
Research Article
Austin J Cancer Clin Res. 2025; 12(1): 1117.
Point-Of-Care Testing with Xpert HPV for Detecting Human Papillomavirus Infections in Women, Both with and without Invasive Cervical Cancer
Muthaiah M1, Brammacharry U2, Ramachandra V2*, Mani BR3 and VidyaRaj CK4
1Department of Microbiology, State TB Training and Demonstration Centre, Intermediate Reference Laboratory, Government Hospital for Chest Diseases, Puducherry, India
2Institute of Basic Medical Sciences, University of Madras, Chennai, Tamil Nadu, India
3Department of Biochemistry, Queen Mary’s College, Madras, Tamil Nadu, India
4Department of Microbiology, Balaji Vidyapeeth (Deemed to be University) Puduchery, India
*Corresponding author: Venkateswari Ramachandra, Department of Medical Biochemistry, Institute of Basic Medical Sciences, University of Madras, Chennai, Tamil Nadu, India Tel: +91 9944737597; Email: cdirlpd@gmail.com
Received: April 12, 2025 Accepted: April 24, 2025 Published: April 28, 2025
Abstract
Human papillomavirus (HPV) infection is a major cause of invasive cervical cancer. The prevalence, risk factors, and genotype distribution among women in Puducherry with and without invasive cervical cancer (ICC) are not well understood. A total of 808 cervical specimens were collected for HPV detection and genotyping using the Xpert HPV assay. Clinical and sociodemographic data were gathered and analysed with statistical methods. The Xpert HPV test shows a sensitivity of 90.2% and specificity of 99.74%, with a positive predictive value of 93.55% and a negative predictive value of 99.61%, leading to an overall accuracy of 99.80%, surpassing the Pap smear test. In our study of 808 patients, 3.8% tested positive for Human papillomavirus infection. Among positive samples, HPV16 was the most common genotype at 80.6%, followed by HPV18 at 9.8%, HPV33 at 6.5%, and HPV45 at 3.2%. Our data indicate that HPV16 infection may be the main cause of ICC among women living in Puducherry. These findings could establish a foundation for creating an effective screening and vaccination strategy for the region.
Keywords: Xpert HPV; Human Papillomavirus; Cervical cancer; Papanicolaou
Abbreviations
HPV: Human Papillomavirus; ICC: Invasive Cervical Cancer; Pap: Papanicolaou; OPD: Out Patients Department; HR: High-Risk; ASCUS: Atypical Squamous Cells of Undetermined Significance; OR: Odds Ratio; CI: Confidence Interval; HSIL: High-grade Squamous Intraepithelial Lesions; WHO: World Health Organization; LSIL: Low-grade Squamous Intraepithelial Lesions; ISCC: Invasive Squamous Cell Carcinoma.
Introduction
Cervical cancer is the fourth most common cancer among women worldwide, posing a significant threat to female health. Persistent infection with high-risk human papillomavirus (HPV) has been identified as the primary cause of cervical cancer. This type of cancer develops in the cervix, the lower part of the uterus. Approximately 99% of cervical cancer cases are linked to infections caused by highrisk types of human papillomavirus, which are commonly transmitted through sexual contact. In 2020, there were approximately 604,000 new cases and 342,000 deaths, with 90% occurring in low- and middle-income countries, where public health access is limited [1]. Women with HIV are six times more likely to develop cervical cancer, contributing to 5% of cases. Asia accounts for over 58% of cases and deaths, particularly in China and India. Persistent infection with high-risk HPV types, such as HPV16 and HPV18, is the main cause of cervical cancer. In China, the infection rate for high-risk HPV is about 14.3%. Preventive measures, including vaccination and screening, are effective but insufficiently accessible in low-income regions, leading to later diagnoses and higher mortality. The World Health Assembly adopted a Global Strategy for cervical cancer elimination in 2022 [2].
India has a population of 511.4 million women aged 15 years and older who are at risk of developing cervical cancer. Current estimates indicate that every year, 123907 women are diagnosed with cervical cancer, and 77348 die from the disease. Cervical cancer is the second most common cancer among women in India and also ranks as the second most frequent cancer among women between the ages of 15 and 44. Approximately 5.0% of women in the general population are estimated to be infected with cervical HPV-16/18 at any given time, and 83.2% of invasive cervical cancers are attributed to HPVs 16 or 18 [3-4]. India has the highest burden of cervical cancer in Asia, followed closely by China, as reported in a recent study published in The Lancet. Of the 40% of global cervical cancer deaths, 23% occurred in India and 17% in China.
The World Health Assembly has adopted a global policy aimed at eliminating cervical cancer as a public health issue. This policy outlines a comprehensive strategy that includes effective screening, prevention, and treatment of pre-cancerous lesions, early diagnosis of cancer, and programs for managing invasive cervical cancer, including palliative care. Current tests for cervical cancer often begin with an abnormal result from an HPV or Papanicolaou (Pap) test, which may lead to further testing to determine the presence of cancer or pre-cancer. It is important to note that the Papanicolaou (Pap) and HPV tests are screening tests and not diagnostic tests; they cannot definitively determine if cervical cancer is present. An abnormal result may indicate the need for additional testing to assess whether cancer or a pre-cancer is present. This comprehensive approach requires ongoing monitoring of cervical cancer prevention and control, focusing on epidemiology, risk factors, screening methods, and effective confirmation through genetic testing, all crucial for paving the way toward the elimination of cervical cancer [5]. With early detection and appropriate care, cervical cancer can be one of the most treatable cancers. A comprehensive approach to prevention, screening, and treatment is essential to eliminate cervical cancer as a public health issue within a generation. This study aims to assess the potential risk factors associated with cervical cancer in the South Indian population to understand the regional variations.
Materials and Methods
Study Setting
Our cross-sectional study is conducted in rural Puducherry, southern India. Residents of rural areas are socioeconomically disadvantaged and lack easy access to cervical cancer screening programs that are available in some regions of the country. This study includes married women and non-pregnant women of eligible age who have an intact uterus, have not been screened previously, and are willing to provide informed written consent. Women diagnosed with cervical pre-cancer or cancer are not eligible to participate in this study.
Sample Size and Sampling Procedure: The sample size is calculated using the assumption of a 5% margin of error and a 95% confidence interval, with a prevalence of 20%, employing a single population proportion formula.
Sample Size and Sampling Procedure: The sample size is calculated using the assumption of a 5% margin of error and a 95% confidence interval, with a prevalence of 20%, employing a single population proportion formula.
Sample Collection and Transportation to the Laboratory
At the Community and Primary Health Centre, a staff nurse explained the self-sampling and assisted sampling processes to each participating woman. They used a Diogenes soft conical brush (Qiagen, Gaithersburg, MD) for this purpose. Two brush specimens, referred to as Specimen A and Specimen B, were placed immediately into separate labelled tubes containing Digene transport medium. After the collection was complete for the day, the samples were transported to the laboratory using a cold chain mechanism.
Laboratory Diagnosis
Conventional Pap smear: All participants underwent as per speculum examination, followed by the collection of cervical samples using an Ayers spatula. A thin smear of exfoliated cervical cells was prepared on a glass slide, dipped into a box containing 3% alcohol, and then transported to the pathology department of the institute. Later, these samples were examined under a microscope for any epithelial abnormalities and reported according to the Bethesda 2001 classification system. The findings ranged from atypical squamous cells of undetermined significance (ASCUS) and atypical squamous cells that cannot exclude high-grade squamous intraepithelial lesions (ASC-H), to low-grade squamous intraepithelial lesions (LSIL), highgrade squamous intraepithelial lesions (HSIL), or invasive squamous cell carcinoma (ISCC) [6].
Human Papillomavirus Testing: The cervical specimens collected in PreservCyt Solution were transported to the Intermediate Reference Laboratory using a cold chain mechanism, with a temperature range of 2–30 ºC. In the event of contamination in the work area or on equipment with samples or controls, thoroughly clean the contaminated area using a 1:10 dilution of household chlorine bleach, followed by a 70% ethanol or 70% isopropanol solution. Ensure that work surfaces are completely dry before proceeding. Cliniciancollected and self-collected samples were tested using the Xpert HPV (CE-IVD) on the GeneXpert system, following manufacturer instructions.
This real-time PCR assay detects 14 types of high-risk HPV DNA across five channels: HPV 16; HPV 18 and/or 45; HPV 31, 33, 35, 52, and/or 58 (P3); HPV 51 and/or 59 (P4); and HPV 39, 56, 66, and/or 68 (P5). To process the sample, first open the lid of the HPV cartridge. Gently mix the sample by either inverting the sample vial 8 to 10 times or briefly vortexing it at half speed for 5 seconds. Next, unwrap the transfer pipette and open the sample vial. Squeeze the bulb of the transfer pipette, insert it into the vial, and then release the bulb to fill the pipette up to the 1 mL line. Turn on the GeneXpert Instrument System and then expel the contents of the pipette into the sample chamber of the HPV cartridge. Finally, close the cartridge lid. First, turn on the GeneXpert Instrument System. Log in to the software using your username and password. Optionally, scan or enter the Patient ID, ensuring the entry is correct since it links to the test results. Next, scan or enter the Sample ID, also double-checking for accuracy. Then, scan the barcode on the Xpert HPV cartridge, which will prompt the Create Test window. The software will automatically populate the following fields: Assay, Reagent Lot ID, Cartridge Serial Number (SN), and Expiration Date. For the GeneXpert System, the cartridge is placed on the conveyor belt. The cartridge is then automatically loaded, and the test runs. Once the test is completed, the used cartridge is placed into the waste container. The GeneXpert Instrument System interprets the results based on the measured fluorescent signals and embedded calculation algorithms. These results are displayed in the Test Result tab of the View Results window [7]. The Xpert HPV test provides results for HPV targets, which are detailed according to the interpretations shown in Table 1.
Demographic variables
Total patients
HPV (+)ve Cases
HPV (-)ve Cases
Odds ratio
p value
n(808)
n(31)3.8%
n(777)96.2%
(95% CI)
Age
< 45
266(32.9%)
8(1.0%)
258(31.9%)
≥ 45
542(67.1%)
23(2.8%)
519(64.3%)
1.43(0.63-3.24)
0.3923
Socioeconomic status
High
157(19.4%)
5(0.9%)
152(18.5%)
low and middle
651(80.6%)
26(3.0%)
625(77.6%)
1.26(0.48-3.35)
0.6364
Education
Literate
565(69.9%)
9(1.1%)
556(68.8%)
Illiterate
243(30.1%)
22(2.7%)
221(27.4%)
6.15(2.79-13.56)
0.0001
Married for
≤ 10 years
245(30.3%)
11(1.3%)
234(28.9%)
>10 years
563(69.7%)
20(2.5%)
543(67.3%)
0.78(0.37-1.66)
0.5246
Residential area
Urban
175(21.7%)
6(0.7%)
169(20.9%)
Rural
633(78.3%)
25(3.1%)
608(75.3%)
1.16(0.47-2.87)
0.7511
Table 1: Demographic and social factors in relation to HPV status.
Results
Pap smear
Value: (95%CI)
Positive
Negative
Sensitivity
Specificity
PPV
NPV
Accuracy
GeneXpert HPV test
Positive
29
2
90.62% (74.98-98.02)
99.74% (99.07-99/97)
93.55% (78.34-98.31)
99.61% (98.87-99.87)
99.38% (98.56-99.80)
Negative
3
774
Table 2: Correlation between HPV positivity detected by GeneXpert and the results of Pap smears reports.
Statistical Analysis
Data analysis was conducted using MedCalc Software Ltd [8]., specifically the Odds Ratio Calculator (Version 23.1.6) [9], while the meta-analysis was performed using online tools. Categorical variables were presented as percentages and counts. A simple logistic regression analysis was conducted to examine the relationship between the dependent variable (unfavourable treatment outcome) and selected independent variables. Independent factors associated with unfavourable treatment outcomes were identified by analysing statistically significant variables from the univariate analysis through multivariate binary logistic regression analysis. The odds ratio (OR) and 95% confidence intervals (CI) were calculated for each variable. Statistical tests were considered significant with a p-value of less than 0.05.
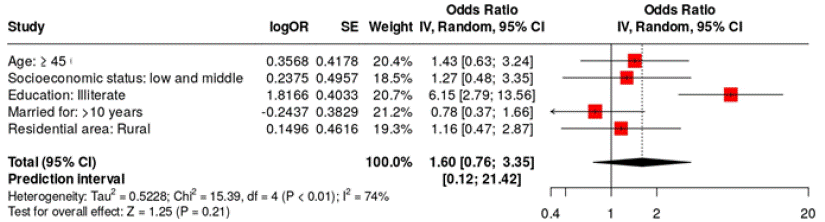
Figure 1: Factors influencing the Human papillomavirus infection.
Results
Clinico-Demographic Characteristics of The Participants and Risk Factor Assessment
The ages of the patients included in this study ranged from 21 to 78 years. Among the 808 women, 542 (67.1%) were aged 45 years or older, and 23 of these women tested positive for HPV. In contrast, only 8 out of 266 women under 45 years old were HPV-positive, which represents 32.9% of that group. A significant portion of the participants, 651 women (80.6%), came from low and middleincome backgrounds, with 26 of these women testing positive for HPV. Conversely, only 5 out of 157 women (19.4%) with higher socioeconomic status were HPV-positive. Additionally, of the 808 women studied, 563 (69.7%) had been married for more than 10 years, and 20 of these women tested positive for HPV. In comparison, 11 out of 245 women (30.3%) who had been married for less than 10 years were HPV-positive. This suggests a strong link between HPV infection and the duration of sexual exposure. Regarding education, 243 women (30.1%) were illiterate, and 22 of these tested positive for HPV. In comparison, only 9 out of 565 literate women (69.9%) were HPV-positive. These findings indicate that factors such as illiteracy are significantly associated with HPV infection. Finally, among the 808 women, 633 (78.3%) lived in rural areas, with 25 of these women testing positive for HPV. In contrast, only 6 out of 175 women (21.7%) living in urban areas were HPV-positive. The prevalence of HPV infection in our study population was 3.8%. Among the samples that tested positive for 31 HPV and were processed for genotyping, the most commonly detected genotype was HPV16, found in 80.6% of the samples (25 out of 31). This was followed by HPV18, which was observed in 9.8% of the samples (3 out of 31). HPV33 and HPV45 were the next most prevalent genotypes, detected in 6.5% (2 out of 31) and 3.2% (1 out of 31) of the samples, respectively.
Correlation between HPV Positivity Detected by Genexpert and the Results of Pap Smears Reports
The geneXpert assay for HPV detection demonstrated a sensitivity of 90.2%, specificity of 99.74%, positive predictive value of 93.55%, negative predictive value of 99.61%, and accuracy of 99.80%, surpassing the Pap smear test. Figure 3 shows a forest plot analysis of five variables that influence HPV infections among the tested cases. The analysis utilized a random effects model with the inverse variance method to compare the odds ratios (OR). The summarized odds ratio was calculated to be 1.72, with a 95% confidence interval ranging from 0.95 to 3.11. The overall effect test did not reveal a significant effect. Additionally, we did not find significant heterogeneity, indicating that the effect sizes across the cohorts were consistent in both magnitude and direction. Patients aged 65 and older had an odds ratio of 1.29 for being infected with HPV compared to those younger than 65. Illiterate patients were 3.91 times more likely to have HPV infection than those who are literate. Additionally, married patients had 1.51 times higher odds of experiencing HPV infection compared to unmarried patients. Furthermore, individuals living in rural areas had 1.91 times higher odds of HPV infection than those residing in urban areas.
Discussion
Puducherry is one of the smallest states in India, covering an area of 294 km² and having an estimated population of 1.616 million. The state faces significant challenges related to public health, awareness, and accessibility. Despite these issues, Puducherry has a well-organized healthcare system that includes nine hospitals, five Community Health Centres, and 21 Primary Health Centres. Our study aims to estimate the prevalence of HPV among asymptomatic women who visited the outpatient department (OPD) of the Primary Health Centre in Puducherry, India. We found that the overall prevalence of HPV infection in our study population was 3.8% (31 out of 808 participants). This is lower than the prevalence reported in Odisha (60.3%) and Bihar (37.3%). Additionally, it is also lower than the figures reported by Sankaranarayanan et al [10]. (10.3%) in Western India and by Franceschi et al [11], in Southern India (9.6%). The literature indicates that there are regional differences in the prevalence of HPV infections, with high-risk HPV strains being the most commonly found in these areas. Multiple studies conducted on cervical cancer specimens from various regions of India have shown that HPV16 is the most prevalent genotype [6]. In our study, an analysis of genotype distribution among women revealed a predominance of high-risk HPV (HR-HPV). In summary, HPV16 was the most prevalent genotype, accounting for 80.6% of cases (25 out of 31). This was followed by HPV18, which represented 9.8% of cases (3 out of 31). These findings are consistent with those reported by Wendland et al., who observed that high-risk HPV infections among women were primarily due to HPV16.
Our study showed low HPV-positivity in women <45 years of age and the incidence increased with increasing age. The patients with > 45 age having higher positivity rate. This study is lined with Pankaj et al [6] and Parvez et al [12], reports in India. The observation that the educated women have the lowest rates of HPV infection suggests that knowledge and awareness campaigns could be beneficial, particularly if they are directed towards pregnant women and those with low education levels in unskilled jobs.
The disparity in HPV infection rates based on educational and employment status highlights the importance of knowledge and empowerment. This aligns with the findings of Onwuamah et al., who assert that education plays a critical role in raising awareness about the dangers of diseases. Our report reveals that HPV prevalence decreases as the education level of women increases, indicating that interventions aimed at enhancing awareness about cervical cancer and its prevention strategies could be highly effective. Additionally, our study found a higher rate of infection among illiterate women working in unskilled jobs.
This suggests two possible implications: first, that knowledge may not be translating into protective actions; and second, that individuals in unskilled positions, often earning low wages, may be driven by economic desperation to engage in risky sexual behaviours, thereby increasing their vulnerability to infections. To address these issues, interventions focused on improving knowledge and empowering women, particularly through financial empowerment, may help them better protect themselves and reduce their risk of infection. Further, our study has reported staying in rural areas, poor socioeconomic status and illiteracy to be associated with higher HPV positivity, which are lined with reports of WHO [13]. Similarly, most of the HPV-positive participants in the research we conducted were from rural areas with most of them being unemployed and from the lower socioeconomic status. However, there was no statistically significant association found between these socio- demographic determinants and HPV infection in our study.
Human papillomavirus testing using the Xpert system is conducted with a single integrated cartridge. This cartridge contains the necessary reagents for DNA extraction, as well as primers and probes for the amplification and detection of HPV DNA. The resources required for performing HPV testing, both in terms of laboratory infrastructure and human expertise, are limited. Therefore, the Xpert HPV test may be particularly beneficial in remote and lowresource countries, as it can be used effectively as a point-of-care test. Although our study involved a small cohort, the ongoing nature of the research allows us to observe trends in HPV infection in this region over time. This information can be valuable for health policy and planning. Estimating HPV infection rates and associated cervical cancer cases among women throughout the state could help in developing an evidence-based strategy for effective disease prevention at the national level.
Conclusion
Our study showed 3.8% HPV prevalence in this cohort study and HPV16 was the most prevalent genotype among women. The Xpert HPV test shows a sensitivity of 90.2% and specificity of 99.74%, with a positive predictive value of 93.55% and a negative predictive value of 99.61%, leading to an overall accuracy of 99.80%, surpassing the Pap smear test. In our study of 808 patients, 3.8% tested positive for HPV. Among positive samples, HPV16 was the most common genotype at 80.6%, followed by HPV18 at 9.8%, HPV33 at 6.5%, and HPV45 at 3.2%. Our data indicate that HPV16 infection may be the main cause of ICC among women living in Puducherry. These findings could establish a foundation for creating an effective screening and vaccination strategy for the region.
Ethical Approval and Consent to Participate
This retrospective study received approval from the Ethics and Scientific Review Committee of the General Hospital Institute in Pondicherry (Approval No. GHIEC/2021/204, dated 18-11-2021), which waived informed consent. The research followed the ethical principles of the Helsinki Declaration, and all data were kept confidential.
Availability of Data and Material
All primary and secondary data are available with the corresponding author and in the Nikshay portal, Government of India. The datasets generated during this study are not publicly accessible but can be requested from the corresponding author.
Contributors
All authors contributed to the conception and design of the study. UB, VR, BRM and CKV all participated in data analysis and interpretation. MM drafted the manuscript, and all authors contributed to revisions and approved the final version.
Acknowledgments
We thank the staff at the Intermediate Reference Laboratory in Puducherry and the medical officers at the TB treatment and primary health centres for their invaluable support during data collection.
References
- Johnson M, Wakefield C, Garthe K. Qualitative socioecological factors of cervical cancer screening use among transgender men. Prev Med Rep. 2020; 17: 101052.
- Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva: World Health Organization; 2022.
- Ramamoorthy T, Sathishkumar K, Das P, Sudarshan KL, Mathur P. Epidemiology of human papillomavirus related cancers in India: findings from the National Cancer Registry Programme. E cancer medical science. 2022; 16: 1444.
- Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. 2023; 11: e197–206.
- Wendland EM, Villa LL, Unger ER, Domingues CM, Benzaken AS; POPBrazil Study Group. Prevalence of HPV infection among sexually active adolescents and young adults in Brazil: The POP-Brazil Study. Sci Rep. 2020; 10: 4920.
- Pankaj S, Rani J, Kumari P, Abhilashi K, Choudhary V, Kumari S, et al. Prevalence, risk factors and genotype distribution of human papillomavirus infection among women with and without invasive cervical cancer: Findings from a hospital-based study in Bihar, India. Natl Med J India. 2024; 37: 13-17.
- Admase A, Joshi S, Borse R, et al. Challenges with the use of Xpert HPV as a screening tool for oral HPV among people living with HIV (PLHIV): experiences from Pune, India. BMC Infect Dis. 2023; 23: 233.
- MedCalc: MedCalc’s Relative risk calculator. MedCalc Software Ltd, 2024.
- Fekete JT, Gyorffy B. MetaAnalysisOnline.com: an Online Tool for the Rapid Meta-Analysis of Clinical and Epidemiological Studies. J Med Internet Res. 2025.
- Sankaranarayanan R, Bhatla N, Gravitt PE, Basu P, Esmy PO, Ashrafunnessa KS, et al. Human papillomavirus infection and cervical cancer prevention in India, Bangladesh, Sri Lanka and Nepal. Vaccine. 2008; 12: M43-52.
- Franceschi S, Plummer M, Clifford G, et al. Differences in the risk of cervical cancer and human papillomavirus infection by education level. Br J Cancer. 2009; 101: 865–870.
- Parvez R, Vijayachari P, Thiruvengadam K, Roy A, Saha MK, Ramasamy J, et al. A population based study on human papillomavirus infection and associated risk factors among women of the remote South Andaman Island, India. BMC Womens Health. 2024; 24: 139.
- World Health Organisation – Global Health Observatory. Women of reproductive age (15−49 years) who are married or in-union who have their need for family planning satisfied with modern methods (%), UNPD. World Health Organisation. 2024.