
Research Article
Austin Biomol Open Access. 2019; 3(1): 1013.
Determination of Monomeric Building Blocks by Py-GC/ MS of Lignins from Agricultural Residues
Hoffmann A*, Bremer M and Fischer S
Institute of Plant and Wood Chemistry, Technische Universität Dresden, Germany
*Corresponding author: Hoffmann A1, Institute of Plant and Wood Chemistry, Technische Universität Dresden, Germany
Received: September 27, 2019; Accepted: October 25, 2019; Published: November 01, 2019
Abstract
Non-woody plants and agricultural residues becomes more important for the production of cellulose. One advantage of non-woody materials is that the plants are growing fast and the cellulose is easy to obtain with a low lignin yield. Neverthless, lignin as a side-product from the soda-pulping project is a low-cost material with a high amount of phenolic structures. Especially for various polymer applications lignin is considered as an alternative to conventional precursors. Because lignin is a very inhomogeneous polymer which can be structured very differently depending on its origin, it is necessary to obtain exact information about its structure. A suitable method is the pyrolysis-GC/MS (pyGC/MS). This short message explains the classification of various pyrolysis products into H/G/S building blocks from the soda lignins of 7 different agricultural residues.
Keywords: Lignin; pyGC/MS; Composition; Building blocks; Soda pulping
Introduction
Because wood pulp is limited in world market, prices for a variety of new cellulose applications are simply too high. For this reason, the search for new, non-wood sources of sulfur-free cellulose production is in the focus of many research groups. [1] Cellulose from non-wood materials could be more interesting because less energy and no sulfur components are needed for the pulping procedure [2]. Also, the extraction of nanocellulose from non-wood materials is an interesting approach. Because the isolation of the fibrillary structures often associated with less effort [3]. The procedure for lignin isolation from agricultural residues has already described by Rossberg et al [4]. After all processes of cellulose production lignin is present as a residue, which is still mainly used thermally for energy [5]. The material use of lignin for various applications such as carbon fibers, polymers or hydrogels is largely determined by its properties [6,7]. The properties of lignin are influenced by the purity, the molecular weight, the functional groups and the ratio of the individual monomers (Hydroxy Phenyl (H), Guaiacyl (G) and Syringyl (S)). For many applications, the H/G/S- ratio plays a particular important role [8,9]. Therefore, its very important to determine this ratio quickly and accurately. Various analytical techniques have been developed, including wet chemistry methods, chromatography, vibration spectroscopy and NMR-techniques, to measure the lignin monomers [10,11,12,13].
The different methods all have different disadvantages. In thioacidolysis, only the beta-O-4 linkages are cleaved and the resulting monomers are detected. C-C linked lignin monomers are not detected [14]. Methods such as NMR, in which the lignin must be solved, are limited, as they measure only the soluble fraction. Spectroscopic methods such as infrared spectroscopy are inaccurate for quantification but well suited for a qualitative evaluation.
Another method of choice for H/G/S determination is pyGC/MS. With a temperature of 450°C, the cleavage takes place primarily on the side chain. The aromatic rings and the substituents are mainly not split during pyrolysis, whereby a correct assignment to the H/G/S monomers is possible [15,16]. The only disadvantage is the low yield of pyrolysis products at this temperature.
Matherials and Methods
Materials
All lignins where obtained from alkaline pulping of the raw material. The alkaline pulping was carried out using a 2-L autoclave. About 250 g of raw material was digested for 30 min in 1,5 L NaOH (3wt%) with a maximum temperature of 160°C (H-factor: 320). After the pulping procedure pulp was separated using a Büchner funnel. The lignin was precipitated at pH=1 from the black liquor using a 1 M HCl. After sedimentation overnight the lignin was separated by centrifugation followed by washing with deionized water. The lignin was dried at room temperature and stored over P2O5 in a desiccator [4].
Pyrolysis GC/MS (pyGC/MS)
PyGC/MS analysis was performed with pyrolysis oven EGA/ Py 3030D (Frontier Lab). Approximately 300μg of the lignin samples were pyrolyzed at 450°C. Separation after pyrolysis was achieved by a GC 7890D (Agilent technologies) on a ZB-5MS capillary column (30 m x 0.25 mm) with a temperature program of 50 – 240°C at 4 K/ min and a second heating to 300°C at 39 K/min with a holding time of 15 min. For Identification of substances the 5977 MSD (SIM) and the NIST 2014 mass spectral library was used.
Results and Discussion
Pyrolysis products
After pyrolysis at 450°C, a large number of products could be identified. These pyrolysis products can be divided into the following categories: carbohydrates and their pyrolysis products such as furfural- derivates, fatty acids and aromatic structures of lignin and extractives such as tannins. In this work, the composition of the aromatic components was investigated.
The aromatic pyrolysis products are mainly derived from lignin. But also the pyrolytically cleavages of extractives such as lignans, tannins or lignin precursors form the same aromatic structures that are also formed from lignin [17]. For this reason, it is not possible to clearly assign the pyrolysis products. This is why the classification occurs only in carbohydrates, fatty acids and aromatic structures. The extractives contents are generally low (‹5 %) [4]. Therefore, the results are changed only insignificantly. If ‘lignin’ is written below, then it is always meant the entirety of the aromatics in the pyrolysis products.
The aromatic structures in the pyrolysis products can be subdivided as follows: firstly, the classification is based on the methoxy groups located on the aromatic ring (ortho-positions o1 and o2 to the phenolic hydroxy group). This means to classify the pyrolysis products in H, G and S monomers (Figure. 1). As a result of the cleavage of the methoxy groups during pyrolysis are also methyl or hydroxy groups in ortho positions of the phenolic hydroxy group. [18] These compounds are assigned to the H/G/S building blocks as if there were methoxy groups at these positions.
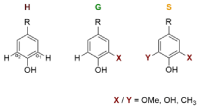
Figure 1: Lignin building blocks: H, G and S. (R = side chain) ortho-positions
of the phenolic hydroxyl group: o1 and o2 can be substituted with a) Methoxylgroup
(OMe) in the precipitated lignins and b) Methoxyl-, Hydroxyl- (OH) or
Methyl-group (CH3) in the pyrolysis-products.
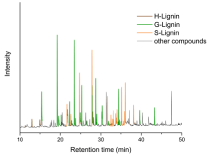
Figure 2: Chromatogramm of pyrolysis products of Soda lignin from wheat
straw. (brown: H-related compounds, green: G-related compounds and
orange: S-related compounds. Black: non-aromatic structures like fatty acids
or pyrolysis products from carbohydrates).
A further classification takes place via the side chain in paraposition to the phenolic hydroxy group (The side chain is labeled with “R” in Figure 1). As side chain (R) different residues can occur. Alkanes (methyl, ethyl, propyl and substituted alkanes such as isopropyl and isobutyl or hydroxy propyl are attached to the various lignin monomers. In addition, there are also unsaturated residues. Alkenes may be bonded to the aromatic ring as ethylene, propylene, and various hydroxy propylenes. Furthermore, there are different carbonyl compounds. Aldehydes occur as C1 aldehyde structures, e.g. Vanillin and Syringaldehyde. Ketones are present as C2, C3 and C4 residues. In addition, a dec-3-en- 5-one residue could be found. Finally, there are various acid and ester groups on the side chain. Formic acid, acetic acid and acrylic acid were found. Also, the methyl esters could be identified for acetic acid and acrylic acid (Table 1).
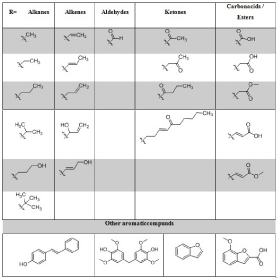
Table 1: Different structures found in the pyGC/MS chromatograms from
different Soda lignins. R=side chain off the different aromatic monomers
H/G/S.
Classification of pyrolysis products in H/G/S
For the determination of the H/G/S ratio of the precipitated lignins the aromatic pyrolysis products were assigned to the corresponding H/G/S monomers. If there is no substituent on orthopositions (o1 and o2 in Figure 1) of the phenolic –OH group, the compound was classified as H-monomer. If there are is substituent (methoxy, hydroxy or methyl) on o1 or o2 , the compund was classified as G-monomer. If there are two substituents, one on each position o 1 and o2 , the compound is classified as S-monomer (a full table with all compounds and the classification can be found in the supplementary materials (Table S1) [18].
Bicyclic compounds such as benzofurans may already be present in the lignin, or may be formed during bond cleavage while pyrolysis. It’s already published in the literature that e.g. 2,3- dihydrobenzofuran is formed while pyrolysis of G-monomers [19].
For catechol residues, a clear distinction between G-units and the pyrolysis products of phenolic extractives such as tannines is not possible. Since in this study we have investigated the pyrolysis products of the precipitations and not only of the lignin, and classified them in the corresponding category of aromatic substitution patterns, we assign catechol residues to the group of G monomers. For this reason, ferulic acid, 4-vinyl-guaiacol and 4-vinyl syringol have also been assigned to the corresponding H/G/S monomers, although these are predominantly monomers in annual plants and are not bound into the lignin structure.
Comparison of H/G/S ratios of different Lignins
The lignins of the studied annual plants show clearly differences in their structure and composition. Lignins from coconut shell powder has the highest amount of H-monomers (21 %). The H-lignin content of the other lignins is between 1 % and 9 %. The highest amount of G-monomers can be found in lignins from oat husks (68.5 %) and miscanthus (64 %) and the lowest amount of G-monomers is in the lignin from sunflower stalks (37%). The highest amount of S-Units is contained in the lignin obtained from sunflower stalks (62 %) and in lignin from hemp shives (51.5 %) (Figure. 4). In addition to the H/G/S ratio, there are also other significant differences in the composition of the investigated lignins. There are three components in the pyrolysis products which cannot clearly assign to lignin or extractives – 2,3-dihydrobenzofuran, catechol and 4-vinyl-guaiacol. They can have their origin in lignin or the lignin precursors but also in other plant components like tannins or coumarin derivates like psoralene. The amounts of these three compounds found by pyrolysis for each lignin can be found in Figure 3. However, benzofuran structures can be found after pyrolysis. The pyrolysis products of lignin from maize straw and oat husks contain up to 9 % 2,3-dihydrobenzofuran, lignin from sunflower stalks contains lower than 1% of 2,3-dihydrobenzofuran. Catechol is another main compound of the precipitated lignin. The pyrolysis products of lignin from coconut shell powder contains about 20% of catechol groups. All other materials show lower amounts between 0 and 2 %. 2-methoxy- 4-vinylphenol be included in every obtained lignin for 5% at least. The highest amount of 2-methoxy-4-vinylphenol can be found in lignins from oat husks and maize straw (up to 13%).
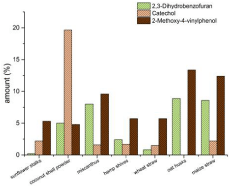
Figure 3: The amounts (area-%) of 2,3-Dihydrobenzofuran, Catechol and
2-Methoxy-4-vinylphenol in the pyrograms of the studied lignins.
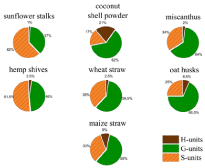
Figure 4: H/G/S-ratios obtained from pyGC/MS of the studied lignins.
Conclusion
This work provides an overview of the soda lignin of various agricultural residues. For the use of lignin the composition and structure is crucial. In particular, the H/G/S ratio is of great importance for polymeric applications. For carbon fiber production, it is advantageous if the lignin has a small amount of methoxy groups, cause of the methoxy groups can lead to faults during carbonization. As a result, lignins with lower S/G ratios are more suitable. Of the lignins studied, the lignins from coconut shell powder (S/G = 0.27) and oats husks (S/G = 0.36) show the lowest S/G ratios. However, high proportions of methoxy groups are advantageous if a high surface area is to be achieved by carbonization. This is the case, for example, with carbon foams for use as electrodes in batteries. For these applications, lignins with high levels of S-monomers can give better results. The lignins of sunflower stalks (S (%) = 62 %) and hemp shives (S (%) = 51.5 %) have the highest S content of the investigated lignins.
Many methods of lignin crosslinking are based on crosslinking via the phenolic OH groups. Especially, the lignin from coconut shell powder shows a significantly high proportion of catechol groups. In addition, the good adsorption of metal ions on catechol groups could be used to make materials with high metal adsorption potential from this lignin. Due to the simple assignment of the H/G/S building blocks, the pyGC/MS is a fast and suitable
method to assess the suitability of lignins for specific applications based on the HGS ratios.
Acknowledgement
The authors are grateful to Projektträger Jülich (PTJ) for funding our research project (PTJ project: FlavorProEco, no. 031B0353C). Special thanks for Deutsche Forschungs Gesellschaft e.V. (DFG) and the saxon government for partially funding our pyGC/MS-device.
References
- Lora JH, Glasser WG. Recent industrial applications of lignin: a sustainable alternative to nonrenewable materials. Journal of Polymers and the Environment 2002; 10: 39-48.
- González-García S, Moreira MT, Artal G, Maldonado L, Feijoo G. Environmental impact assessment of non-wood based pulp production by soda-anthraquinone pulping process. Journal of Cleaner Production 2010; 18: 137-145.
- Phanthong P, Reubroycharoen P, Hao X, Xu G, Abudula A, Guan G. Nanocellulose: Extraction and application. Carbon Resources Conversion 2018; 1: 32-43.
- Rossberg C, Bremer M, Machill S, Koenig S, Kerns G, Boeriu C, Windeisen E, Fischer S. Separation and characterisation of sulphur-free lignin from different agricultural residues. Industrial Crops and Products 2015; 73: 81-89.
- Hamaguchi M, Kautto J, & Vakkilainen E. Effects of hemicellulose extraction on the kraft pulp mill operation and energy use: Review and case study with lignin removal. Chemical Engineering Research and Design 2013; 91: 1284-1291.
- Berlin A, Balakshin M. Industrial lignins: analysis, properties, and applications. In: Bioenergy Research: Advances and Applications. Elsevier. 2014; 315- 336.
- Passauer L, Struch M, Schuldt S, Appelt J, Schneider Y, Jaros D, Rohm H, Dynamic moisture sorption characteristics of xerogels from water-swellable oligo (oxyethylene) lignin derivatives. ACS applied materials & interfaces 2012; 4: 5852-5862.
- Hosseinaei O, Harper D, Bozell J, Rials T. Improving processing and performance of pure lignin carbon fibers through hardwood and herbaceous lignin blends. International journal of molecular sciences 2017; 18: 1410.
- Pospiech D, Korwitz A, Eckstein K, Komber H, Jehnichen D, Suckow M, et al. Fiber formation and properties of polyester/lignin blends. Journal of Applied Polymer Science 2019; 48257.
- Harman-Ware AE, Foster C, Happs RM, Doeppke C, Meunier K, Gehan J, et al. A thioacidolysis method tailored for higher-throughput quantitative analysis of lignin monomers Biotechnology journal. 2016; 10: 1268-1273.
- Jose C, Gutiérrez A, Rodríguez IM, Ibarra D, Martinez AT. Composition of non-woody plant lignins and cinnamic acids by Py-GC/MS, Py/TMAH and FT-IR. Journal of Analytical and Applied Pyrolysis 2007; 2: 39-46.
- Huang Y, Wang L, Chao Y, Nawawi DS, Akiyama T, Yokoyama T, et al. Analysis of lign in aromatic structure in wood based on the IR spectrum. Journal of wood chemistry and technology 2012; 4: 294-303.
- Zeng J, Helms GL, Gao X, Chen S. Quantification of wheat straw lignin structure by comprehensive NMR analysis. Journal of agricultural and food chemistry. 2013; 46: 10848-10857.
- Yue F, Lu F, Regner M, Sun R, Ralph J. Lignin-derived thioacidolysis dimers: reevaluation, new products, authentication, and quantification. ChemSusChem. 2017; 5: 830-835.
- Kim YM, Jae J, Myung S, Sung BH, Dong JI, Park YK. Investigation into the lignin decomposition mechanism by analysis of the pyrolysis product of Pinus radiata. Bioresource technology. 2016; 219: 371-377.
- Brebu M, Tamminen T, Spiridon I. Thermal degradation of various lignins by TG-MS/FTIR and Py-GC-MS. Journal of analytical and applied pyrolysis. 2013; 104: 531-539.
- Pinto O, Romero R, Carrier M, Appelt J, Segura C. Fast pyrolysis of tannins from pine bark as a renewable source of catechols. Journal of analytical and applied pyrolysis. 2018; 136: 69-76.
- Huang J, Li X., Wu D, Tong H, Li W. Theoretical studies on pyrolysis mechanism of guaiacol as lignin model compound. Journal of Renewable and Sustainable Energy. 2013; 4: 043112.
- Custodis VB, Hemberger P, Ma Z, van Bokhoven, JA. Mechanism of fast pyrolysis of lignin: studying model compounds. The Journal of Physical Chemistry B. 2014; 29: 8524-8531.