
Special Article: Fibrinogen
J Blood Disord. 2023; 10(2): 1075.
Fibrinogen Cleavage by Lectin Pathway Proteases: Roles and Implications
Madhuri M¹; Kumari P¹; Ali A¹; Roy D¹; Hajela S²; Kumari B¹; Hajela K³*; Sharma S¹*
1All India Institute of Medical Sciences, Patna, India
2Mahakaushal University, Jabalpur, Madhya Pradesh, India
3School of Life Sciences, DAVV, Indore, India
*Corresponding author: Sadhana Sharma Department of Biochemistry, All India Institute of Medical Sciences, Patna - 801507, Bihar, India Tel: 9631280481 E-mail: drsadhanas@aiimspatna.org, sharmasadhana.7@gmail.com
Received: June 07, 2023 Accepted: July 06, 2023 Published: July 13, 2023
Abstract
The lectin pathway proteases (MASPs) are diverse in regard to their substrate selectivity and also have substrates outside the complement pathway. These proteases have been reported to cleave proteins of the coagulation cascade. MASP-1 has thrombin-like activity and therefore cleaves fibrinogen, fibrin and prothrombin. MASP-2 also participates in coagulation by cleaving prothrombin. Thus, these proteases are key players in the process of thrombosis and thrombolysis, therefore have implications in thrombotic vascular diseases. The cleaved fragments of fibrinogen and fibrin, consequently produced during coagulation and fibrinolysis, are important in the repair process and hence may have involvement in inflammatory and fibro-proliferative diseases. Also, altered levels of MBL and its associated serine proteases reported to be involved in different diseases such as cardiovascular diseases, diabetes, cancer, sepsis, stroke, COVID-19 etc. may have a role in thrombosis and thrombolysis. Upregulated lectin pathway-associated proteins predispose towards autoimmune disease susceptibility and may also exhibit off-target activities resulting in clot formation. Thus, the lectin pathway and its associated proteases are of great importance in normal circumstances where it plays a role in the maintenance of hemostasis and homeostasis, playing a preventive role in infections. Modulation in the level of MASPs proteases could be developed as a promising approach to treat a variety of infections and diseases.
Keywords: Fibrinogen; MBL; MASPs; Thrombin; Coagulation; Fibrinolysis
Abbreviations: MASPs: MBL Associated Serine Protease; rMASP- Recombinant MASP; FPA and FPB: Fibrinopeptide A and B, Respectively; TAFI: Thrombin-Activated Fibrinolysis Inhibitor; DIC: Disseminated Intravascular Coagulation; CVD: Cardiovascular Diseases; LP: Lectin Pathway.
Introduction
Fibrinogen is a serum glycoprotein which is synthesized by the liver. It is a large molecular weight (340kDa) protein with three pairs of non-identical polypeptide chains (Hexameric homodimer) [1]. Such a structure of fibrinogen supports its complex roles in hemostasis and homeostasis [2]. During an inflammatory response, this protein is upregulated and expressed more. Different variants of fibrinogen are also formed as a result of alternative splicing. These have unique properties to contribute to the coagulation process following any vascular injury [3]. During coagulation, fibrinogen conversion to fibrin occurs via thrombin-mediated proteolytic cleavage. It produces intermediate protofibrils followed by mature fibers which eventually provide stable hemostatic clots and prevent blood loss at sites of tissue damage [4]. The processes of hemostasis and thrombosis involve interactions between fibrinogen and/or fibrin and plasma proteins and receptors on platelets, leukocytes, endothelial cells, and other cells [4]. Disorders in fibrinogen concentration and/or function increase the risk of bleeding, thrombosis, and infection [5]. Also, recent studies suggest that abnormal thrombin generation patterns produce abnormally structured clots that are associated with an increased risk of bleeding or thrombosis [6,4].
Further, a network of circulating blood cells and soluble proteins constitutes the host’s immune system which not only provides protection from various diseases but maintains homeostasis too. The complement system is one component of the immune system, which is a set of soluble proteins, which get activated in response to pathogens and help in clearing infection. There are three types of complement pathways, namely Classical, Alternative and Lectin. The lectin pathway [Mannan-Binding Lectin (MBL)], of complement activation, plays a key role in all types of infections and diseases. MBL, a pathogen recognition molecule of the innate immune system, remains associated with three serine proteases as MASP-1, MASP-2 and MASP-3 (MASP: MBL associated serine protease) [7].
The purple notches on top are CRD (carbohydrate recognition domain) through which MBL binds PAMPs on pathogen surface and associated serine proteases MASP-1, MASP-2 and MASP-3 are activated leading to lysis of pathogen [8].
Their role in the lectin pathway has been reported extensively; however, these serine proteases are pretty diverse in regard to their substrate selectivity and have substrates outside the complement pathway also. MASPs have been reported to share similar substrate specificity as that of thrombin which is also a serine protease. Hence, proteins of the coagulation pathway like fibrinogen, fibrin and prothrombin are cleaved by MASPs, however, the sites may be different [9].
During coagulation and fibrinolysis, the other cleaved fragments of fibrinogen and fibrin are released to perform various other roles in the repair process such as cell adhesion and spreading, vasoconstriction and chemotaxis [10]. These cleaved fragments have also been reported to act as mitogens for several cell types including fibroblasts, endothelial and smooth muscle cells. Such roles of these fragments open newer strategies to develop molecules for tissue repair or some preventive strategy for fibrosis in inflammatory and fibro-proliferative diseases where endothelial cell damage or chronic leakage of blood proteins is a feature [11].
This review deals with the unconventional cleavage of fibrinogen by MBL-associated serine proteases and its implications.
The Basic Mechanism of Fibrinogen Cleavage by Thrombin
Thrombin, a serine protease, plays a pivotal role in the blood coagulation pathway. It acts to convert various factors, including fibrinogen, into their active forms [6]. A significant part of this process is the conversion of soluble fibrinogen into insoluble strands of fibrin, which is critical for blood clot formation [12]. Thrombin accomplishes this by catalyzing the cleavage of fibrinopeptides A and B from the respective Aa and Bβ chains of fibrinogen, thus forming fibrin monomers [1]. This conversion process is one among a cascade of reactions that thrombin catalyzes in the coagulation pathway.
Fibrinogen, the substrate for this reaction, is a complex molecule composed of three pairs of different polypeptide chains: Aa, Bβ, and γ, held together by disulfide bonds [13]. The specific sites at which thrombin cleaves fibrinogen are located at the N-terminus of the Aa and Bβ chains. Following the cleavage, small peptides known as fibrinopeptides A and B are released. The specific cleavage sites are situated after residues Arg16-Gly17 on the Aa chain (releasing fibrinopeptide A) and after Arg14-Gly15 on the Bβ chain (releasing fibrinopeptide) [2].
Upon the cleavage of fibrinopeptides by thrombin, fibrinogen undergoes a transformation to fibrin monomers. This process exposes new polymerization sites on the fibrinogen molecule, allowing it to polymerize into fibrin, the main protein of blood clots [2]. It is this transformation that changes the soluble fibrinogen into insoluble fibrin strands. The fibrin strands then interact via "knobs" exposed by fibrinopeptide removal in the central region, with "holes" always exposed at the ends of the molecules. The resulting half-staggered, double-stranded oligomers lengthen into protofibrils, which aggregate laterally to make fibers [12]. These fibers then branch, yielding a three-dimensional network that forms the basis of a blood clot [2]. This entire process is crucial for hemostasis, the cessation of bleeding following vascular injury [13].
MASP-1 in Coagulation
The activation of serine proteases namely MASP-1, 2, and 3 of the lectin pathway of the complement system is an important event for its functioning to achieve pathogen clearance. MASP-1 forms an association with MBL/Ficolins in response to foreign moieties and auto-activates itself, downstream of that it also activates other associated serine proteases such as MASP-2 [14]. Further, the complement system, through the mediation of the coagulation components, builds a greater response to invading pathogens [15,16], probably causing aggregation of pathogens [17], thus limiting the dissemination of infection. Such studies have clearly demonstrated that the complement system and the coagulation cascade are intricately interlinked. Furthermore, in vitro and in vivo studies have also supported that MBL/Ficolins-MASP-1/2 have a pivotal role in the coagulation system [9,18].
In the thrombotic pathway, the involvement of MASP-1 in the lectin pathway is crucial [19] as it plays a predominant role in the coagulation system [20]. MASP-1 interacts with a broad range of proteins involved in the coagulation system such as coagulation Factor XIII (FXIII), fibrinogen, prothrombin etc. [21,17,22] (discussed in Figure 3). In coagulation pathway, prothrombin gets activated to thrombin in a conventional way by activated Factor X (FXa), however, recombinant MASP-1(rMASP-1) and rMASP-2 also have shown to catalyse the same reaction [21,17]. In this catalytic cleavage, the underlined mechanism of prothrombin cleavage by rMASPs may be different from FXa. Functional studies by [21] have shown that MASP-1 may potentially activate the prothrombin by cleaving the arginine (R) at either 271 or 393 position however, in the conventional pathway of thrombin generation, prothrombin cleavage occurs at R155 and R284, which are crucial sites, however, it is not true for MASP-1 [21]. Apart from this, prothrombin cleavage by MASP-1 at R320 can also activate it to thrombin. We were the first to demonstrate that MASP-1 also possesses thrombin-like activity [23] and shares similar substrate specificity as thrombin but with reduced catalytic potential [17]. Biochemical evidence suggests that both MASP-1 and thrombin act on fibrinogen. These share an identical cleavage site for the β-chain but a differential cleavage site for the a-chain of the fibrinogen during its polymerization. During its cleavage, MASP-1 leads to the generation of fragmented γ2-chain along with a few oligomers of a-chain. Upon cleavage of the fibrinogen, MASP-1 mainly releases fibrinopeptide B in contrast to thrombin, which releases both fibrinopeptide A and B. In terms of target specificity (a-chain), MASP-1 seems to be less specific than thrombin [17].
Cleavage of fibrinogen by thrombin results in the polymerization of the alpha chain (alpha n) with the complete disappearance of the alpha chain band. The γ chain dimmers are formed by factor XIII present as a contaminant present in fibrinogen. Factor XIII is converted to Factor XIIIa by the action of thrombin in the presence of calcium. Fibrinogen cleavage by purified MASP-1 essentially required Ca++ as in the absence of Ca++ fibrinogen cleavage is not observed. (Figure 02 lane 4, lane 6). However, no difference is observed in fibrinogen cleavage by recombinant MASP-1 in the presence and absence of Ca++ (data not shown). In both cases, only the alpha chain is cleaved. It is to be noted that in our studies recombinant MASP-1 lacks the Ca++ binding EGF domain. The fibrinogen cleavage by MASP-1 was found to be completely inhibited in the absence of Ca++ or the presence of 5 mM EDTA (lane 6) or 5 mM EGTA (lane 8). In our study presence of Mg in place of Ca++ could not restore the fibrinogen-cleaving activity of MASP-1(Lane 10). The fibrinogen cleavage by serum-purified MASP-1 is stopped completely by a C-1 inhibitor [23].
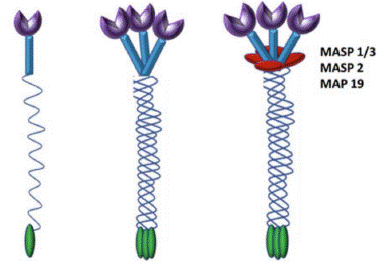
Figure 1: Cartoon representation of MBL: Three identical 32 kDa polypeptides form a triple helix. Higher oligomers of different sizes are formed, tetramer being the most common one.
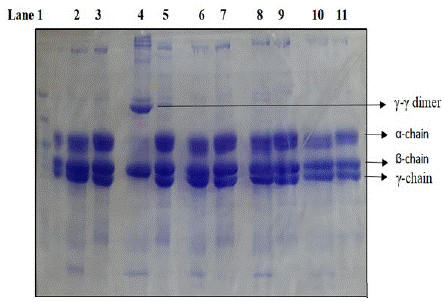
Figure 2: SDS PAGE analysis of cleavage of fibrinogen by purified MASP-1 in the presence or absence of different metal ions/chelators. Lane 1 marker. Lane 2 cleavage in HEPES buffer without any ion, Lane 4 in the presence of 5mM Ca++ (see the complete absence of γ bands with prominent γ-γ dimer at the top), Lane 6 in the presence of 5mM EDTA, Lane 8 in the presence of 5mM EGTA and Lane 10 in presence 5mM Mg++. Lanes 3,5,7,9 and 11 are fibrinogen controls in respective buffers. Cleavage was done for 4 hours at 37C. Native MASP-1 was isolated from human serum by PEG precipitation followed by affinity chromatography on the antibody column (K Hajela, unpublished results).
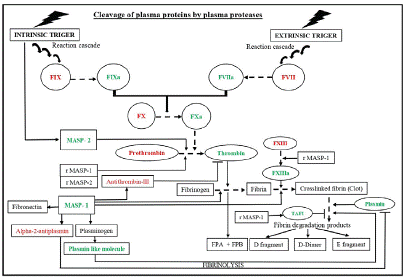
Figure 3: Schematic diagram showing the interplay of Lectin pathway proteases in coagulation. During thrombosis, the proteases such as prothombinase, MASP-1, and MASP-2 lead to fibrin clot formation through cascade of reactions. Additionally, MASP-1 also contributes to plasmin mediated fibrinolysis by inactivating alpha-2-antiplasmin or by activating plasminogen to produce plasmin like molecules and generates different fibrin degradation products.
Abbreviations: MASPs: MBL Associated Serine Protease; rMASP: Recombinant MASP; FPA and FPB: Fibrinopeptide A and B, respectively; TAFI: Thrombin-Activated Fibrinolysis Inhibitor. The substrates for MASP-1 and 2 are indicated with solid arrows. Sequential conversion is indicated with dotted arrows. Symbol “’’ denotes pathway inhibition. Green and red colour denotes activated and inactivated states, respectively. Sequential arrows in intrinsic and extrinsic pathways show reaction cascade.
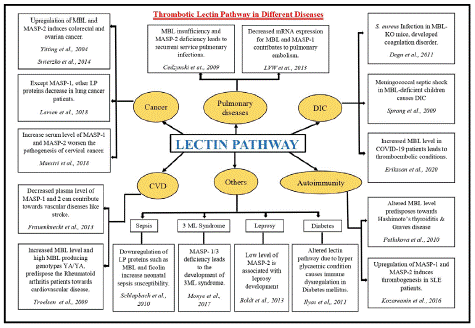
Figure 4: Dysregulated components of Lectin Pathway and their implications in different diseases.
Abbreviations: DIC: Disseminated Intravascular Coagulation; CVD: Cardiovascular Diseases; LP: Lectin Pathway; KO: Knockout; SLE: Systemic Lupus Erythematosus
In vitro studies by Gulla et al., (2010) have also revealed that L-Ficolins or MBL-MASP-1 complexes have similar fibrinogen cleavage and clot generation potential like recombinant MASP-1. Although MASPs and thrombin-generated clots are chemically similar in terms of cross-linking potential, the latter is more rigid [9]. In-vitro studies have demonstrated fibrin clot formation by Ficolins-MASP-1 or MBL-MASP-1 complex in the presence of calcified plasma and the same was further confirmed by plasmin digestion [9]. Literature suggests that human MASP-1 has similar enzymatic activity and specificity to thrombin against artificially synthesized substrates such as Val-Pro-Arg-Aminomethylcoumarin (VPR-AMC), FGR-AMC [24,25]. It has been reported that MASP activation due to its ligand binding contributes to the fibrin clot generation which is indistinguishable from the one generated by the conventional coagulation pathway [9]. Therefore, MASP-1 can be regarded as a key player in the coagulation system because it potentially carries out the cleavage and polymerisation of fibrinogen moiety [23] which is a prerequisite during thrombogenesis.
MASP-2 in Coagulation
MASP-2 behaves differently in the coagulation pathway. It does not have direct proteolytic activity towards fibrinogen or factor XIII but can mediate the turnover of these molecules by activating prothrombin [15]. MASP-2, however, is not functionally efficient compared to the prothrombinase complex; hence the formation of large blood clots and the subsequent hindrance of clearance of the pathogenic complexes by phagocytes is prevented. MASP-2 activates prothrombin close to the surface where MBL or the ficolins can bind. These surfaces can be transported in the bloodstream. A low activation potential of MASP-2 during coagulation is advantageous as it generates a limited amount of thrombin with a short activation period [23]. This results in insufficient fibrin clot production and a localised action of thrombin, as the latter is inhibited before it can be disseminated to a distant site. Subsequently, a localized release of fibrinopeptides A & B and fibrin deposits serve as adhesion points to attract phagocytes and immune cells for immune clearance [15].
It is also noteworthy that activated platelets when binding to Ficolin-1, 2 and 3 (but not to MBL), activate MASP-1 and MASP-2 via fibrin and plasmin-generated fibrin fragment D-dimer [26] and lead to thrombo-inflammation.
Substrates of MASPs in the Coagulation Pathway
Human plasma has numerous substrates of lectin pathway proteases [22]. However, few of them are from the coagulation pathway (Table 1). Bhagwat et al., (2022) [22] have shown in vitro cleavage of three plasma proteins from the coagulation pathway i.e. a2-Antiplasmin (A2AP), a1-Acid Glycoprotein (A1AG), and anti-thrombin III by MASP-1 [22]. Various substrates of Lectin pathway proteases have been listed in Table I along with their roles in the coagulation pathway.
MASPs
Coagulation Pathway Substrates
Roles
References
MASP-1
Coagulation factor XIII (FXIII)
Clot stabilization by cross-linking fibrin molecules
[21]
Fibrinogen
Clot contraction and wound healing
[17]
Prothrombin
Zymogen of thrombin eventually helps in fibrin generation.
[21]
Fibronectin
Thrombosis regulation and haemostasis maintenance.
[35,40]
Kininogen-1
Help in positioning of prekallikrein and factor XI next to factor XII during intrinsic pathway of blood coagulation; inhibits thrombin- and plasmin-induced thrombocytes aggregation.
[41,42]
PAR-4
Maintenance of hemostasis by platelets activation during coagulation cascade.
[43]
a2-antiplasmin (A2AP)
Inhibitor of fibrinolysis by plasmin.
[22]
a1-acid glycoprotein (A1AG)
Maintenance of hemostasis during coagulation and homeostasis during inflammation.
[22,44]
Anti-thrombin III
Inhibitor of MASP-1& 2 and thrombin, prevents coagulation
[22]
MASP-2
Prothombinase-like activity
[14,17]
MASP-3
Mainly involved in Complement System. Pro-Factor D activator in Complement Pathway.
[45]
Thrombin-activatable fibrinolysis inhibitor (TAFI)
Inhibitor of fibrinolytic system
[46]
Plasminogen
Zymogen of plasmin which enables fibrinolysis.
[47]
MASP-2
Prothrombin*
Zymogen of thrombin eventually helps in fibrin generation.
[17]
MASP-3
No natural substrate identified yet
Table 1: Reported substrates of MASP-1 and 2 in Coagulation Pathway.
Among various substrates, anti-thrombin III is an endogenous anticoagulant that neutralizes the activity of thrombin and other serine proteases through irreversible binding [27]. Apart from inhibiting MASPs, it prevents coagulation and thrombosis, which is evident in patients with severe thromboembolic states and antithrombin deficiency [28] an acute phase glycoprotein, which increases during tissue injury, infection, and inflammation [29] has an anti-inflammatory role too, as it inhibits human neutrophil aggregation and superoxide anion generation [30]. This indicates its possible link with MASP-1 which increases neutrophil adhesion through various cell adhesion molecules, leading to neutrophil aggregation [31]. In fact, MBL/MASP is known to facilitate platelet aggregation [32]. Increased A1AG in tissue injury inhibits platelet aggregation, which possibly increases MASP-1 as a homeostatic mechanism, cleaving A1AG and promoting platelet aggregation.
Various other substrates participate in MASPs-mediated fibrinolysis/thrombolysis. A2AP, a plasmin inhibitor regulates intravascular fibrinolysis [33]. MASP-1, by cleaving A2AP, may promote plasmin-mediated fibrinolysis [22]. The plasmin/A2AP complex levels are increased in acute stroke, myocardial infarction, and arterial fibrillation [34]. rMASP-1 dissolves clots by activating fibrin-associated plasminogen [35]. Thrombin activates FXIII which helps in forming crosslinks between fibrin monomers, and eventually an insoluble fibrin clot. The generation of such clots is counter-balanced by the fibrinolytic system, where plasmin dissolves the clot and generates soluble fibrin fragments [36,36]. Among the three chains (a, β, and γ) of fibrinogen, the ϒ-chain is cleaved by thrombin, forming ϒ-ϒ dimers [17,37]. When fibrin is treated with plasmin, it forms the digestion products ϒ’-ϒ’ dimer and β” band. When A2AP, an inhibitor of plasmin, is earlier treated with MASP-1, the plasmin is still able to perform fibrinolysis, which is demonstrated by the presence of ϒ’-ϒ’ dimer and β” band [22], whereas when the A2AP was not treated with MASP-1, the plasmin activity was inhibited.
Fibronectin is an important regulator of hemostasis and thrombosis. It inhibits platelet aggregation in the presence of fibrinogen and von Willebrand Factor, and induces platelet aggregation and thrombosis in the presence of fibrin [38,39]. During the latter, fibronectin incorporates itself into the clot to strengthen it [40]. Fibronectin is known to be acted upon and digested by recombinant MASP-1 [35], suggesting that MASP-1 may have a role in thrombolysis.
MASP-1 has a broad range of substrates in the coagulation pathway. Most of them have been discussed here. The involvement of MASP-2, in the coagulation pathway is also well documented. Numerous substrates for MASP-2 have been reported in the complement pathway but in the coagulation pathway only known substrate to date is prothrombin. It leads to fibrin clot generation by acting on prothrombin and further its activation to thrombin.
Fibrin(ogen) Lysis and by-Product Peptides
The fibrinolytic system involves the conversion of soluble fibrinogen to insoluble fibrin and then its further breakdown into D and E fragments or D dimer by plasmin [47]. Dissolution of a fibrin clot is achieved by activated plasmin due to the action of tissue plasminogen activator or urokinase on the plasminogen [48]. The role of MASP-1 is well-documented in thrombosis whereas recent studies have also shown its involvement in thrombolysis [22,35]. MASP-1 converts plasminogen (zymogen) into a plasmin-like molecule which potentially cleaves the fibrin clot [35]. Another study by Bhagwat et al., (2022) [22], also emphasized the fact that MASP-1 promotes plasmin-mediated fibrinolysis as alpha-2 antiplasmin is its substrate. Proteolytic degradation of fibrinogen or fibrin gives rise to several products in blood which is known as Fibrin Degradation Products (FDPs) [47]. FDPs mainly include FpA, FpB, D-D dimer, Fragment D and E which are involved in immune modulation, chemotaxis, and numerous disease pathophysiology (discussed in detail in Table II). The FpA and FpB are very small in size i.e. 16 and 14 amino acids respectively whereas fragments D and E are quite large with molecular wt. of 90kDa and 60kDa, respectively [49,50].
Fibrin Degradation Products
Implications on Diseases
References
Fibrinopeptide A (FpA)
· Biomarker for activation of coagulation system.
[51]
· Increased level have pathological effect on cardiovascular system.
[47]
· Proinflammatory role by inducing CRP expression.
[52]
· Disease biomarker for acute-coronary thrombosis, ischemic heart disease and liver cirrhosis.
[53-55]
· N-terminal truncated FpA derivatives i.e. FpA-3 and FpA-6 are cancer biomarker.
[56]
· Induces secretion of inflammatory chemokines and AMP, involved in pathophysiology of psoriasis.
[57]
Fibrinopeptide B (FpB)
· Chemoattractant, involved in inflammation and angiogenesis.
[58]
· Increased level is an indicator of pulmonary embolism and deep vein thrombosis.
[59]
· Induces platelet aggregation by inhibiting the PGI2 and PGD2 activity.
[60]
· Both FpA and FpB are markers for early diagnosis of mesenteric ischemia.
[61]
D-D dimer
· Marker for hypercoagulopathy and thrombosis.
[62]
· Increased level leads to organ dysfunction in early stage of sepsis.
[63]
· Prognostic Marker (Covid-19).
[64]
· Diagnostic marker for pulmonary embolism.
[65,66]
· Predictive marker for cardiovascular diseases and cancer.
[67]
Fragment D and E
· Biomarkers of inflammation.
[68]
· Fragment D and E are associated with tumour progression.
[49]
· Fragment E acts as chemoattractant for myofibroblast formation (TGF-β mediated), also involved in wound healing and fibrosis.
[49]
Table 2: Implications of fibrinolysis by-products in different diseases.
Role of MASP-3
MASP-3 is a splicing variant of MASP-1 that is transcribed from the MASP-1 gene. It circulates in an active form in a complex with ficolin-3 rather than with MBL or ficolin-2. It has no known inhibitors. It has been reported that MASP-3 plays a role in the activation of the complement system by activating complement Factor D (FD), an important step in Alternative Pathway (AP) and also has a role in embryonic development. However, its role in coagulation has not been reported yet. A study has shown that MASP-3 forms a complex with the pattern recognition molecules of the lectin pathway but its functional benefits need to be explored yet [69]. MASP-3 is relatively naïve and least explored among the three MASPs as it shares great structural similarities with other MASPs, it might be playing some hidden role in the coagulation system which requires further attention.
Implications
Although the lectin pathway was discovered two decades ago its unconventional role in fibrinogen cleavage by MBL-associated serine proteases is relatively a novel concept that needs to be explored further for better understanding. MBL and Ficolins (H, L, and M) play a prominent role in activating the innate immune system in response to carbohydrate and protein moieties of the pathogen such as bacteria, viruses, and altered cells [70]. Misregulation of MBL and its associated serine proteases have shown to be involved in different diseases such as cardiovascular diseases, diabetes, cancer, sepsis, stroke, COVID-19 etc. [70-77] (discussed in Figure 4). MASP-1 levels are higher in patients with sub-acute myocardial infarction, [76] suggesting that MASP-1 levels that increase later support thrombolysis. This property can be used to develop therapeutics for cardiovascular and thrombotic diseases [78].
Genomic and proteomic dysregulation of lectin proteases exacerbate the disease consequences as upregulation of MASP-1, 2, and 3 eventually increases the embolism possibility [78,79]. Upregulation of lectin pathway-associated proteins predisposes towards autoimmune disease susceptibility such as Hashimoto’s thyroiditis [80] and Systemic Lupus Erythematosus (SLE) [26]. Increased MBL levels trigger the lectin serine protease auto-activation [81,7] and complex formation that ultimately aggravate the thromboembolic complications in individuals [77]. Upregulated Complement MASP proteases may exhibit off-target activities and result in unnecessary clot formation. Enhanced level of MBL and decreased level of MASP-2 is consistently reported in stroke patients [76] where MASP-2 can be used as a diagnostic marker as well. Several studies suggests that not only altered plasma level but genetic variants of MBL and MASPs also predispose towards several diseases including COVID-19 [8,71]. Cancer patients are predisposed towards the risk of thromboembolism [82] which imparts negative effects on the treatment modalities such as surgery, which also have an influence on the expression of genes involved in the lectin pathway [83]. Another study by Larsen et al., (2018) [84] also discussed the low expression of MASP proteases namely MASP-2/3 in lung cancer patients, indicating that in such cases clotting phenomena are primarily achieved by either thrombin or MASP-1 mediated pathway. In COVID-19 infection, the overwhelmed response of complement activation contributed to disseminated intravascular coagulation [77]. We found significantly elevated serum, C4b levels in COVID-19 patients with elevated MBL and MASP-2, which came down to normal once the infection subsided [85].
Thus, the Lectin pathway and its associated proteases are of great importance in normal circumstances where it plays a role in the maintenance of hemostasis, homeostasis, and also a preventive role in infections. Under circumstances where it is erroneously upregulated and downregulated, it contributes towards medical illness. Modulation in the level of MASPs proteases could be developed as a promising approach to treat a variety of infections as these are involved in the coagulation cascade and fibrinolysis impairment. Therefore, targeting the MBL pathway and its associated serine proteases can be developed as a novel therapeutic target for antithrombotic treatment in several diseases including COVID-19.
Conclusion
The role of lectin pathway proteases in the coagulation cascade, fibrinolysis and their consequences, generate sufficient basis to develop targeted therapies for antithrombotic treatment in several diseases.
Author Statements
Acknowledgements
We thank Indian Council of Medical Research, Govt. of India for financial support (Grant No. 61/2/2020-IMM/BMS). Special mention to Mr. Raj Kumar for his technical assistance during the manuscript preparation. We apologize that due to space limitation, not all of the work related to this field could be discussed or cited. We acknowledge and thank Dr R.B.Sim, MRC Immunochemistry Unit, Department of Biochemistry University of Oxford, Oxford, UK posthumously for facilities and guidance for experiment presented in Figure 2.
References
- Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005; 3: 1894-904.
- Weisel JW, Litvinov RI. Fibrin formation, structure and properties. Subcell Biochem. 2017; 82: 405-56.
- Stubbs MT, Bode W. A model for the specificity of fibrinogen cleavage by thrombin. Semin Thromb Hemost. 1993; 19: 344-51.
- Wolberg AS. 1 RAC. Thrombin generation, fibrin clot formation and hemostasis. Transfus Apher Sci. 2008; 38: 15-23.
- Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. 2020; 105: 284-96.
- Huntington JA. Molecular recognition mechanisms of thrombin. J Thromb Haemost. 2005; 3: 1861-72.
- Beltrame MH, Catarino SJ, Goeldner I, Boldt ABW, Reason IJde M. The lectin pathway of complement and rheumatic heart disease. Front Pediatr. 2015; 2: 1-14.
- Sharma S, Patel PK, Choudhary K, Phadnavis PP, Bhagwat SR, et al. Lectins in health and diseases: mannan-binding lectin and infectious diseases. Lectins. 2021: 185-214.
- Gulla KC, Gupta K, Krarup A, Gal P, Schwaeble WJ, et al. Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology. 2010; 129: 482-95.
- Sarah Herrick b, Olivier Blanc-Brude b. Andrew Gray a GL b. Fibrinogen. Int J Biochem Cell Biol. 2009; 31: 741-6.
- Ghatak S, Maytin EV, Mack JA, Hascall VC, Atanelishvili I, et al. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int J Cell Biol. 2015; 2015: 834893.
- Weisel JW. Structure of fibrin: impact on clot stability. J Thromb Haemost. 2007; 5: 116-24.
- Wolberg AS. Determinants of fibrin formation, structure, and function. Curr Opin Hematol. 2012; 19: 349-56.
- Héja D, Kocsis A, Dobó J, Szilágyi K, Szász R, et al. Revised mechanism of complement lectin-pathway activation revealing the role of serine protease MASP-1 as the exclusive activator of MASP-2. Proc Natl Acad Sci USA. 2012; 109: 10498-503.
- Krarup A, Wallis R, Presanis JS, Gál P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLOS ONE. 2007; 2: e623.
- Tomo S, Kumar KP, Roy D, Sankanagoudar S, Purohit P, et al. Complement activation and coagulopathy - an ominous duo in COVID19. Expert Rev Hematol. 2021; 14: 155-73.
- Krarup A, Gulla KC, Gál P, Hajela K, Sim RB. The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim Biophys Acta. 2008; 1784: 1294-300.
- Endo Y, Nakazawa N, Iwaki D, Takahashi M, Matsushita M, et al. Interactions of ficolin and mannose-binding lectin with fibrinogen/fibrin augment the lectin complement pathway. J Innate Immun. 2010; 2: 33-42.
- Megyeri M, Harmat V, Major B, Végh á, Balczer J, et al. Quantitative characterization of the activation steps of mannan-binding lectin (MBL)-associated serine proteases (MASPs) points to the central role of MASP-1 in the initiation of the complement lectin pathway. J Biol Chem. 2013; 288: 8922-34.
- Dobó J, Schroeder V, Jenny L, Cervenak L, Závodszky P, et al. Multiple roles of complement MASP-1 at the interface of innate immune response and coagulation. Mol Immunol. 2014; 61: 69-78.
- Jenny L, Dobó J, Gál P, Schroeder V. MASP-1 of the complement system promotes clotting via prothrombin activation. Mol Immunol. 2015; 65: 398-405.
- Bhagwat SR, Choudhary K, Pandya N, Sharma S, Srivastava S, et al. Identification of substrates of MBL Associated serine Protease-1 (MASP-1) from human plasma using N-terminomics strategy. Mol Immunol. 2022; 151: 114-25.
- Hajela K, Kojima M, Ambrus G, Wong KH, Moffatt BE, et al. The biological functions of MBL-associated serine proteases (MASPs). Immunobiology. 2002; 205: 467-75.
- Morita T, Kato H, Iwanaga S, Takada K, Kimura T. New fluorogenic substrates for a-thrombin, factor Xa, kallikreins, and urokinase. J Biochem. 1977; 82: 1495-8.
- Presanis JS, Hajela K, Ambrus G, Gál P, Sim RB. Differential substrate and inhibitor profiles for human MASP-1 and MASP-2. Mol Immunol. 2004; 40: 921-9.
- Kozarcanin H, Lood C, Munthe-Fog L, Sandholm K, Hamad OA, et al. The lectin complement pathway serine proteases (MASPs) represent a possible crossroad between the coagulation and complement systems in thromboinflammation. J Thromb Haemost. 2016; 14: 531-45.
- Nugent MA, Forsten-Williams K, Karnovsky MJ, Edelman ER. Mechanisms of cell growth regulation by heparin and heparan sulfate. Chem Biol Heparin Heparan Sulfate. 2005: 533-70.
- Annichino-Bizzacchi JM, de Paula EV. Blood coagulation and endothelium. Endothelium Cardiovasc Dis Vasc Biol Clin Syndr. 2018: 147-52.
- Ceciliani F, Lecchi C. The immune functions of a 1 acid glycoprotein. Curr Protein Pept Sci. 2019; 20: 505-24.
- Costello M, Fiedel BA, Gewurz H. Inhibition of platelet aggregation by native and desialised alpha-1 acid glycoprotein (13). Nature. 1979; 281: 677-8.
- Jani PK, Schwaner E, Kajdácsi E, Debreczeni ML, Ungai-Salánki R, et al. Complement MASP-1 enhances adhesion between endothelial cells and neutrophils by up-regulating E-selectin expression. Mol Immunol. 2016; 75: 38-47.
- Laura R La Bonte, Vasile I Pavlov, Ying S Tan, Kazue Takahashi, Minoru Takahashi, et al. Mannose-binding lectin-associated serine protease-1 is a significant contributor to coagulation in a murine model of occlusive thrombosis. J Immunol. 2012; 188: 885–91.
- Urano T, Suzuki Y, Iwaki T, Sano H, Honkura N, et al. Recognition of plasminogen activator inhibitor Type 1 as the primary regulator of fibrinolysis. Curr Drug Targets. 2019; 20: 1695-701.
- Hou Y, Okada K, Okamoto C, Ueshima S, Matsuo O. Alpha2-antiplasmin is a critical regulator of angiotensin II-mediated vascular remodeling. Arterioscler Thromb Vasc Biol. 2008; 28: 1257-62.
- Choudhary K, Patel PK, Are VN, Makde RD, Hajela K. Mannose-binding lectin-associated serine protease-1 cleaves plasminogen and plasma fibronectin: prefers plasminogen over known fibrinogen substrate. Blood Coagul Fibrinolysis. 2021; 32: 504-12.
- Fuss C, Palmaz JC, Sprague EA. Fibrinogen: structure, function, and surface interactions. J Vasc Interv Radiol. 2001; 12: 677-82.
- Smith EL, Cardinali B, Ping L, Ariëns RAS, Philippou H. Elimination of coagulation factor XIII from fibrinogen preparations. J Thromb Haemost. 2013; 11: 993-5.
- Reheman A, Yang H, Zhu G, Jin W, He F, et al. Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor. Blood. 2009; 113: 1809-17.
- Wang Y, Carrim N, Ni H. Fibronectin orchestrates thrombosis and hemostasis. Oncotarget. 2015; 6: 19350-1.
- Wang Y, Reheman A, Spring CM, Kalantari J, Marshall AH, et al. NH. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest. 2014; 124: 4281-93.
- Dobó J, Major B, Kékesi KA, Szabó I, Megyeri M, et al. Cleavage of Kininogen and subsequent bradykinin release by the complement component: mannose-binding lectin-associated serine protease (MASP)-1. PLOS ONE. 2011; 6: e20036.
- Ponczek MB. High molecular weight kininogen: a review of the structural literature. Int J Mol Sci. 2021; 22: 1-9.
- Posma JJ, Grover SP, Hisada Y, Owens AP, Antoniak S, et al. Roles of coagulation proteases and PARs (protease-activated receptors) in mouse models of inflammatory diseases. Arterioscler Thromb Vasc Biol. 2019; 39: 13-24.
- Sumanth MS, Jacob SP, Abhilasha KV, Manne BK, Basrur V, et al. Different glycoforms of alpha-1-acid glycoprotein contribute to its functional alterations in platelets and neutrophils. J Leukoc Biol. 2021; 109: 915-30.
- Sekine H, Machida T, Fujita T, Factor D. Immunol Rev. 2023: 1-12.
- Sillen M, Declerck PJ. Thrombin activatable fibrinolysis inhibitor (Tafi): an updated narrative review. Int J Mol Sci. 2021; 22.
- Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015; 29: 17-24.
- Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005; 129: 307-21.
- Kolodziejczyk J, Ponczek MB. The role of fibrinogen, fibrin and fibrin(ogen) degradation products (FDPs) in tumor progression. Contemp Oncol (Pozn). 2013; 17: 113-9.
- Senior RM, Skogen WF, Griffin GL, Wilner GD. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986; 77: 1014-9.
- Balme S. Fibrinopeptide A family biomarker identification at single molecule level. ACS Cent Sci. 2023; 9: 131-3.
- Xu S, Zhao J, Liu J, Gou W. Fibrinopeptide A induces expression of C-reactive protein via the ROS-ERK1/2/ P38-NF-κB signal pathway in vascular smooth muscle cells. Cell Physiol Biochem. 2018; 47: 266-78.
- Eisenberg PR, Sherman LA, Schectman K, Perez J, Sobel BE, et al. Fibrinopeptide A: A marker of acute coronary thrombosis. Circulation. 1985; 71: 912-8.
- Alemán-Gómez JA, López-Candalez A, Freytes CO, Mignucci M, Linares E. Usefulness of single fibrinopeptide A determination in patients with acute ischemic coronary artery syndromes. Bol Asoc Med P R. 1992; 84: 134-8.
- Coccheri S, Mannucci PM, Palareti G, Gervasoni W, Poggi M, et al. Significance of plasma fibrinopeptide A and high molecular weight fibrinogen in patients with liver cirrhosis. Br J Haematol. 1982; 52: 503-9.
- Bhalla S, Verma R, Kaur H, Kumar R, Usmani SS, et al. CancerPDF: A repository of cancer-associated peptidome found in human biofluids. Sci Rep. 2017; 7: 1511.
- Matsuura T, Sato M, Nagai K, Sato T, Arito M, et al. Serum peptides as putative modulators of inflammation in psoriasis. J Dermatol Sci. 2017; 87: 36-49.
- Riedel T, Suttnar J, Brynda E, Houska M, Medved L, et al. Fibrinopeptides A and B release in the process of surface fibrin formation. Blood. 2011; 117: 1700-6.
- Morris TA, Marsh JJ, Burrows CM, Chiles PG, Konopka RG, et al. Urine and plasma levels of fibrinopeptide B in patients with deep vein thrombosis and pulmonary embolism. Thromb Res. 2003; 110: 159-65.
- Moore PK, Hussaini I, Bhardwaj R. Effect of fibrinopeptides A and B on human and rat platelet aggregation in vitro. Eur J Pharmacol. 1988; 153: 231-8.
- Cikot M, Gemici E, Isiksacan N, Kones O, Kasapoglu P, et al. Fibrinopeptide-A and fibrinopeptide-B may help to D-dimer as early diagnosis markers for acute mesenteric ischemia. Turk J Biochem. 2019; 44: 654-60.
- Chandel A, Patolia S, Looby M, Bade N, Khangoora V, et al. Association of D-dimer and fibrinogen with hypercoagulability in COVID-19 requiring extracorporeal membrane oxygenation. J Intensive Care Med. 2021; 36: 689-95.
- Iba T, Kidokoro A, Fukunaga M, Sugiyama K, Sawada T, et al. Association between the severity of sepsis and the changes in hemostatic molecular markers and vascular endothelial damage markers. Shock. 2005; 23: 25-9.
- Yao Y, Cao J, Wang Q, Shi Q, Liu K, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J Intensive Care. 2020; 8: 49.
- Nemec HM, Ferenczy A, Christie BD, Ashley DW, Montgomery A. Correlation of D-dimer and outcomes in COVID-19 patients. Am Surg. 2022; 88: 2115-8.
- Logothetis CN, Weppelmann TA, Jordan A, Hanna C, Zhang S, et al. D-dimer testing for the exclusion of pulmonary embolism among hospitalized patients with COVID-19. JAMA Netw Open. 2021; 4: e2128802.
- Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, et al. D-dimer predicts long-term cause-specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease LIPID study. Circulation. 2018; 138: 712-23.
- Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, et al. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med. 2011; 17: 568-73.
- Kusakari K, Machida T, Ishida Y, Omori T, Suzuki T, et al. The complex formation of MASP-3 with pattern recognition molecules of the lectin complement pathway retains MASP-3 in the circulation. Front Immunol. 2022; 13: 907023.
- Degn SE, Jensenius JC, Bjerre M. The lectin pathway and its implications in coagulation, infections and auto-immunity. Curr Opin Organ Transplant. 2011; 16: 21-7.
- Troelsen LN, Garred P, Christiansen B, Torp-Pedersen C, Christensen IJ, et al. Double role of mannose-binding lectin in relation to carotid intima-media thickness in patients with rheumatoid arthritis. Mol Immunol. 2010; 47: 713-8.
- Ilyas R, Wallis R, Soilleux EJ, Townsend P, Zehnder D, et al. High glucose disrupts oligosaccharide recognition function via competitive inhibition: A potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011; 216: 126-31.
- Ytting H, Jensenius JC, Christensen IJ, Thiel S, Nielsen HJ. Increased activity of the mannan-binding lectin complement activation pathway in patients with colorectal cancer. Scand J Gastroenterol. 2004; 39: 674-9.
- Swierzko AS, Szala A, Sawicki S, Szemraj J, Sniadecki M, et al. Mannose-binding lectin (MBL) and MBL-associated serine protease-2 (MASP-2) in women with malignant and benign ovarian tumours. Cancer Immunol Immunother. 2014; 63: 1129-40.
- Schlapbach LJ, Mattmann M, Thiel S, Boillat C, Otth M, et al. Differential role of the lectin pathway of complement activation in susceptibility to neonatal sepsis. Clin Infect Dis. 2010; 51: 153-62.
- Frauenknecht V, Thiel S, Storm L, Meier N, Arnold M, et al. Plasma levels of mannan-binding lectin (MBL)-associated serine proteases (MASPs) and MBL-associated protein in cardio- and cerebrovascular diseases. Clin Exp Immunol. 2013; 173: 112-20.
- Eriksson O, Hultström M, Persson B, Lipcsey M, Ekdahl KN, et al. Mannose-binding lectin is associated with thrombosis and coagulopathy in critically ill COVID-19 patients. Thromb Haemost. 2020; 120: 1720-4.
- Jenny L, Noser D, Larsen JB, Dobó J, Gál P, et al. MASP-1 of the complement system alters fibrinolytic behaviour of blood clots. Mol Immunol. 2019; 114: 1-9.
- Damoah CE, Snir O, Hindberg K, Garred P, Ludviksen JK, et al. High levels of complement activating enzyme MASP-2 are associated with the risk of future incident venous thromboembolism. Arterioscler Thromb Vasc Biol. 2022; 42: 1186-97.
- Potlukova E, Jiskra J, Freiberger T, Limanova Z, Zivorova D, et al. The production of mannan-binding lectin is dependent upon thyroid hormones regardless of the genotype: A cohort study of 95 patients with autoimmune thyroid disorders. Clin Immunol. 2010; 136: 123-9.
- Wallis R. Interactions between mannose-binding lectin and MASPs during complement activation by the lectin pathway. Immunobiology. 2007; 212: 289-99.
- Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006; 119: 60-8.
- Van Till JW, Boermeester MA, Modderman PW, Van Sandick JW, Hart MHL, et al. Variable mannose-binding lectin expression during postoperative acute-phase response. Surg Infect. 2006; 7: 443-52.
- Larsen JB, Troldborg A, Christensen TD, Hvas CL, Thiel S, et al. The lectin pathway and coagulation in lung cancer patients undergoing lobectomy – A randomised controlled trial. Thromb Res. 2018; 163: 92-9.
- Kumari B, Hajela K, Ali A, Sharma AK, Yadav RK, et al. Evaluation of C4b as an adjunct marker in symptomatic RT-PCR negative Covid-19 cases. Indian J Clin Biochem. 2023; 38: 102-9.