
Special Article - Bioprocessing
Austin J Biotechnol Bioeng. 2022; 9(1): 1115.
Conventional and Emerging Novel Techniques for the Extraction of Pectin and Applications of Pectin
Sharma N, Pooja and Yadav SK*
Center of Innovative and Applied Bioprocessing (CIAB), Sector- 81 (Knowledge city), Mohali, India
*Corresponding author: Sudesh Kumar Yadav, Center of Innovative and Applied Bioprocessing (CIAB), Sector-81 (Knowledge city), Mohali, 140306, India
Received: February 22, 2022; Accepted: April 01, 2022; Published: April 08, 2022
Abstract
Agricultural crops and its by-product utilization is an emerging area in Food Industry. The waste generated from processing industries is generally disposed off, ultimately hampering the environment. However, this waste is a rich source of many valuable components, pectin being one of them. Pectin, a heteropolysaccharide has numerous nutritional and functional properties making its extraction a matter of utmost importance. Although it is present in cell wall of most of the plants but its amount, structure and chemical composition differs between plants and makes its recovery tedious. In order to commercialize, yield and quality of pectin are important parameters to be considered during extraction. Therefore, selection of a convenient technique is essential. However, conventional extraction method is being widely used; it has some limitations such as laborious handling and certain environmental concerns. Therefore, there is a need to exploit novel pectin extraction techniques. In addition to that, there are many other parameters (increase in cost and skilled labor) to be considered in order to have clear insight of the possibilities to scale up the process. The present review discusses the chemical structure and classification of pectin, its properties and source of recovery with primary focus on innovative pectin extraction techniques.
Keywords: Agricultural crops; Pectin; Heteropolysaccharide; Laborious handling; Ohmic heating
Introduction
Pectin is a natural biopolymer categorized as heteropolysaccharide and is present mostly in the primary cell wall of plants [1]. In some of the plants, it is reported to be present in the middle lamella part of the plant cell wall. Pectin is a combination of various complex polysaccharides and provides mechanical strength to the plant tissue [2]. The main building block of pectin is galacturonic acid and unit is associated with other compounds present in plant cell wall like lignin, cellulose or polyphenols [3]. The pectin content is substantially more in the cell walls of some fruits and vegetables. Pectin helps in ion homeostasis as well as regulates the properties such as ion balance, pH, porosity, and surface change [4]. Also, pectin oligosaccharides help to activate plant defense responses [5]. Pectinase and pectinesterase are the enzymes responsible to hydrolyze the structure of the pectin during ripening process. Pectinase work by cleaving the main pectin chain and its side branches to disrupt the whole structure of the pectin and converting it to a simple soluble polymer [6]. The content, chemical constituents and structure of the pectin depends upon the source, and condition of the plant or plant part.
Chemical Structure of Pectin
Pectin is a multifunctional component of the plant cell wall consisting of linear polysaccharide (composed of a-galacturonic acid monomer) having molecular weight approx. 60,000-130,000 g/mol [7]. The carboxyl groups of uronic acid residues exist either in free form or as a salt form with calcium, sodium or other small counter ions and in some cases as naturally esterified groups, mainly with methanol. The reason of pectin being acidic in nature is the presence of free carboxyl groups. Pectin is both polydisperse and polymolecular. Hence, it is heterogeneous in its chemical structure and molecular weight [8]. Isolation of pectin is very tough as it changes according to storage, processing and source of the plant material [7].
Galacturonic acid sub-units in pectin are attached by a-1,4- glycosidic bonds. The carboxylic groups in galacturonic acid are fully or partly neutralized by sodium, ammonia or potassium ions and partly esterified by methyl groups. Pectin is composed of very complex set of polysaccharides covalently linked to each other. Homogalacturonan (HG) and rhamnogalacturonan I (RG-I) are the most abundant classes. Whereas, other classes include rhamnogalacturonan II (RGII), xylogalacturonan (XGA), and apiogalacturonan (AGA) [9]. As mentioned above, homogalacturonan are the most abundant form that are partially carboxylated at C-6 and acetylated at O-2 or O-3. The ability to interact and industrial applications are partly determined by methyl esterification of homogalacturonan regions. Methyl esterification is equivalent to the degree of methylation (DM) as a percentage, which is an important attribute to indicate the ability of the pectin to form gel. Rhamnogalacturonan-I (RGI) is a type of pectin with a backbone of the continuous units of disaccharide i.e. (1-4)-a-D-galacturonic acid-(1, 2)-a-L-rhamnose. Rhamnogalacturonan II (RG-II) type of pectin has a complex structure comprising of highly branched structure of polysaccharide. RG-II exists as a dimer in the primary walls of plants. While xyloglacturoanan and apiogalacturonan are much less complex regions. The homogalacturonan structure in pectin is substituted with xylose for xylogalacturonan and monosaccharide or disaccharide apiofuranosyl for apiogalacturonan. RG-II plays a crucial role in the structure of plant cell walls as small structure alterations of RG-II lead to the reduction in the dimers formation and can cause severe growth defects [10-12].
Structural Classifications of Pectin
Pectin can be classified according to their degree of esterification, acetylation and amidation as follows:
Degree of esterification (DE) in pectin
The Degree of esterification (DE) is defined as the percentage of esterified carboxyl groups present in the structure of pectin. Different properties like gelling, emulsifying and texturizing are dependent upon DE. It is an important parameter to determine further applications of pectin. With the increase in DE, the water solubility decreases due to the hydrophobic nature of esters. Therefore, increase in DE improves the gelation rate and results in the rapid gelation of pectin [8].
Pectin can be divided into two types depending upon their degree of esterification (DE) i.e., low methoxyl pectin (DE<50) or high methoxyl pectin (DE>50) [13]. These two types of pectin form the gels by different mechanisms. High methoxyl pectin (HMP) has DE higher than 50%. Such pectins are used mostly in the food industry because of their gelling and thickening properties. HMP is very sensitive to acidity and for gelation it requires large amount of sugar. Due to the presence of hydrogen bonding and hydrophobic interactions between pectin chains, they form gel at low pH within a narrow range of around 3.0 and high concentration of soluble solids. Gelation occurs when HG portions are cross-linked to form three dimensional crystalline networks in which water and other solutes are trapped. These gels are thermally reversible and are soluble in hot water. It is reported that formation of HMP gel occurs by different mechanisms, such as self-aggregation, esterification and entanglement under alkaline pH. Due to electronic attraction, dissociated carboxyl groups in HMP are bound to Na+ or K+, and thereby, enable HMP molecules to move close to each other and improve the gel network formation [8,14]. Low methoxyl pectin (LMP) has DE less than 50%. Generally, it is formed by the de-esterification of HMP and is not sensitive to pH. For gel formation, they require no sugar content and limited quantities of divalent cations such as Ca2+ [15]. Mechanism of gelation in LMP occurs by the formation of calcium bonds between two carboxyl groups. It has been observed that high concentration of calcium and pH values close to the isoelectric point (pH=3.50) improves the gel strength by building calcium bridges at dissociated carboxyl groups. LMP is commonly used in food industry to form low-sugar jams as it requires no sugar content for gelation [8].
Degree of acetylation in pectin
The degree of acetylation (DAC) can be defined as the total percentage of acetyl groups attached to the hydroxyl groups of galacturonosyl residues by ester bonds. It has been shown widely that acetylation of pectin is a stabilizing and emulsifying effects which concomitantly decreases the gel forming ability of the pectin [16- 18]. Studies have shown that pectins with a degree of acetylation up to 25% possess the reduced gelling properties [19]. Specifically, the multiple acetyl groups in sugar beet pectin provide it a surfactant group (COOCH3) with ammonia [20,21].
Degree of amidation in pectin
The percentage of amide groups present in the pectin is termed as the degree of amidation. The amidated pectin are thermo-reversible and resistant to variations of calcium preventing it from precipitation. Amidation prevents the syneresis and increases the solubility of pectin in water [21].
Various Sources of Pectin
In higher plants, one third of the dry weight of the cell wall is composed of pectin. The gelation property of pectin depends upon the molecular size and degree of esterification (DE). Pectin extracted from distinct sources have unique gelling properties owing to difference in these above- mentioned parameters. So, a fruit cannot be qualified as a source of commercial pectin solely on the basis of high pectin content [22]. At present, main source of commercial pectin is apple pomace and citrus peels. Both are by-products of cider (or juice) production. Apple pomace encompasses 10-15% of pectin, whereas citrus peels contain 20-30% pectin on a dry matter basis [23]. Citrus and apple pectin are basically equivalent from an application point of view. Citrus pectin appears light tan in color whereas apple pectin is often dark in color. Alternate sources such as eggplant peel, chamomile waste, cocoa pod husk, mango peel, banana peel or tomato husk have been considered to acquire pectin-based polymers of better quality. Also, tropical fruits have been recommended as compelling pectin sources. Due to the innumerable applications of pectin in various industries, the extraction of pectin from several biomasses and their waste has been widely studied. Table 1 summarizes various reported studies based on the extraction of pectin from different sources.
Source (common name/Scientific name)
% Pectin content (wet weight)
Apple (Malus spp.)
0.5-1.6
Apple pomace
1.5-2.5
Banana (Mus acuminate L.)
0.7-1.2
Beet pulp (Beta vulgaris)
1
Carambola (Averroha carambola)
0.66
Carrot (Daucus carota)
0.2-0.5
Gauva (Psidium guajava L.)
0.77-0.99
Lemon pulp (Citrus limon)
2.5-4.0
Lychee (Litchi chinesis L.)
0.42
Mango (Mangifera indica L.)
0.26-0.42
Orange peel (Citrus sinesis)
3.5-5.5
Papaya (Carica papaya)
0.66-1.0
Passion fruit (Passiflora edulis S.)
0.5
Passion fruit rind
2.1-3.0
Peaches (Prunus persica)
0.1-0.9
Pineapple (Ananas comosus L.)
0.04-0.13
Strawberries (Fragaria ananassa)
0.6-0.7
Tamarind (Tamarindus indica L.)
1.71
Thimbleberry (Rubus rosalfolius)
0.72
Tomato fruit (Lycopersicon esculentum)
0.2-0.6
Table 1: Different raw materials and percentage pectin content [77].
Properties of Pectin
Pectin is water soluble in nature. Monovalent cationic salts of pectic and pectinic acids are generally water soluble, whereas diand trivalent cation of salts are water insoluble or sparsely soluble in water. Dry pectin in powder form has the tendency to form clumps when added to water. The clumps so formed comprise of partially dry packets of pectin enclosed in a covering of moist outer envelope. It has been found that by improving dispersibility of pectin using special treatment during manufacturing or by mixing dry pectin with water soluble carrier material can prevent clump formation [14]. Dilute pectin solutions are usually Newtonian in nature but they also exhibit in non-Newtonian, pseudo plastic behavior at moderate concentrations. Gelation, viscosity and solubility are inter-connected. For example, components which increase the firmness of gels can lead to reduction in solubility increase in viscosity and thereby the overall proclivity to form gel easily.
The most important application of pectin is gel formation. HMpectin forms the gel in the presence of sugar at low pH. The structure of HM-pectin has some limitations due to the insufficient acid groups in HM-pectin to form gel or precipitate with calcium ions. However, other ions such as copper or aluminium can help in precipitation at certain conditions [14]. It has been reported that in order to form gel, hydrogen bonding and hydrophobic interactions are very significant forces [24]. Gel is formed by the interaction of free carboxyl groups with the hydroxyl groups of neighboring molecules through hydrogen bonding. Majority of the unesterified carboxyl groups are existing as partially ionized salts in neutral environment. At acidic pH, the carboxyl groups are transformed into unionized carboxylic acid moieties. Therefore, the lowering of number of negative charges leads to a decrease in repulsive forces between pectin molecules and reduces the bonding between the pectin and water molecules. Addition of sugar leads to competition between pectin and sugar to bind with water. Sugar being more soluble competes out pectin and further decreasing its solubility in water.
All the above-mentioned factors reduce the potential of pectin molecules to stay in a dispersed state and therefore aggregate together. As the pectin solution is cooled, the instability of less hydrated pectin molecules helps in the formation of a gel, a conspicuous matrix holding the aqueous solution. Moreover, Degree of esterification directly affects the rate of gel formation. A higher DE leads to faster and rapid gel formation. Pectin with DE of above 72% set rapidly whereas pectin with DE of 58-65% set comparatively slowly. It has been reported that for LM-pectin, the divalent cations are vital for appropriate gel formation. The process of LM-pectin gel formation works principally on the mechanism of widely accepted ‘egg-box’ model [25]. This model involves the formation of junction zones by the side-by-side aligned clusters of galacturonans, where the electrostatic and ionic bonding of carboxyl groups forms the intermolecular links between adjacent or parallel chains of GalA monomer. Now it is widely accepted that these junctions are composed of dimers in helical symmetry, similar to the model proposed for alginates [26]. The free-electron pairs of the oxygen atoms of the hydroxyl groups, the pyranose ring, and the glycosidic bonds of the component sugar units aid the bonding process [27]. The strength of the electrostatic bonds has a direct impact on the longevity of the junctions. The presence of a minimum of seven continuous carboxyl groups on the internal face of each participating chain is essential for the stability of the electrostatic bonds [28]. Interestingly, it has been reported that all LM-pectin gels develop similar type of junction zones [29]. Moreover, it has been also observed that the gelling ability of the LMpectin improves on amidation. Amidated pectin requires less calcium levels for gel formation and is resistant to precipitate formation at higher calcium quantities [23]. The Degree of Esterification strongly affects the strengths of such ionic bonded gels. The gel formation ability of pectin is also affected by monovalent cations like sodium as they can react with free carboxyl group and reduce the crosslinkage with calcium and ameliorate the solubility of LM-pectin in the presence of calcium [30]. It has been accepted that sugar is not necessary for the formation of gel with LM-pectins but around 10- 20% of sugar protects the gel from syneresis and provides the desired strength to these gels [31]. Moreover, the amount of calcium required to form gels can be reduced with addition of low quantities of sugar. According to the reports the higher concentrations of sugar (60% or more) are not favorable because it interferes with gel formation as the sugar undergoes dehydration which assists hydrogen bonding and removes cross-linking by divalent cations.
Pectin Extraction Methods: Conventional Extraction and Emerging New Innovative Extraction Technologies like Microwaveassisted Extraction, Enzyme-assisted Extraction, Ultrasound-assisted Extraction, Dielectric Barrier Discharge Extraction, Subcritical Water Extraction, Ohmic Heating Extraction, Moderate electric field Extraction, High Pressure Processing Extraction
Pectin is gaining more attention now-a-days because of its diverse applications in food sector. Therefore, extraction of pectin using different techniques has become more challenging and is compiled as shown in Figure 4.
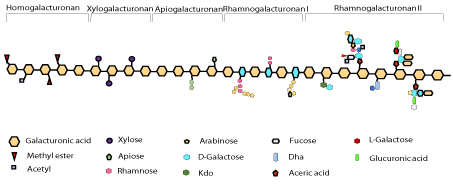
Figure 1: Pectin structure.
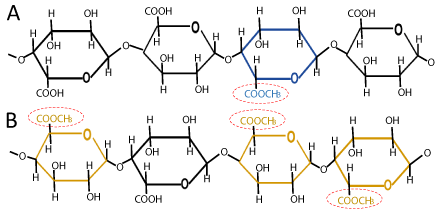
Figure 2: (A) Low-methoxyl and (B) high-methoxyl pectin structures.
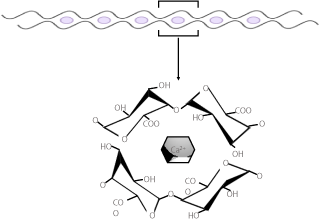
Figure 3: Gelation of LM pectin in the presence of Ca2+ cations.
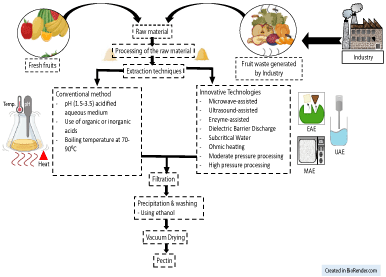
Figure 4: Conventional and Innovative technologies to extract pectin.
Conventional extraction method for pectin
Pectin is insoluble is nature when present in the cell wall of plants and known as “protopectin”. Protopectin can be extracted by hydrolysis with a hot diluted mineral acid. This helps in breaking the bonds between the cell wall and the sugars present on the side chains and then releasing the pectin into the aqueous medium [32]. The pectin is precipitated and purified in various ways and finally dried. Due to the high yield and good quality of pectin, citrus peels are considered to be the main source to obtain pectin at industrial level [33]. The most common method to extract pectin from orange peels is hydrothermal extraction which involves extraction under acidic conditions, extraction time (60-300 min), and high temperatures (75- 95°C). The acidic medium reduces the presence of other bioactive compounds like polyphenol which increases the yield of pectin and helps in improving the quality of extracted pectin. In order to obtain higher yield, conventional extraction using boiling water requires several hours [34,35]. During the extensive heating process, pectin degrades by debranching which leads to the poor quality of pectin. Therefore, appropriate conditions to extract pectin is acidic medium (pH 1.5-3), heating between 75 and 100 °C with continuous stirring for 1-3 h. Generally, pectin is extracted in industry using strong acid solutions under the heating environment such as hydrochloric acid, sulphuric acid, nitric acid and phosphoric acid. The use of such strong acids possesses a risk to environment and increases the cost of the process. Use of mineral acids has some other drawbacks as well, such as loss of some volatile compounds, and degradation of pectin. As the new concept of “green technology” and “green chemistry” is emerging rapidly. The attention is now moving towards the use of organic acids (citric acid and acetic acid) [36]. Research has shown that during extraction of pectin, use of mineral acids lead to more hydrolysis and more depolymerization as compared to the use of organic acids. The HCl has documented to be more pectin degrading agent compared to citric acid. Pectin extracted using mineral acid is expected to have lower molecular weight as compared to that of pectin extracted from organic acid.
There are many factors, such as pH, solid to liquid ratio, solvent properties, temperature, time, diffusion rate and particle size which are important for conventional extraction. Some pretreatments like washing with water, blanching in order to inactivate the enzymes, drying to remove excess water and grinding to increase the surface area are also done in order to get high yield of pectin [37]. After all these pretreatments, pectin is subjected to acidic aqueous solution and released pectin was precipitated with the help of alcohol. The precipitated pectin is then separated, washed and dried under vacuum oven, and finally crushed into fine powder. From the commercial point of view, there are other steps that can be performed in between in order to get desired quality of pectin. For example, between the filtration and washing step, removal of color of pectin can be done by using activated carbon/charcoal, degradation of residual starch can be carried out by using the amylase. Moreover, LM- pectin can be obtained with the help of chemical, acid, and/or alkaline de-esterification. Acidic aqueous solution at low pH helps in releasing protopectin easily and also removes Ca2+ and Mg2+, thus allowing isolation of HG enriched pectin with higher yield. Structure of alkaline pectin have many RG-I oligomers with arabinan and galactan side chains and usually have low DM and low yields [38]. As the heating time increases, degree of esterification in pectin decreases. Pectin extracted through different extraction techniques has different DEs. Considering the extraction time, prolonged extraction procedure can lead to pectin degradation. It should be in the range where solvent dissolves appropriate amount of the target molecule. Therefore, in order to get high yield and better quality product, desired conditions should be optimized properly. The solid/liquid ratio is also an important attribute for the extraction of pectin and should be maintained between 1:10 and 1:50. Solid/liquid ratio lower than 1:10 leads to lower yield of pectin. This is because the quantity of solvent is not enough to dissolve the biomass properly and extract the pectin from it. It has been reported that increase in solid/liquid ratio leads to the rise in dissolution capacity which generate high yield of pectin [17]. Various studies on pectin extraction by conventional acid extraction method are compiled in Table 2.
Sources
Treatments
Extraction conditions
Yield (%)
References
S/L ratio
Solvent
pH
Time (min)
Temp (°C)
Grapefruit peel
CE
1:50
HCl
1.5
90min
80°C
23.5
[78]
Grapefruit peel
CE
1:50
HCl
1.5
1.5h
80°C
-
[79]
Passion fruit
CE
1:30
HCI
2
60min
98.7°C
14.8
[17]
Citron peels
CE
1:30
Citric acid
1.5
95
95°C
28.31
[80]
Pomelo peels
CE
1:30
HNO3
2
90
90°C
23.19
[81]
Beet pulp
CE
1:50
HCl
1
3h
80°C
20
[82]
Lime peel
CE
1:40
HCl
-
1h
95°C
15.91
[83]
Papaya peel
CE
1:50
HCl
2
60min
80°C
16
[84]
Potato pulp
CE
1:15
Citric acid
2.04
60min
90°C
14.34
[85]
Sugar beet pulp
CE
1:20
-
1.5
1h
80°C
7.1
[86]
Carrot pomace
CE
-
-
1.3
79.8min
90°C
15.2
[87]
Apple pomace
CE
1:25
HNO3
1.5
70
90°C
25.3
[88]
Pomegran-ate peel
CE
1:20
HNO3
1.7
80min
86°C
8.5
[85]
Water-melon peel
CE
1:20
H2SO4
1
150min
90°C
17.6
[89]
Apple pomace
CE
1:40
HNO3
-
10min
BT
15.04
[90]
CE: Conventional Extraction.
Table 2: Pectin extraction from different sources by conventional method.
Emerging new innovative extraction technologies for pectin
Several other techniques have been examined to decrease the time of extraction of pectin from citrus by-products. Further investigations are required to obtain new extraction procedures for the improvement of pectin quality and reduction of extraction time. The model extraction method which is extensively used has several drawbacks like longer extraction time, lower yield and chances of degradation of pectin. To address the aforementioned issues, different approaches have been explored which could increase the yield, lessen the solvent consumption and protect the functional properties of pectin [37]. Microwave-assisted extraction (MAE), Enzyme-assisted extraction (EAE), Ultrasound-assisted extraction (UAE), Subcritical fluids, and high hydrostatic pressure are some of the current alternatives. These methods are sustainable and much efficient than the classic method of pectin extraction [39]. The pursuit for inventive and greener extraction methods has led the investigations into amalgamation of different techniques with the goal of consolidating their advantages. Furthermore, the development of new methods has provided investment opportunities for industries and with these superior techniques they can obtain specific extractions of high value added purified compounds. Though the expenses of microwave or ultrasound based equipment seem to be higher but the energy consumption, time of extraction, cost of reagents are nullified and responsible for higher yield. Hence, these methods will prove to be profitable in the long run. Some of the above-mentioned techniques are discussed below:
Microwave-assisted extraction (MAE) for pectin: It is an environment-friendly technique and uses the polar solvent to absorb microwave energy enclosed in electromagnetic field [40]. The microwave radiation heats up the polar solvent very fast to make the extraction process less time consuming. This makes the process more efficient as compared to the traditional heating methods. It has been reported that pectin depolymerization which occurs during acid extraction process can be reduced by MAE technique. Additionally, energy from microwave irradiation can enhance cell lysis through an escalated internal pressure inside the cells of the plant sample and an abrupt increase in temperature, which disintegrates the sample surface and as a result pectin exudes from the plant cells into the solvent [41].
Enzyme-assisted extraction (EAE) for pectin: Currently, enzymes are also being utilized to ameliorate the extraction process by dissolving the matrix of the plant cell wall. Application of enzymes helps in increasing the cell permeability by destroying the cell wall. Several factors like concentration of enzyme, reaction temperature, time and type of enzyme and particle size of sample play a key role in enzyme-assisted extraction [42]. Pectinases are the enzymes which are employed for the extraction of pectin from the plant cell wall. Pectinases are isolated majorly form fungi. Enzymes used extensively in EAE are namely, cellulase, protease, alcalase, hemicellulase, pectin lyase, xylanase, a-amylase, β-glucosidase, endo- and exopolygalacturonase and pectinesterase. These enzymes serve as the agents for breaking the glycosidic bonds between the monomers of pectin. Breaking of the glycosidic bond reduces the viscosity of the solution which further eases the centrifugation and filtration. According to Rhein-Knudsen et al. [43], the most credible benefit of using this green technique for the extraction of pectin is the preservation of structural traits and functional properties of the target polysaccharides. Enzyme extraction is considered to be least polluting extraction method compared to other methods. However, the isolation and procurement of the enzymes is somewhat exorbitant as well as controlling enzymatic reactions at commercial level is also a tedious task. Different enzymes have a unique response to changing environmental conditions like temperature and nutrient availability which can ultimately effect the yield of pectin [44].
Ultrasound-assisted extraction (UAE) for pectin: Soundwaves with frequencies above 20kHz are called Ultrasounds. Ultrasonic waves, in the range of 20 to 100 kHz, are widely used in UAE [45,46]. Ultrasounds (Us) have been profoundly utilized in the food industry because of their chemical and/or physical properties. Unlike electromagnetic waves, sound waves require a medium for propagation. In UAE method, when sound waves pass through a liquid medium, they create cycles of compression and expansion [47]. For a liquid medium, the expansion cycle creates bubbles/cavities which grows and subsequently experience collapse as the negative pressure exerted exceeds the local tensile strength of the liquid. This process of formation, growth and collapse of bubbles is known as “cavitations”, and is the basis for ultrasound-assisted extraction (UAE) [48]. There are several advantages of UAE such as decreased extraction time, equipment size, and energy consumption, lower use of solvent and improved extraction yield, and it is also acknowledged more eco-friendly than the conventional acid extraction method [49].
Dielectric barrier discharge extraction for pectin: Another method is Dielectric Barrier Discharge Extraction which involves the innovative use of plasma, the fourth state of matter. Plasma contains gases which are partially ionized consisting of reactive species like anions, cations, photons, free radicals and gas atoms. Dielectric Barrier Discharge is one among several other methods to generate cold plasma [50]. This method can employ the plasma’s active constituents as the means to destroy particular bonds leading to overall modification and degradation of macro biomolecules like proteins and polysaccharides. It has been observed that the chemically active species from DBD plasma produce hydroxyl free radicals which attack the pectin chains and generate molecules of smaller sizes [51]. Though DBD plasma has tremendous potential, pectin extraction by this technique has not captivated the imaginations of researchers. Therefore, there are very sparse studies and data on this subject. In near future DBD plasma can be used to alter the pectin structures.
Subcritical water extraction for pectin: Subcritical water extraction involves the use of water at higher pressure reaching a temperature more than its normal boiling temperature without undergoing any phase alteration. When such a solvent is utilized in extraction, the process is called subcritical water extraction (SWE) which is also known as pressurized hot water extraction (PHWE) and superheated water extraction (SHWE) [52]. Hence this principle has been applied within the food and environmental fields reported with many other different names. The rise in temperature of water for extraction has many advantages like low viscosity, low surface tension, more mass transfer rate, and high diffusion. It may also help to extract both non-ionic and ionic compounds as the dielectric constant of water reduces from 79 at 25°C to 33 at 200°C [53]. Recent studies have shown work on SWE processes for pectin from many different plants. Ueno et al. [54] have shown the comparison between SWE and conventional acid extraction method to isolate pectin from the flavedo part of Citrus junos. Authors observed that the subcritical water at temperature 160°C increases the extraction rate quickly. This can be explained that the solvent at high temperature with low dielectric point helps in enhancing the solubility of pectin in water. A reported study showed lower yield of pectin at temperature more than 80°C due to the degradation of pectin. Chen et al. showed in their study that subcritical water aid in increasing mass transfer and optimum temperature can be helpful to elude the acid hydrolysis step in conventional acid extraction. This eco-friendly method offers many benefits such as rapid extraction process, exclude solvent, and higher quality extracts [52,54]. Generally, subcritical water as a solvent is considered to be as safe (GRAS) and makes this method appropriate for food and pharmaceutical compounds like pectin.
Ohmic heating extraction for pectin: Ohmic heating is a cutting edge technology in which electric current is passed through the desired food material producing the heat according to Joule’s law [55]. This technique is one of the advanced thermal process which is quick and constant in nature. This method has been employed to sterilize foods and to recover different valuable compounds from plants. Ohmic heating rapidly heats the heterogeneous system through volumetric heating causing the proper mass and heat transfer during the extraction. Thus, minimizes the variations in pectin properties and decreases the processing time. So, this feasible technique can be used to improve the quality of pectin [56].
Moderate electric field extraction for pectin: It is yet another non-thermal method which uses an electric field (low frequencies) at relatively low temperatures that helps in electroporation and improves diffusion. Application of voltage distresses the cells membrane and improves their permeability. Pulse electric field is similar to MEF and works by producing electroporation on cytoplasmic membranes and enhances its intracellular constituents by evading unwanted changes in plant material [56].
High pressure processing extraction for pectin: One of the commonly used non-thermal processing techniques in food industry is high pressure processing (HPP). This method includes sealing of the sample in special packages and keeping them in a vessel under isostatic pressure above 300 MPa. High pressure damages the plant cells which leads to the proper diffusion of solvents and increases mass transfer and liberates the extracts [57]. This extraction technique can be applied in synergetic extraction to increase the yield of pectin and other bioactive compounds by decreasing the extraction time.
Regardless of extensive work in laboratories on these emerging extraction techniques, still there are various promising challenges for the industrial application. Like the requirement of professional operators, high capital expenditure, and less suppliers of these machinery. Currently various efficient and effective novel technologies are being investigated to extract pectin from different agricultural byproducts and food wastes such as microwave, ultrasound, subcritical water, enzymes and thermal or non-thermal methods, with different levels of successes shown in table 3. Apparently, all above mentioned techniques have provided very promising results both qualitatively and quantitatively at laboratory scale, so far, sometimes unsatisfactory and inadequate knowledge of their applicability upon scale-up can hamper their commercialization. Therefore, proper professional training and knowledge of up-scaling should be kept in mind by the scientists in this area of research.
Sources
Treatments
Extraction conditions
Yield (%)
References
S/L ratio
Solvent
pH
Time (min)
Temp (°C)
Lime peel
EAE
1:30
Citrate buffer
3.5
4h
50°C
22.5
[91]
Beetroot
EAE
0.11111
Citrate buffer
-
20h
30°C
-
[92]
Chicory root
EAE
-
Sodium acetate buffer
5.5
4h
50°C
-
[91]
Butternut squash
EAE
0.11111
Citrate buffer
-
20h
-
-
[92]
Green tea leaf
EAE
-
HCl
4.5
3h
3°C
8.5
[93]
Eggplant peel
UAE
1:20
Citric acid
1.5
30min
-
33.64
[94]
Prickly pear
UAE
1:30
-
1.5
70min
70°C
18.14
[95]
Pomegranate peels
UAE
1:15
Citrate buffer
5
20min
-
24.8
[96]
Grapefruit
UAE
1:50
HCl
1.5
25min
70°C
17.92
[97]
Grapefruit peel
UAE
1:50
HCl
1.5
27.9min
66.7°C
27.46
[78]
Apple peel waste
UAE
1:23
HCl
2.36
18min
63°C
8.93
[98]
Dragon fruit
MAE
1:56
Citric acid
2.9
12min
75°C
17.01
[99]
Sweet lemon peel
MAE
-
Citric acid
1.5
3min
-
25.31
[100]
Grapefruit
MAE
1:50
HCl
-
6min
-
27.81
[97]
Banana peels
MAE
1:50
HCl
3
100s
-
2.18
[101]
Watermelon rinds
MAE
0.11111
Acetic acid
2
12min
-
5.76
[102]
Lime peel
MAE
1:40
HCl
-
-
-
23.32
[83]
Pistachio green hull
MAE
1:15
-
1.5
165s
-
18.13
[103]
Pomelo peel
MAE
1:30
HCl
-
2min
-
20.5
[16]
Orange waste
Ohmic heating
1:20
-
1.5
15s
90°C
10.69
[104]
EAE: Enzyme-Assisted Extraction; UAE: Ultrasound-Assisted Extraction; MAE: Microwave-Assisted Extraction.
Table 3: Different innovative extraction treatments and conditions to extract pectin.
Applications of Pectin in Various Sectors
Applications of pectin in food industry
Generally, in the food industry pectin is used as a thickening, gelling, emulsifying and stabilizing agent [58-61]. The property of pectin to form hydrogels makes its use in viscous and hydrated foods. Pectin is popularly used in fruit juices, jams, jellies, desserts and many other dairy products. The use of pectin as a stabilizing agent in colloidal dispersions differs between fruit drinks having high protein content, foods fortified with antioxidants, and acidic milk drinks [62,63]. Nowadays, pectin is getting attention because of its property to make films. Such films are used in the food industry to make edible coatings and packaging materials [61,64]. This ability of pectin defines its usage in the preparation of biodegradable food packaging. Edible coatings formed from pectin are biodegradable, renewable and biocompatible. Hence, such coatings are very useful for food preservation [65]. This concept is related to green chemistry and will help to support efforts of the sustainable world. Edible coating of food products has various applications such as it helps in extending the shelf life of the food, retaining the firmness, control water loss and minimize the decay in fruits [66]. However, still there are some drawbacks in the use of edible coatings for meat products. While scope of usage of such coatings has been realized for fruits and vegetables. Edible coating has many positive features when used to produce stand-alone films and also shows promising results when assimilated with active compounds, or even integrated with other polymers [67].
Applications of pectin in pharmaceutical industry
Pectin is an extremely valuable asset for the pharmaceutical industry. Pectin is found to have a positive effect on cholesterol levels in blood. It has been well-known that pectin helps to decrease blood cholesterol levels [68]. The intake of approximately 6g/day pectin has been shown to remarkably decrease the cholesterol levels in blood. Quantities below 6g/day of pectin have been reported to be ineffective [69]. Reports claim that a 13% reduction in blood cholesterol level has been observed within 2 weeks [70]. Pectin can also function against fatal cations, as a prophylactic agent. Studies have reported pectin to be highly efficacious in removal of mercury and lead from the respiratory and gastrointestinal organs [27]. Pectin is found to act as an anticoagulant when injected in the bloodstream for the prevention of haemorrhage [71]. In newborns and children, a blend of pectin and other colloids has been used considerably to aid diarrhea. Pectin can also act against E. coli as bactericidal agent as documented through in vitro conditions [22]. Pectin slows down the movement of food components in the intestine thereby slowing the speed of digestion, as an outcome the absorption of food usually decreases. The density of pectin coating inversely affects the food absorption as it forbids any exposure of food to the intestinal enzymes [72]. Pectin has a great proclivity for water binding and gives a feeling of fullness as well as simultaneous decrease in food intake. Pectin is a fascinating compound and can be put to pharmaceutical use in several ways, e.g. as an excipient, a vehicle for range of drugs in monitored-release operations. Gel coating and ionotropic gelation are some methods which have been used to develop pectin-based carrier systems. The ease of working with pectin and its chemically inert nature makes it an astounding candidate as an excipient for industrial applications. In the medicinal industry, the utilization of pectin in the manufacturing of controlled-release of drugs is ongoing for example, as a vehicle material in colon-targeted drug-delivery complexes [68]. While using pectin as a coating agent in drug delivery, the methyl content of pectin becomes an important factor. Mostly HM pectin are favored for encapsulation because of high molecular weight and low solubility in water. In cases where there is a risk of early erosion of gel coating LM pectin are used [73]. Recent reports have suggested that a blend of pectin with natural polymers like chitosan, or bioactive compounds, such as curcumin and cysteine can increase gel resistance, reduce water solubility, and reduce erosion of coating gels [74-76].
Future Trends of Research for Pectin
With the recent advancement in agro-processing for valorization, huge waste is continuously generated. Such biomass has several dimensions of exploration for various purposes. One of the most thought product is the pectin for lots of commercial opportunities in the food, nutraceutical and pharmaceutical industries. Different biomasses have different levels of molecular complexities and therefore, to recover pectin from such biomasses need optimization of process technology. We further need to explore the techniques and methods described above as well as to invent more economical and viable techniques for the same. Characterization of obtained pectin is very important in terms of its ultimate use for various applications. Research efforts should also be focused on the optimization of process in fortification of pectin based products.
Conclusion
The market for hydrocolloids like pectin is growing rapidly. In this review, various properties of pectin and extraction techniques along with their applications in pharmaceutical and food industry has been discussed. Some of the applications of pectin are well-known but many more are yet to be discovered. As the demand for pectin is increasing there is a need to upgrade the extraction processes in such a manner that the speed and reproducibility of the process can be enhanced. Currently there are a number of efficient and effective innovative technologies being studied for the recovery of pectin, including microwave, enzyme-assisted, ultrasound, subcritical water, high pressure and ohmic heating, with unprecedented levels of accomplishments. However, all these innovative techniques are helpful at laboratory scale only, but to achieve similar results upon scale-up is tricky. Also these novel technologies, when shifted on a larger scale will increase the cost and new challenges can arise. Thus proper knowledge and understanding of such innovative technologies is absolutely necessary for scaling them up. Therefore, for successful commercialization, these limitations should be contemplated in future work.
References
- Martau GA, Mihai M, Vodnar DC. The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness, and biodegradability. Polymers. 2019; 11: 1837.
- Jarvis MC, Briggs SPH, Knox JP. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003; 26: 977-989.
- Harholt J, Suttangkakul A, Vibe Scheller H. Biosynthesis of pectin. Plant physiology. 2010; 153: 384-395.
- McNeil M, Darvill AG, Fry SC, Albersheim P. Structure and function of the primary cell walls of plants. Annual review of biochemistry. 1984; 53: 625- 663.
- Hahn MG, Darvill AG, Albersheim P. Host-pathogen interactions: XIX. The endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiology. 1981; 68: 1161- 1169.
- Pedrolli DB, Monteiro AC, Gomes E, Carmona EC. Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnology Journal. 2009: 9-18.
- Srivastava P, Malviya R. Sources of pectin, extraction and its applications in pharmaceutical industry- An overview. 2011.
- Mellinas C, Ramos M, Jimenez A, Garrigos MC. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials. 2020; 13: 673.
- Gawkowska D, Cybulska J, Zdunek A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers. 2018; 10: 762.
- Belkheiri A, Forouhar A, Ursu AV, Dubessay P, Pierre G, Delattre C, et al. Extraction, characterization, and applications of pectins from plant byproducts. Applied Sciences. 2021; 11: 6596.
- do Nascimento Oliveira A, de Almeida Paula D, de Oliveira EB, Saraiva SH, Stringheta PC, Ramos AM. Optimization of pectin extraction from Uba mango peel through surface response methodology. International journal of biological macromolecules. 2018; 113: 395-402.
- Sharma BR, Naresh L, Dhuldhoya NC, Merchant SU, Merchant UC. An overview on pectins. Times Food Processing Journal. 2006; 23: 44-51.
- BeMiller JN. An introduction to pectins: structure and properties. 1986.
- Sriamornsak P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn University International Journal. 2003; 3: 206-228.
- Fishman ML, Chau HK, Qi PX, Hotchkiss AT, Garcia RA, Cooke PH. Characterization of the global structure of low methoxyl pectin in solution. Food Hydrocolloids. 2015; 46: 153-159.
- Wandee Y, Uttapap D, Mischnick P. Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocolloids. 2019; 87: 237-244.
- Kulkarni SG, Vijayanand P. Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.). LWT-Food Science and Technology. 2010; 43: 1026-1031.
- Stephen AM, Phillips GO. Food polysaccharides and their applications. CRC press. 2016.
- Williams PA. (Ed.). Renewable resources for functional polymers and biomaterials. Polysaccharides, proteins and polyesters. Royal society of chemistry. 2011.
- Matia-Merino L, Lau K, Dickinson E. Effects of low-methoxyl amidated pectin and ionic calcium on rheology and microstructure of acid-induced sodium caseinate gels. Food Hydrocolloids. 2004; 18: 271-281.
- Chen J, Niu X, Dai T, Hua H, Feng S, Liu C, et al. Amino acid-amidated pectin: Preparation and characterization. Food chemistry. 2020; 309: 125768.
- Thakur BR, Singh RK, Handa AK, Rao MA. Chemistry and uses of pectin. A review. Critical Reviews in Food Science & Nutrition. 1997; 37: 47-73.
- May CD. Industrial pectins: sources, production and applications. Carbohydrate polymers. 1990; 12: 79-99.
- Oakenfull DG. The chemistry of high-methoxyl pectins. The chemistry and technology of pectin. 1991: 87-108.
- Grant GT, Morris ER, Rees DA, Smith PJ, Thom D. Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS letters. 1973; 32: 195-198.
- Axelos MAV, Thibault JF. The chemistry of low-methoxyl pectin gelation. The chemistry and technology of pectin. 1991; 6: 109-108.
- Kohn R. Binding of divalent cations to oligomeric fragments of pectin. Carbohydrate Research. 1987; 160: 343-353.
- Powell DA, Morris ER, Gidley MJ, Rees DA. Conformations and interactions of pectins: II. Influence of residue sequence on chain association in calcium pectate gels. Journal of molecular biology. 1982; 155: 517-531.
- Filippov MP, Komissarenko MS, Kohn R. Investigation of ion exchange on films of pectic substances by infrared spectroscopy. Carbohydrate polymers. 1988; 8: 131-135.
- Axelos MAV. Ion complexation of biopolymers. Macromolecular structure and viscoelastic properties of gels. In Makromolekulare Chemie. Macromolecular Symposia. 1990; 39: 323-328. Basel: Huthig & Wepf Verlag.
- Christensen SH. Pectins. In Food hydrocolloids. 2020: 205-230. CRC Press.
- Turakhozhaev MT, Khodzhaev MA. Plant pectin substances. Methods of isolating pectin substances. Chemistry of Natural Compounds. 1993; 29: 558-565.
- Chavan P, Singh AK, Kaur G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. Journal of Food Processing and Engineering. 2018; 41: e12895.
- Li DQ, Jia X, Wei Z, Liu ZY. Box-Behnken experimental design for investigation of microwave-assisted extracted sugar beet pulp pectin. Carbohydrate Polymers. 2012; 88: 342-346.
- Mao G, Wu D, Wei C, Tao W, Ye X, Linhardt RJ, et al. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends in Food Science & Technology. 2019; 94: 65-78.
- Picot-Allain MCN, Ramasawmy B, Emmambux MN. Extraction, characterization, and application of pectin from tropical and sub-tropical fruits: a review. Food Reviews International. 2020: 1-31.
- Maric M, Grassino AN, Zhu Z, Barba FJ, Brncic M, Brncic SR. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound, microwaves, and enzymeassisted extraction. Trends in Food Science & Technology. 2018; 76: 28-37.
- Yang JS, Mu TH, Ma MM. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food chemistry. 2018; 244: 197-205.
- Adetunji LR, Adekunle A, Orsat V, Raghavan V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocolloids. 2017; 62: 239-250.
- Routray W, Orsat V. Microwave-assisted extraction of flavonoids: a review. Food and Bioprocess Technology. 2012; 5: 409-424.
- Maran JP, Sivakumar V, Thirugnanasambandham K, Sridhar R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydrate polymers. 2014; 101: 786-791.
- Poojary MM, Orlien V, Passamonti P, Olsen K. Enzyme-assisted extraction enhancing the umami taste amino acids recovery from several cultivated mushrooms. Food chemistry. 2017; 234: 236-244.
- Rhein-Knudsen N, Ale MT, Meyer AS. Seaweed hydrocolloid production: an update on enzyme assisted extraction and modification technologies. Marine drugs. 2015; 13: 3340-3359.
- Munarin FABIOLA, Tanzi MC, Petrini PAOLA. Advances in biomedical applications of pectin gels. International journal of biological macromolecules. 2012; 51: 681-689.
- Pena-Pereira F, Tobiszewski M. (Eds.). The application of green solvents in separation processes. Elsevier. 2017.
- Li L, Fang Y, Vreeker R, Appelqvist I, Mendes E. Reexamining the egg-box model in calcium-alginate gels with X-ray diffraction. Biomacromolecules. 2007; 8: 464-468.
- Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, et al. Techniques for extraction of bioactive compounds from plant materials: A review. Journal of food engineering. 2013; 117: 426-436.
- Luque-Garcia JL, De Castro ML. Ultrasound: a powerful tool for leaching. TrAC Trends in Analytical Chemistry. 2003; 22: 41-47.
- Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics sonochemistry. 2017; 34: 540-560.
- Misra NN, Tiwari BK, Raghavarao KSMS, Cullen PJ. Nonthermal plasma inactivation of food-borne pathogens. Food Engineering Reviews. 2011; 3: 159-170.
- Misra NN, Martynenko A, Chemat F, Paniwnyk L, Barba FJ, Jambrak AR. Thermodynamics, transport phenomena, and electrochemistry of external field-assisted nonthermal food technologies. Critical Reviews in Food Science and Nutrition. 2018; 58: 1832-1863.
- Zakaria SM, Kamal SMM. Subcritical water extraction of bioactive compounds from plants and algae: applications in pharmaceutical and food ingredients. Food Engineering Reviews. 2016; 8: 23-34.
- Brunner G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. The Journal of Supercritical Fluids. 2009; 47: 373- 381.
- Ueno H, Tanaka M, Hosino M, Sasaki M, Goto M. Extraction of valuable compounds from the flavedo of Citrus junos using subcritical water. Separation and Purification Technology. 2008; 62: 513-516.
- Gavahian M, Chu YH, Farahnaky A. Effects of ohmic and microwave cooking on textural softening and physical properties of rice. Journal of Food Engineering. 2019; 243: 114-124.
- Gavahian M, Mathad GN, Pandiselvam R, Lin J, Sun DW. Emerging technologies to obtain pectin from food processing by-products: A strategy for enhancing resource efficiency. Trends in Food Science & Technology. 2021.
- Balasubramaniam VM, Martinez-Monteagudo SI, Gupta R. Principles and application of high pressure-based technologies in the food industry. Annual review of food science and technology. 2015; 6: 435-462.
- Wang W, Chen W, Zou M, Lv R, Wang D, Hou F, et al. Applications of power ultrasound in oriented modification and degradation of pectin: A review. Journal of Food Engineering. 2018; 234: 98-107.
- Verkempinck SHE, Salvia-Trujillo L, Denis S, Van Loey AM, Hendrickx ME, Grauwet T. Pectin influences the kinetics of in vitro lipid digestion in oil-inwater emulsions. Food chemistry. 2018; 262: 150-161.
- Sun D, Chen X, Zhu C. Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: A comparison of different extraction methods. International Journal of Biological Macromolecules. 2020; 158: 1239-1247.
- Espitia PJP, Du WX, de Jesus Avena-Bustillos R, Soares NDFF, McHugh TH. Edible films from pectin: Physical-mechanical and antimicrobial properties-A review. Food hydrocolloids. 2014; 35: 287-296.
- Douglas TE, Hempel U, Zydek J, Vladescu A, Pietryga K, Kaeswurm JA, et al. Pectin coatings on titanium alloy scaffolds produced by additive manufacturing: Promotion of human bone marrow stromal cell proliferation. Materials Letters. 2018; 227: 225-228.
- Jindal M, Kumar V, Rana V, Tiwary AK. Aegle marmelos fruit pectin for food and pharmaceuticals: Physico-chemical, rheological and functional performance. Carbohydrate polymers. 2013; 93: 386-394.
- Sakooei-Vayghan R, Peighambardoust SH, Hesari J, Peressini D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food chemistry. 2020; 311: 125978.
- Chaturvedi K, Sharma N, Yadav SK. Composite edible coatings from commercial pectin, corn flour and beetroot powder minimize post-harvest decay, reduces ripening and improves sensory liking of tomatoes. International journal of biological macromolecules. 2019; 133: 284-293.
- Anastas PT, Warner JC. Green chemistry. Frontiers. 1998; 640: 1998.
- Mendes JF, Norcino LB, Manrich A, Pinheiro ACM, Oliveira JE, Mattoso LHC. Characterization of pectin films integrated with cocoa butter by continuous casting: physical, thermal and barrier properties. Journal of Polymers and the Environment. 2020; 28: 2905-2917.
- Sriamornsak P. Pectin: The role in health. Journal of Silpakorn University. 2001; 21: 60-77.
- Ginter E, Kubec FJ, Vozar J, Bobek P. Natural hypocholesterolemic agent: pectin plus ascorbic acid. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin-und Ernahrungsforschung. Journal international de vitaminologie et de nutrition. 1979; 49: 406-412.
- Miettinen TA, Tarpila S. Effect of pectin on serum cholesterol, fecal bile acids and biliary lipids in normolipidemic and hyperlipidemic individuals. Clinica Chimica Acta. 1977; 79: 471-477.
- Joseph GH. Pectin: Bibliography of pharmaceutical literature. Ontario: Sunkist Growers. 1956.
- Flourie B, Vidon N, Florent CH, Bernier JJ. Effect of pectin on jejunal glucose absorption and unstirred layer thickness in normal man. Gut. 1984; 25: 936- 941.
- Wicker L, Kim Y, Kim MJ, Thirkield B, Lin Z, Jung J. Pectin as a bioactive polysaccharide-Extracting tailored function from less. Food Hydrocolloids. 2014; 42: 251-259.
- Hwang SW, Shin JS. Pectin-coated curcumin-chitosan microparticles crosslinked with Mg2+ for delayed drug release in the digestive system. International Journal of Polymer Science. 2018.
- Bai F, Diao J, Wang Y, Sun S, Zhang H, Liu Y, et al. A new water-soluble nanomicelle formed through self-assembly of pectin-curcumin conjugates: preparation, characterization, and anticancer activity evaluation. Journal of agricultural and food chemistry. 2017; 65: 6840-6847.
- Majzoob S, Atyabi F, Dorkoosh F, Kafedjiiski K, Loretz B, Bernkop-Schnürch A. Pectin-cysteine conjugate: synthesis and in-vitro evaluation of its potential for drug delivery. Journal of pharmacy and pharmacology. 2006; 58: 1601- 1610.
- Shivamathi CS, Gunaseelan S, Soosai MR, Vignesh NS, Varalakshmi P, Kumar RS, et al. Process optimization and characterization of pectin derived from underexploited pineapple peel biowaste as a value-added product. Food Hydrocolloids. 2022; 123: 107141.
- Wang W, Ma X, Xu Y, Cao Y, Jiang Z, Ding T, et al. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food chemistry. 2015; 178: 106- 114.
- Wang W, Ma X, Jiang P, Hu L, Zhi Z, Chen J, et al. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocolloids. 2016; 61: 730-739.
- Pasandide B, Khodaiyan F, Mousavi Z, Hosseini SS. Pectin extraction from citron peel: optimization by Box-Behnken response surface design. Food science and biotechnology. 2018; 27: 997-1005.
- Methacanon P, Krongsin J, Gamonpilas C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters and its properties. Food Hydrocolloids. 2014; 35: 383-391.
- Mesbahi G, Jamalian J, Farahnaky A. A comparative study on functional properties of beet and citrus pectins in food systems. Food Hydrocolloids. 2005; 19: 731-738.
- Rodsamran P, Sothornvit R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food chemistry. 2019; 278: 364-372.
- Altaf U, Immanuel G, Iftikhar F. Extraction and characterization of pectin derived from papaya (Carica papaya Linn.) peel. International Journal of Science, Engineering and Technology. 2015; 3: 970-974.
- Yang X, Nisar T, Hou Y, Gou X, Sun L, Guo Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocolloids. 2018; 85: 30-38.
- Pi F, Liu Z, Guo X, Guo X, Meng H. Chicory root pulp pectin as an emulsifier as compared to sugar beet pectin. Part 1: Influence of structure, concentration, counterion concentration. Food Hydrocolloids. 2019; 89: 792-801.
- Jafari F, Khodaiyan F, Kiani H, Hosseini SS. Pectin from carrot pomace: Optimization of extraction and physicochemical properties. Carbohydrate polymers. 2017; 157: 1315-1322.
- Yapo BM, Besson V, Grah AMB, Kouassi K, Dago G. Macromolecular and viscoelastic properties of low methoxy pectin from cashew apple pomace. Journal of Food and Nutrition Science. 2014; 2: 1-6.
- Sato MDF, Rigoni DC, Canteri MHG, Petkowicz CLDO, Nogueira A, Wosiacki G. Chemical and instrumental characterization of pectin from dried pomace of eleven apple cultivars. Acta Scientiarum. Agronomy. 2011; 33: 383-389.
- Jiang LN, Shang JJ, He LB, Dan JM. Comparisons of microwave-assisted and conventional heating extraction of pectin from seed watermelon peel. In Advanced Materials Research. 2012; 550: 1801-1806. Trans Tech Publications Ltd.
- Dominiak M, Sondergaard KM, Wichmann J, Vidal-Melgosa S, Willats WG, Meyer AS, et al. Application of enzymes for efficient extraction, modification, and development of functional properties of lime pectin. Food Hydrocolloids. 2014; 40: 273-282.
- Fissore EN, Rojas AM, Gerschenson LN, Williams PA. Butternut and beetroot pectins: Characterization and functional properties. Food Hydrocolloids. 2013; 31: 172-182.
- Zhang C, Zhu X, Zhang F, Yang X, Ni L, Zhang W, et al. Improving viscosity and gelling properties of leaf pectin by comparing five pectin extraction methods using green tea leaf as a model material. Food Hydrocolloids. 2020; 98: 105246.
- Kazemi M, Khodaiyan F, Hosseini SS. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. Lwt. 2019; 105: 182-189.
- Bayar N, Bouallegue T, Achour M, Kriaa M, Bougatef A, Kammoun R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chemistry. 2017; 235: 275-282.
- Talekar S, Patti AF, Vijayraghavan R, Arora A. Complete utilization of waste pomegranate peels to produce a hydrocolloid, punicalagin rich phenolics, and a hard carbon electrode. ACS Sustainable Chemistry & Engineering. 2018; 6: 16363-16374.
- Bagherian H, Ashtiani FZ, Fouladitajar A, Mohtashamy M. Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chemical engineering and processing: Process Intensification. 2011; 50: 1237-1243.
- Shivamathi CS, Moorthy IG, Kumar RV, Soosai MR, Maran JP, Kumar RS, et al. Optimization of ultrasound assisted extraction of pectin from custard apple peel: Potential and new source. Carbohydrate polymers. 2019; 225: 115240.
- Dao TAT, Webb HK, Malherbe F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwaveassisted heating. Food Hydrocolloids. 2021; 113: 106475.
- Dao TAT, Webb HK, Malherbe F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwaveassisted heating. Food Hydrocolloids. 2021; 113: 106475.
- Swamy GJ, Muthukumarappan K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chemistry. 2017; 220: 108-114.
- Sari AM, Ishartani D, Dewanty PS. Effects of microwave power and irradiation time on pectin extraction from watermelon rinds (Citrullus lanatus) with acetic acid using microwave assisted extraction method. In IOP Conference Series: Earth and Environmental Science. 2018; 102: 012085. IOP Publishing.
- Kazemi M, Khodaiyan F, Labbafi M, Hosseini SS, Hojjati M. Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food chemistry. 2019; 271: 663-672.
- Saberian H, Hamidi-Esfahani Z, Ahmadi Gavlighi H, Banakar A, Barzegar M. The potential of ohmic heating for pectin extraction from orange waste. Journal of Food Processing and Preservation. 2018; 42: e13458.