 in melanoma patients. This randomized trial involved 104 participants. The findings indicated that Vitamin D3 supplementation delivered substantial benefits to patients with a Breslow value of less than 3mm, reducing the risk of relapse. However, patients with a Breslow value greater than 3mm experienced fewer benefits, possibly due to alterations in RNA expression associated with cancer progression and control. Despite the potential therapeutic benefits of Vitamin D in cancer, its utilization remains limited, particularly in countries like Tanzania. Factors such as the absence of health insurance coverage and insufficient research may contribute to the underutilization of this promising avenue of treatment."
Carotenoids and Tocopherols
Carotenoids and tocopherols, which include vitamin E compounds, beta carotene, zeaxanthins,, have been extensively studied for their potential roles in cancer chemoprevention. These phytochemicals, commonly found in fruits, vegetables, and nuts, exhibit antioxidant properties that contribute to their anti-cancer effects.. They can neutralize reactive oxygen species (ROS), protecting cells from oxidative damage that may lead to cancer initiation. Additionally, carotenoids have been associated with the regulation of cell growth, immune function, and inflammation, all of which are relevant in cancer development. A prospective controlled trial study conducted by Antwi et al. conducted to evaluate the association between the carotenoids intake and risk of prostate cancer development [46], in their study the intervention group were administered with daily lycopene ranging from 15mg/kg/day to 120mg/kg/day of tomato sauce and followed for a period of 3months and 6 months to evaluate the results in relation to the control group. Consequently, Results from this analysis showed that participants with higher cis-lutein/ zeaxanthin level at 3 months had statistically lower mean PSA level at 3 months. Additionally, participants with higher plasma levels of α tocopherol,cryptoxanthin, all trans-lycopene, and higher antioxidant score at 3 months, had signifificantly lower mean PSA level at 6 months. Furthermore, there are some studies that have been conducted to evaluate the mechanism behind that correlate with chemoprevention of these carotenoids, which includes inhibition of androgen sensitive LNCaP Prostate tumor cell growth, down regulation of androgen activity and anti-inflammatory activities [47-49] cancer cells. Also, another Population based case-controlled study of 952 rectal cancer cases and 1205 controls reported that the carotenoids used at different doses that includes lutein 1400mcg -4158mcg, beta-carotene at a dosage range of 2137mcg to 7493mcg, in combination with vitamin C and Vitamin E at a dosage range of 7.7mg -18.8mg and vitamin C at a dosage not less than 200mg were significantly associated with decreased risk for rectal cancers mostly in women [50]. Furthermore, there are some studies that have reported that these carotenoids not only could be used in rectal cancers but could also be used to mitigate nephrotoxicity induced by cytotoxic agents [51].
All these findings have provided valuable information on the potential chemoprevention effects of carotenoids on cancer chemoprevention but amid of these findings still their use in clinical settings is not commonly realised as therapeutic approach in patient management. This could be brought by some various factors including awareness, health insurance coverage and lack of extensive studies have been conducted to bring to stage the beneficial therapeutic effects of these agents among cancer patients. Furthermore, till to date are some few studies conducted to evaluate the chemopreventive effects of these agents.
Conclusion
Based on the study's findings, we recommend further research, including large-scale clinical trials, to validate the use of antioxidants and free radical scavengers in cancer prevention and treatment. Additionally, efforts should be made to raise awareness among healthcare professionals and the community regarding the potential benefits of these compounds in fighting cancer and other forms of Non communicable disease.
Supporting Information
SI checklist, PRISMA checklist, PRISMA, Preferred reporting items for systematic reviews and meta-Analyses.
Acknowledgements
We would like to thank the following for their contribution:
Author Contributions
Conceptualization, M.M. M and K.B.Z.; methodology, M.M.M., K.B.Z., S.B.; formal analysis and data curation, M.M.M, K.B.Z. ; writing—original draft preparation, M.M.M; writing—review and editing, S.B., M.M.M, K.B.Z. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
References
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014; 94: 329–354.
- Young B, Purcell C, Kuang YQ, Charette N, Dupré DJ. Superoxide dismutase 1 regulation of CXCR4-mediated signaling in prostate cancer cells is dependent on cellular oxidative state. Cellular Physiology and Biochemistry. 2015; 37: 2071–2084.
- Liou GY, Storz P. Reactive oxygen species in cancer. Vol. 44, Free Radical Research. 2010: 479–496.
- Boivin B, Zhang S, Arbiser JL, Zhang ZY, Tonks NK. A modified cysteinyllabeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc Natl Acad Sci USA. 2008; 105: 9959–9964.
- Ward PS, Thompson CB. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell. Cell Press; 2012; 21: 297– 308.
- Cosentino C, Grieco D, Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO Journal. 2011; 30: 546–555.
- Weitzman SA, Weitberg AB, Clark EP, Stossel TP. Phagocytes as carcinogens: Malignant transformation produced by human neutrophils. Science (1979). 1985; 227: 1231–1233.
- Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: Reconciling chemical mechanisms and biological fates. International Journal of Cancer. 2011; 128: 1999–2009.
- He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cellular Physiology and Biochemistry. S. Karger AG; 2017; 44: 532–553.
- Slezák J, Kura B, Frimmel K, Zálešák M, Ravingerová T, Viczenczová C, et al. Preventive and therapeutic application of molecular hydrogen in situations with excessive production of free radicals. Physiological Research. Czech Academy of Sciences; 2016; 65: S11–28.
- Indo HP, Yen HC, Nakanishi I, Matsumoto KI, Tamura M, Nagano Y, et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. Journal of Clinical Biochemistry and Nutrition. The Society for Free Radical Research Japan. 2015; 56: 1–7.
- Oršolić N, Jazvinšćak Jembrek M. Molecular and Cellular Mechanisms of Propolis and Its Polyphenolic Compounds against Cancer. International Journal of Molecular Sciences. MDPI; 2022. 23.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ. BMJ Publishing Group; 2021. 372.
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. In: Research Synthesis Methods. John Wiley and Sons Ltd. 2021: 55–61.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ. BMJ Publishing Group; 2021; 372.
- Polireddy K, Dong R, Reed G, Yu J, Chen P, Williamson S, et al. High dose parenteral ascorbate inhibited pancreatic cancer growth and metastasis: Mechanisms and a phase I/IIa study. Sci Rep. 2017; 7.
- Wang L, Sesso HD, Glynn RJ, Christen WG, Bubes V, Manson JAE, et al. Vitamin E and C supplementation and risk of cancer in men: Posttrial followup in the Physicians’ Health Study II randomized trial. American Journal of Clinical Nutrition. 2014; 100: 915–923.
- Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ, Levine M. Intravenously administered vitamin C as cancer therapy: Three cases. CMAJ Canadian Medical Association Journal. 2006; 174: 937–942.
- Gaman AM, Buga AM, Gaman MA, Popa-Wagner A. The role of oxidative stress and the effects of antioxidants on the incidence of infectious complications of chronic lymphocytic leukemia. Oxid Med Cell Longev. 2014; 2014.
- Amini L, Chekini R, Nateghi MR, Haghani H, Jamialahmadi T, Sathyapalan T, et al. The Effect of Combined Vitamin C and Vitamin e Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res Manag. 2021; 2021.
- Durak Y, Kokcu A, Kefeli M, Bildircin D, Çelik H, Alper T. Effect of vitamin C on the growth of experimentally induced endometriotic cysts. Journal of Obstetrics and Gynaecology Research. 2013; 39.
- Nadă ES, Bratu OG, Mihai D, Brătilă E. Alternative Treatment in Endometriosis. Research and Science Today. 2019; (Supplement 2).
- S. G, N. M, D. S, A. C, A. A. Oxidative stress and its role in female infertility and assisted reproduction: Clinical implications. Int J Fertil Steril. 2009; 2.
- Parazzini F, Viganò P, Candiani M, Fedele L. Diet and endometriosis risk: A literature review. Reproductive BioMedicine Online. 2013; 26.
- Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. New England Journal of Medicine. 2015; 373: 1618–1626.
- Minocha R, Martin AJ, Chen AC, Scolyer RA, Lyons G, McKenzie CA, et al. Nicotinamide for skin cancer chemoprevention: effects on tumour immune infiltrates and melanoma biology. Australasian journal of dermatology. 2017; 58.
- Thompson BC, Halliday GM, Damian DL. Nicotinamide enhances repair of arsenic and ultraviolet radiation-induced DNA damage in HaCaT keratinocytes and Ex Vivo human skin. PLoS One. 2015; 10.
- Hwang S, Zhao C, Fernandez-Penas P. Meta-analysis: interventions for preventing nonmelanoma skin cancers (NMSC) in patients with one or more previous NMSC. Australasian journal of dermatology. 2016; 57.
- McKenzie CA, Scolyer RA, Damian DL. Pathological re-classification of basal cell carcinomas from the ontrac skin cancer chemoprevention study and the new who skin blue book. Pathology. 2019; 51.
- Lee BJ, Tseng YF, Yen CH, Lin PT. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr J. 2013; 12.
- Liu HT, Cheng S Bin, Huang YC, Huang YT, Lin PT. Coenzyme Q10 and oxidative stress: Inflammation status in hepatocellular carcinoma patients after surgery. Nutrients. 2017; 9.
- Chan MY, Lee BJ, Chang PS, Hsiao HY, Hsu LP, Chang CH, et al. The risks of ubiquinone and β-carotene deficiency and metabolic disorders in patients with oral cancer. BMC Cancer. 2020; 20.
- Rauchová H, Battino M, Fato R, Lenaz G, Drahota Z. Coenzyme Q-pool function in glycerol-3-phosphate oxidation in hamster brown adipose tissue mitochondria. J Bioenerg Biomembr. 1992; 24.
- Yoneda T, Tomofuji T, Kawabata Y, Ekuni D, Azuma T, Kataoka K, et al. Application of coenzyme Q10 for accelerating soft tissue wound healing after tooth extraction in rats. Nutrients. 2014; 6.
- Yoshiji H, Noguchi R, Ikenaka Y, Kaji K, Aihara Y, Yamazaki M, et al. Combination of branched-chain amino acids and angiotensin-converting enzyme inhibitor suppresses the cumulative recurrence of hepatocellular carcinoma: A randomized control trial. Oncol Rep. 2011; 26.
- Kakazu E, Kondo Y, Kogure T, Ninomiya M, Kimura O, Iwata T, et al. Supplementation of branched-chain amino acids maintains the serum albumin level in the course of hepatocellular carcinoma recurrence. Tohoku Journal of Experimental Medicine. 2013; 230.
- Lam VW, Poon RT. Role of branched-chain amino acids in management of cirrhosis and hepatocellular carcinoma. In: Hepatology Research. 2008.
- Cha JH, Bae SH, Kim HL, Park NR, Choi ES, Jung ES, et al. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 2013; 8.
- Yoshiji H, Noguchi R, Namisaki T, Moriya K, Kitade M, Aihara Y, et al. Branched-chain amino acids suppress the cumulative recurrence of hepatocellular carcinoma under conditions of insulin-resistance. Oncol Rep. 2013; 30: 545–552.
- Kim EK, Cho JH, Kim EJ, Kim YJ. Ursodeoxycholic acid inhibits the proliferation of colon cancer cells by regulating oxidative stress and cancer stem-like cell growth. PLoS One. 2017; 12.
- Ward JBJ, Lajczak NK, Kelly OB, O’Dwyer AM, Giddam AK, Ní Gabhann J, et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am J Physiol Gastrointest Liver Physiol. 2017; 312.
- Huo X, Juergens S, Zhang X, Rezaei D, Yu C, Strauch ED, et al. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-κ{green}b activation in benign barrett’s epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011; 301.
- Johansson H, Spadola G, Tosti G, Mandalà M, Minisini AM, Queirolo P, et al. Vitamin d supplementation and disease-free survival in stage ii melanoma: A randomized placebo controlled trial. Nutrients. 2021; 13.
- Vahedpoor Z, Jamilian M, Bahmani F, Aghadavod E, Karamali M, Kashanian M, et al. Effects of Long-Term Vitamin D Supplementation on Regression and Metabolic Status of Cervical Intraepithelial Neoplasia: a Randomized, Double-Blind, Placebo-Controlled Trial. Horm Cancer. 2017; 8: 58–67.
- Vahedpoor Z, Jamilian M, Bahmani F, Aghadavod E, Karamali M, Kashanian M, et al. Effects of Long-Term Vitamin D Supplementation on Regression and Metabolic Status of Cervical Intraepithelial Neoplasia: a Randomized, Double-Blind, Placebo-Controlled Trial. Horm Cancer. 2017; 8: 58–67.
- Antwi SO, Steck SE, Zhang H, Stumm L, Zhang J, Hurley TG, et al. Plasma carotenoids and tocopherols in relation to prostate-specific antigen (PSA) levels among men with biochemical recurrence of prostate cancer. Cancer Epidemiol. 2015; 39: 752–762.
- Lycopene and Vitamin E interfere with autocrine: paracrine loops in the Dunning prostate cancer model. FASEB J. 2004.
- Willis MS, Wians FH. The role of nutrition in preventing prostate cancer: A review of the proposed mechanism of action of various dietary substances. Vol. 330, Clinica Chimica Acta. Elsevier; 2003: 57–83.
- Wertz K, Siler U, Goralczyk R. Lycopene: Modes of action to promote prostate health. Vol. 430, Archives of Biochemistry and Biophysics. Academic Press Inc. 2004: 127–134.
- Murtaugh MA, Ma KN, Benson J, Curtin K, Caan B, Slattery ML. Antioxidants, Carotenoids, and Risk of Rectal Cancer. Am J Epidemiol. 2004; 159: 32–41.
- Sahin K, Tuzcu M, Sahin N, Ali S, Kucuk O. Nrf2/HO-1 signaling pathway may be the prime target for chemoprevention of cisplatin-induced nephrotoxicity by lycopene. Food and Chemical Toxicology. 2010; 48: 2670–2674.