
Research Article
Austin J Biomed Eng. 2025; 8(1): 1049.
Modeling and Biomechanical Testing of the Piercing Force of Nanosecond Pulse Electric Field Tumor Ablation Electrode
Ting Dong¹, Yougan Zhang², Yonggang Chen², Gang Dong¹* and Xinhua Chen³*
1Department of Ultrasound Intervention, First Affiliated Hospital of Zhengzhou University, Henan, Zhengzhou, 450052, China
2Pulsed Electric Field Technology Medical Translational Laboratory, Hangzhou, Zhejiang 310003, China
3Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital of Zhejiang University, Hangzhou, Zhejiang, 310003, China
*Corresponding author: Dong Gang, Department of Ultrasound Intervention, First Affiliated Hospital of Zhengzhou University, China Email: dgcsjr@126.com
Xinhua Chen, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital of Zhejiang University, China Email: xinhua_chen@zju.edu.cn
Received: March 26, 2025 Accepted: April 14, 2025 Published: April 17, 2025
Abstract
Nanosecond pulsed electric fields represent an emerging technique for thyroid ablation, where the puncture force of the ablation electrode is one of the key factors determining the success of the surgery. This paper aims to establish significant foundational biomechanical methods and data for the future development of a large-scale biomechanical model through modeling analysis and biomechanical testing, with the goal of providing a more accurate and practical clinical ablation decision-making model.
Keywords: Nanosecond pulsed electric fields; Solid tumor; Ablation; Puncture force; Puncture force; Artificial skin
Introduction
Driven by innovative medical devices, the field of rapid rehabilitation surgery is experiencing rapid development. Among these advancements, minimally invasive interventional procedures have gradually become the preferred choice for clinical treatment due to their significant clinical advantages, such as minimal invasiveness, faster postoperative recovery, and shorter hospital stays [1]. Notably, with the widespread implementation of day surgery models, the core role of minimally invasive surgeries in perioperative management has been further strengthened, elevating its clinical value to a new strategic level. The technological evolution of solid tumor ablation therapy is closely related to breakthroughs in imaging diagnostic techniques. In the early stages of this technology's development, it primarily relied on traditional open surgical methods. However, with the clinical application of precise imaging technologies like multi-planar CT reconstruction and MRI diffusion-weighted imaging, sub-millimeter spatial localization of tumor lesions has been achieved, driving percutaneous ablation therapy towards more precise directions [2]. Currently, ultrasound-guided percutaneous ablation techniques for solid tumors have formed a standardized operational system, with their clinical application scope continuously expanding. The academic community is enhancing complete tumor response rates (CR) through the iterative upgrade of ablation devices and optimization of ablation parameters. Minimally invasive interventional ablation therapy for solid tumors aligns with the development philosophy of rapid rehabilitation surgery, and relevant research findings have accumulated substantial evidence-based medical evidence [3-5].
"Ultrasound-guided nanosecond pulse electric field ablation of solid tumors" is an innovative day surgery procedure. Unlike traditional thermal ablation techniques that rely on high temperatures to denature proteins, such as radiofrequency/microwave, the pulsed electric field ablation mechanism based on electrostatic potential energy achieves tissue destruction through non-Joule heating effects, with the advantage of precisely preserving normal tissue structure. This method can still ensure safety when performing ablation treatments in areas adjacent to nerves, blood vessels, bile ducts, trachea, and esophagus. Its mechanism of action stems from: according to differences in tissue bioelectric impedance characteristics, a highvoltage ultra-short pulse electric field (peak voltage> 30 kV) is selectively induced between two electrodes, inducing non-thermal apoptosis in solid tumors. Compared to traditional thermal ablation techniques, this technology exhibits three major innovative features:
Firstly, a real-time ultrasound-guided three-dimensional spatial positioning system is used to accurately place bipolar ablation electrodes into the target lesion via percutaneous puncture. By combining specific patient impedance parameters, a personalized treatment model is established, enabling dynamic optimization of ablation parameters; Secondly, based on the physical characteristics of differences in electrical conductivity between biological tissues, high-voltage pulses cannot form closed loops when applied to high-resistance vascular systems (such as blood vessels and nerves), effectively avoiding collateral damage to surrounding important anatomical structures; Thirdly, the entire procedure can be completed under local anesthesia (such as subcutaneous infiltration of lidocaine), significantly reducing perioperative time (including both surgical duration and postoperative recovery period), aligning with the modern concept of rapid recovery in minimally invasive surgery [6].
The clinical application value of this technology is mainly reflected in: breaking through the traditional thermal diffusion limitations through non-thermal ablation mechanisms, enhancing the controllability of the ablation boundary; using the principle of impedance matching to achieve precise differentiation between "target area and safe area," providing safety guarantees for nodule ablation in adjacent hazardous areas; personalized treatment plans based on bioelectric parameters can be adaptively adjusted for different pathological types of solid tumors, demonstrating significant potential for clinical translation.
Puncture of solid tumors, as a core operational step in high-voltage pulsed electric field ablation of solid tumors, directly affects surgical safety and clinical success through the mechanical characteristics of the ablation electrode puncture. To address this critical technical aspect, our study systematically conducts comprehensive research on the mechanical properties of ablation electrode puncture [7]. Given that ablation electrodes must withstand high-voltage loads in medical scenarios, their puncture mechanical stability and durability are closely related to material mechanical performance parameters [8]. This study employs an interdisciplinary analysis method to verify the puncture force of ablation electrodes under ultra-high voltage and ultra-short pulse electric fields, considering key parameters that influence conductive safety and effectiveness.
Experimental Materials and Methods
Test Device and Materials
The experiment used a puncture test device equipped with high precision mechanical sensors (model: YFZ02-D, Shanghai Yuanzi Electronic Technology Co., LTD.), and the linear drive speed was set to 100 mm/min, and the pressure sensor range was 0-50 N. The experimental system was certified by the National Metrology Institute, and the measurement accuracy reached ±0.1% FS.
Simulated Skin
Polyurethane-based artificial skin (SIMULAB Corporation, USA) was selected and the area was controlled at 10x10mm² after clamping. The chemical composition of this material was confirmed by Fourier transform infrared spectroscopy analysis, and its elastic modulus (2.1±0.3MPa) showed good biomechanical similarity with human epidermal tissue (Figure 1).

Figure 1: Test System.
The ablation electrode diameter is selected as 19G, with the electrode tip chamfer angle set at 30°±1° (calibrated using a Olympus SZX16 stereomicroscope). Sample pretreatment includes three standardized steps: cleaning (wiping with 75% ethanol), constant temperature equilibrium (bathing in 37±0.5oc physiological saline for 30 minutes), and surface tension measurement. Displacementforce curves are synchronized. The sample screening criteria include uniform thickness (2.0±0.2 mm), surface roughness (Ra≤5 μm), and no visible defects as determined by microscopy.
Test Procedures
Before the experiment, artificial skin samples were pre-stretched to physiological strain range using a stretching fixture that applied constant tension. The puncture angle was controlled at 90°±0.3°. Each sample underwent 10,000 repeated tests in a (23±1) ? temperaturecontrolled environment. The entire process was independently performed by two physicians who had undergone standardized operational training. The experimental procedures strictly followed a three-tier quality control system, including pre-experiment calibration, dual-person verification during the process, and blind data analysis.
Result
Repeated Verification of the Key Effectiveness and Safety of Ablation Electrodes
Through systematic experimental design and data analysis, the following important research results were obtained: The experimental data are shown in the table. In the ten-thousand times fatigue test, the key electrical parameters reached the effective index of clinical discharge, and the number of arc breakdown was 0 times, which verified the safety, with no significant statistical difference (P>0.05).
Test Results of Puncture Force of Artificial Skin Electrode Needles
As shown in table1. The experimental design adopted a multifactorial analysis scheme, conducting puncture tests under standard temperature (23±2o) and humidity (50±5% RH). The needle tip angle was set at different gradients from 15 to 45 °, with diameters covering the clinically common range of 19G,15cm to 25cm. The puncture speed was controlled within the range of 1 to 5 mm/s according to clinical operation standards. By integrating a camera system and force sensor, the deformation characteristics of the needle tip and three-dimensional mechanical parameters were simultaneously obtained during the puncture process. The data analysis showed that when the blade angle increased, the maximum puncture force decreased significantly by 19.3% (p<0.01). This quantitative result is well consistent with feedback from clinical operators, confirming that the artificial skin model can effectively simulate the biomechanical response characteristics of real tissues.
In the 19G needle (Figure 2), tissue elasticity retraction was observed to cause a reduction in needle diameter of 13%-21%. The average error of the measured values was controlled within 7.2%. Based on feature importance analysis, it was confirmed that the needle diameter contributes 67.3% to the puncture force, significantly higher than the angle of attack (21.5%) and speed (12.2%). This conclusion provides a basis for parameter optimization.
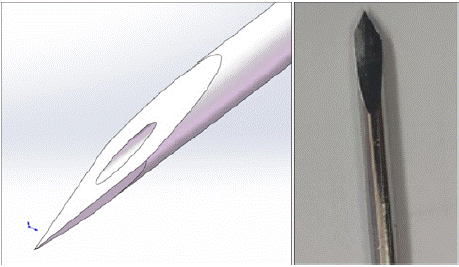
Figure 2: Electrode Design.
Prediction of Puncture Force
Several key factors affecting puncture force of pulse electrode include blade Angle arc value, needle diameter and puncture speed. These three key process parameters have significant influence on the mechanical properties of puncture of electrode (p<0.05) (Table 1).
Typical sample
Puncture force (N)
Material quality
Length
Diameter
Organizational resilience is reduced by%
Conductivity
Arc breakdown count
Sample 1
2.04
304 steel
15cm
19G
13.2±3.4%
100%
0
Sample 2
1.94
304 steel
25cm
19G
21.3±4.1%
100%
0
Table 1: The Test of Mechanical and Electric Characters.
Discussion
High-voltage nanosecond pulse electric field ablation technology applied to solid tumor treatment falls under a special clinical medical scenario. Given that the ablation electrode must carry high voltage at 30 kV and achieve precise control over steep pulse rise time, square wave pulse duration, and fall time within nanoseconds, it is also necessary to perform percutaneous puncture on relaxed neck skin tissue to avoid mechanical damage to surrounding blood vessels and nerves. Research on the modeling of puncture force for solid tumor biopsy needles requires manual skin puncture experiments, focusing on analyzing the impact of geometric parameters such as needle tip angle and diameter on maximum puncture force. This study can provide quantitative evaluation criteria for optimizing clinical percutaneous puncture instruments and establish a biomechanical evaluation system for standardized training in puncture operations.
The ablation electrode of pulsed electric fields not only has the function of discharging but also requires a puncture process. It is necessary to combine Joule heating effects with electromagneticforce coupling field analysis to optimize the geometric parameters of the electrode needle to balance the efficiency of electrical pulse transmission and mechanical strength [9-10]. This study provides a biomechanical basis for material selection in clinical pulsed electric field interventional devices. It is recommended that subsequent research establish a quantitative model between the stiffness of puncture needle materials and tissue penetration performance to optimize the selection criteria for puncture instruments in clinical operations.
It is worth noting that the accurate modeling of physical tumor puncture force needs to integrate in vitro experiments, molecular dynamics simulation and nonlinear finite element analysis to construct a multi-scale biomechanical model, which will provide scientific guidance for the development of instruments and operation specifications for minimally invasive surgery of solid tumors, and finally achieve the synergistic improvement of puncture safety and surgical efficiency [11].
This study employs a medical-engineering interdisciplinary analysis method to verify the puncture force of ablation electrodes under ultra-high voltage and ultra-short pulse electric fields. It considers key parameters affecting the mechanical properties that influence conductivity safety and effectiveness, with electrode diameter serving as the core indicator for directional guidance during puncture. The experimental results show that the structural design adopted in this study does not dissipate electric field energy while enhancing blade sharpness. By reducing contact area, it optimizes the utilization of puncture force, effectively improving puncture accuracy and ensuring the straightness of the puncture path. Both typical electrodes designed in this study meet the positioning characteristics required for tumor ablation surgery.
Acknowledgement
This study is supported by the National Natural Science Foundation of China (82027803).
References
- Selim M, Dresscher D, Abayazid M. Virtual Needle Insertion with Enhanced Haptic Feedback for Guidance and Needle-Tissue Interaction Forces. Sensors (Basel). 2024; 24: 5560.
- Cseh M, Katona G, Berkó S, Budai-Szucs M, Csóka I. A Stereolithography- Based Modified Spin-Casting Method for Faster Laboratory-Scale Production of Dexamethasone-Containing Dissolving Microneedle Arrays. Pharmaceutics. 2024; 16: 1005.
- Bloemberg J, Trauzettel F, Coolen B, Dodou D, Breedveld P. Design and evaluation of an MRI-ready, self-propelled needle for prostate interventions. PLoS One. 2022; 17: e0274063.
- Knull E, Bax JS, Park CKS, Tessier D, Fenster A. Design and validation of an MRI-compatible mechatronic system for needle delivery to localized prostate cancer. Med Phys. 2021; 48: 5283-5299.
- Khan JM, Rogers T, Eng MH, Lederman RJ, Greenbaum AB. Guidewire electrosurgery-assisted trans-septal puncture. Catheter Cardiovasc Interv. 2018; 91: 1164-1170.
- Norman JJ, Choi SO, Tong NT, Aiyar AR, Patel SR, Prausnitz MR, Allen MG. Hollow microneedles for intradermal injection fabricated by sacrificial micromolding and selective electrodeposition. Biomed Microdevices. 2013; 15: 203-210.
- Majewicz A, Marra SP, van Vledder MG, Lin M, Choti MA, Song DY, Okamura AM. Behavior of tip-steerable needles in ex vivo and in vivo tissue. IEEE Trans Biomed Eng. 2012; 59: 2705-2715.
- Yang B, Tan UX, McMillan A, Gullapalli R, Desai JP. Design and Control of a 1-DOF MRI Compatible Pneumatically Actuated Robot with Long Transmission Lines. IEEE ASME Trans Mechatron. 2011; 16: 1040-1048.
- Eggener SE, Scardino PT, Carroll PR, Zelefsky MJ, Sartor O, Hricak H, et al. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007; 178: 2260-2267.
- Sun D, Willingham C, Durrani A, King P, Cleary K, Wood B. A novel endeffector design for robotics in image-guided needle procedures. Int J Med Robot. 2006; 2: 91-97.
- Adebar TK, Greer JD, Laeseke PF, Hwang GL, Okamura AM. Methods for Improving the Curvature of Steerable Needles in Biological Tissue. IEEE Trans Biomed Eng. 2016; 63: 1167-1177.