Research Article
Austin J Biomed Eng. 2014;1(4): 1019.
A Biomimetic Reverse Thermal Gel for 3-Dimensional Neural Tissue Engineering
Yun D1, Laughter MR and Park D1*
Department of Bioengineering, University of Colorado Denver Anschutz Medical Campus, USA
*Corresponding author: :Park D, Department of Bioengineering, University of Colorado Denver Anschutz Medical Campus, 12700 E, 19th Avenue, Aurora, CO 80045-2560, USA.
Received: July 10, 2014; Accepted: August 10, 2014; Published: August 11, 2014
Abstract
Injuries to the nervous system, associated with permanent functional loss, often result in irregularly shaped lesions. With an aim to develop surgical implantation that fits to irregular shapes of injuries, we synthesised a biomimetic reverse thermal gel (or thermo-responsive gel). We initially synthesizedpoly( serinolhexamethylene urea)-co-poly(N-isopropylacrylamide) (PSHU-NIPAAm) and subsequently conjugated a pentapeptide, Gly-Arg-Gly-Asp-Ser(GRGDS) (PSHU-NIPAAm-GRGDS), which showed ideal temperature-dependent thermal gelling properties. We investigated3D neuronal growth capacity of PSHU-NIPAAm-GRGD Sousing PC12 cell. The concentration of the gels significantly affected cellular behaviour, where a greater degree of proliferation was observed with lower gel concentration. Cellular assays demonstrated enhanced cell viability and differentiation in PSHU-NIPAAm-GRGDS compared to non-functionalized PSHU-NIPAAm.
Keywords: Nerve Regeneration; 3d Culture; Reverse Thermal Gel; Polymer Scaffold; Neuritis Outgrowth
Abbreviations
ACA: 4,4’-Azobis(4-cyanovaleric acid); CNS:Central Nervous System; DCM: Dichloromethane; DMF: N,N-Dimethylformamide; ECM: Extracellularmatrix; EDC: N-(3-Dimethylaminopropyl)- N-Ethylcarbodiimide Hydrochloride; GRGDS:Gly-Arg-Gly-Asp-Ser; HDI: Hexamethylene Diisocyanate; LCST: Lower Critical Solution Temperature; NHS: N-Hydroxysuccinimide; NIPA Am: N-isopropylacrylamide; PSHU: Poly(serinolhexamethylene urea); SCI: Spinal Cord Injury; TFA: Trifluoroacetic Acid
Introduction
The annual incidence of spinal cord injury (SCI) is estimated at about 15-40 cases per million people worldwide [1]. SCI results in the loss of sensory and motor function and ultimately leads to the formation of irregularly shaped tissue injuries [2,3]. Despite major advances in the medical care of SCI patients, there is no clinical treatment that can restore lost function. Long-term treatment after SCI focuses on rehabilitation, pain management, and the prevention of complications. Unfortunately, since the inherent regenerative capabilities of the adult central nervous system (CNS) are limited, most SCI patients still face substantial neurological dysfunction and lifelong disability. Therefore, therapeutic strategies aiming to enhance and ultimately restore regenerative potential represent promising treatment modalities.
One of the primary goals in the treatment of SCI is to bridge the injured spinal cord using implantable scaffolds [4,5]. In particular, injectable thermo-responsive polymers can bridge the lesion cavities, providing a permissive environment for axonal regeneration by simple injection of their solutions into target sites. Thermo-responsive polymers undergo temperature-dependent solution-to-gel (sol-gel) phase transitions, facilitating a minimally invasive treatment due to an in situ gelation mechanism [6,7]. Over the past few decades, great interest has been given to grating natural polymers onto synthetic scaffolds to mimic the extra cellular matrix (ECM) environment. Although natural polymers have inherent bioactivity that aids in nerve regeneration, it is not easy to control their physicochemical properties [8,9]. In contrast, synthetic polymers have easy to control properties such as stiffness or degradation rate. However, most of them lack the endogenous factors that promote cell behaviour [10]. The ECM in biological systems provides cues for cells to interact and migrate [11]. It might be desirable if the synthetic scaffold mimics the biological activity of the ECM, promoting cell adhesion, proliferation, and differentiation. Recently we reported that GRGDS-modified PSHU greatly improved PC12 cell survival and differentiation in 2D conditions [12].
Herein, we aim to further modify this polymer with poly (N-iso propylacrylamide) (PNIPAAm) for injectable 3D neural tissue engineering. PNIPAAm is a thermo-responsive segment with a lower critical solution temperature (LCST) of around 32°C. After conjugation of PNIPAAm, we investigated thermal gelling properties of the polymer, and subsequently 3D neuronal growth was performed using PC12 cells [13,14].
Materials/Methods
Materials
N-BOC-Serinol, urea, hexamethylene diisocyanate (HDI), anhydrous chloroform, and anhydrous N,N-dimethylformamide (DMF), N-isopropylacrylamide (NIPAAm), and 4,4‘-azobis(4- cyanovaleric acid) (ACA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). N-(3-Dimethylaminopropyl)-Nethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and trifluoroacetic acid (TFA) were purchased from Alfa Aesar (Ward Hill, MA, USA). Anhydrous diethyl ether was purchased from Fisher Scientific (Pittsburgh, PA, USA). Anhydrous dichloromethane (DCM) was purchased from JT Baker (Phillipsburg, NJ, USA). The pentapeptide Gly-Arg-Gly-Asp-Ser (GRGDS) was purchased from Biomatik (Wilmington, DE). Dialysis tube (Spectra/ Por) was obtained from Spectrum Labs (Houston, TX).
Synthesis of poly (serinol hexamethylene urea) (PSHU)
PSHU was synthesized as described previously [12]. Briefly, in a dried 50ml round bottom flask, N-BOC-serinol (6mmol) and urea (6mmol) were dissolved in anhydrous DMF (5ml). Then HDI (12mmol) was added drop-wise to the mixture and the solution was stirred for 7 days at 90°C under a nitrogen atmosphere. After cooling to ambient temperature, anhydrous DMF was removed by rotary evaporation at 45°C, the product mixture was then dissolved in 3ml anhydrous chloroform and precipitated with an excess of anhydrous diethyl ether under vigorous stirring to yield a pale yellowish solid. The purification process was carried out twice and the precipitates were washed in 100ml of anhydrous diethyl ether overnight to remove unreacted reagents. PSHU was obtained after drying at 45°C under vacuum (yield: 90.8%).
Conjugation of PNIPA Am and GRGDS
PSHU of 1g was dissolved in 100ml mixture of DCM/TFA (1:1, v/v). The BOC de protection reaction was carried out at room temperature for 45 min. The mixture was rotary-evaporated at 45oC to remove methylene chloride and TFA. The product was dissolved in anhydrous DMF (1ml). Once fully dissolved, this solution was purified by precipitation in excess cooled ether (100ml). Finally, the product was rotary-evaporated at 45°C, dried, and stored at room temperature. PNIPA Am-COOH was synthesized as described previously [15]. Briefly NIPA Am (44.19mmol) and ACA (0.22mmol) were dissolved in 25ml dry methanol. The solution was bubbled with nitrogen for 30 min and then stirred at 68°C for 3h. The solution was then dropped into hot water (60°C) to precipitate the PNIPA Am-COOH. After washing twice with hot water, the polymer was dissolved in double distilled water (25°C) and further purified by dialysis (MWCO: 3500Da) against water at room temperature for 2 days and then freeze-dried (yield: 92%, Mw 11,000).
For conjugation, PNIPA Am-COOH (0.04mmol) was dissolved in2 ml anhydrous DMF with stirring. EDC (0.048mmol) and NHS (0.048mmol) dissolved in 1ml anhydrous DMF were added to the PNIPA Am-COOH solution. After 24h, 100mg of de protected PSHU (0.1956mmol amine groups) was slowly added and the conjugation was performed for 24h. Subsequently, the reactant was mixed with GRGDS (0.16mmol) that was pre-activated with EDC/NHS (0.192mmol) for 24h. The conjugation was performed for another 24h. The product mixture was then precipitated with an excess of anhydrous diethyl ether under vigorous stirring to yield a white solid. The purification process was carried out twice and the precipitates were washed in 100ml of anhydrous diethyl ether overnight. The product was dissolved in water, dialysed (MWCO: 12,000Da) in water for 3 days, and finally lyophilized.
Sol-gel phase transition
The sol-gel phase transition of the PSHU-NIPAAm-GRGDS solutions was determined by test tube inversion method. The polymer was dissolved in PBS with various concentrations and the each polymer solution (2ml) was placed in a glass vial. The initial temperature of a water bath was set to 25°C and heated up to 50°C with 1°C/min. The sol-gel phase transition was determined by inverting the vial horizontally at each temperature.
3D cell culture
PC12 cells (2×103 cells) were suspended in a solution of 3 or 5% (w/w) PSHU-NIPAAm-GRGDS or PSHU-NIPAAm in complete media. The cell suspension in the polymer solutions was placed in a 16-chamber slide (Lab-Tek, Naperville, IL, USA), and then cultured in RPMI complete media (with glutamine, 2mM glucose, 2mM sodium bicarbonate, Thermo Scientific Hyclone) supplemented with 10% heat-inactivated horse serum (HS), 5% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS) solution at 37°C in a 5% CO2 incubator.
Cell proliferation assay
The number of undifferentiated PC12 cells in the gel was determined at every odd day for 9 days. The 3D cultures were rinsed twice with PBS and fixed with 4% paraformaldehyde for 24 h at 37°C. Cultures were embedded in sucrose and frozen in Tissue- Tek optimum cutting temperature (O.C.T.) compound (Miles Laboratories), and were cut in 40μm slices using a cryostat for immunostaining. Nuclei were stained with PBS containing 5 μg/ml Hoechst dye for 5 min at room temperature. Immunofluorescence staining was visualized and analyzed on a Nikon E600 fluorescent microscope (Kanagawa, Japan) using SPOT Advanced software. All immunofluorescence images were recorded at 400× magnification, and ten to twenty random visual fields were selected and counted for each sample.
Measurement of Neurite Outgrowth
PC12 cells (2×103 cells) were mixed with 100 μl differentiation medium containing PSHU-NIPAAm-GRGDSor PSHU-NIPAAm and NGF (100 ng/ml). At different times (7 and 14 days after the initial NGF addition), the sections were prepared as described in 2.6. Cells were stained with β-III tubulin and visualized by fluorescent microscope.Primary antibody localization was performed using goat anti-rabbit and anti-mouse IgG conjugated to Alexa 488 (1:300, Invitrogen).The neurite length was calculated using SPOT Advanced software. Length was defined as the distance from the tip of the neurite to the junction between the cell body and neurite. In the case of branched neurites, the length of the longest branch was measured, and then each branch was measured from the tip of the neurite to the neurite branch point. Average neurite length was calculated by dividing the sum of neurite length by the number of neurite. Independent experiments were performed three times and average neurite length were measured in ten random images for each condition (n = 10).
Statistical Analysis
In all experiments, results are presented as means ± standard deviation. All quantitative results were analyzed using analysis of variance (ANOVA) and, if necessary, follow-up analysis by Tukey’s test. Statistical significance was considered at p < 0.05.
Results and Discussions
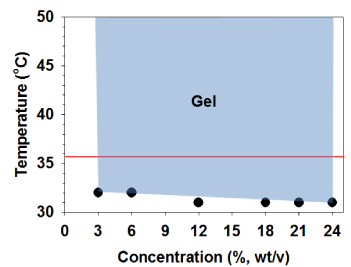
Since the PSHU-NIPAAm-GRGDS was designed to show temperature-dependent solution-to-gel phase transition, its thermal gelling properties were examined. Although not significant, the gelling temperature of PSHU-NIPAAm-GRGDS decreased from 32 to 31°C as the concentration of aqueous solution increased from 3 to 24% (wt) (Figure 1). Most importantly all aqueous solutions remained gel at 37°C indicating that PSHU-NIPAAm-GRGDS is a promising temperature-dependent injectable material. Moreover, the gel status was maintained up to the highest temperature (50°C) with no phase separation.
Figure 1: Thermal gelling property of PSHU-NIPAAm-GRGDS. All solutions turned to physical gel upon temperature increase and maintained gel status in a broad range (blue area) of temperature.
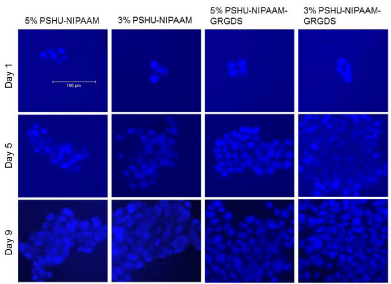
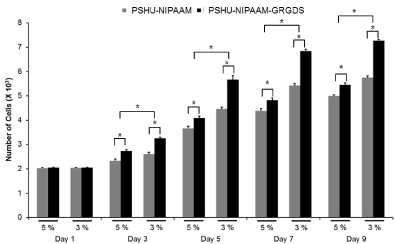
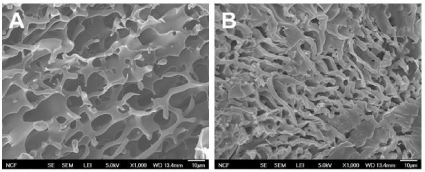
ECM plays an essential role in cell survival and tissue growth [16,17]. ECM consists of several proteins, such as collagen and fibronectin. These ECM proteins can be directly conjugated onto polymeric materials to fabricate artificial ECM environments. However, several limitations such as expensive production cost, vulnerability to denaturation, and immunogenicity diminish their practical applications [18]. To overcome these limitations, ECM-derived short peptides that act as receptor binding motifs have been introduced to polymeric materials to mimic ECM environments for tissue engineering [19,20]. GRGDS is a peptide sequence found in fibronectin, which promotes cell attachment, survival, and differentiation. Thus, to fabricate an injectable temperature-dependent material that mimics growth-permissive ECM environment, we conjugated GRGDS onto PSHU-NIPAAm, and further investigated its potential as a scaffold for neural tissue engineering by culturing PC12 cells in 3D condition. Figures 2 and 3 show the change in number of PC12 cells in the 3 and 5% gel with/ withoutGRGDSpeptide sequence. The results indicate that the degree of PC12 cell growth cultured in PSHU-NIPAAm-GRGDS was higher than that cultured in PSHU-NIPAAm with no GRGDS.Thus, it is likely that PC12 cells could recognize the binding sites of the GRGDS ligands in the PSHU-NIPAAm-GRGDSwhich played crucial role for cell growth.Interestingly, 3% gel showed a higher cell growth than 5% gel after three days, which might be caused by changes in inner morphologies of the gels. The SEM images of the gel cross-sections are good evidence that a higher gel concentration led to the smaller pore sizes (Figure 4), which may restrict cell migration.
Figure 2: PC12 cells cultured in PSHU-NIPAAm and PSHU-NIPAAm- GRGDS with different concentrations. A higher increase in cell number was observed in PSHU-NIPAAm-GRGDS. Also cells in lower concentrations were more proliferative.
Figure 3: Cell viability assessed by quantification of cell numbers. At all time points except day 1, a significantly higher cell numbers were observed in PSHU-NIPAAm-GRGDS. * corresponds to p<0.0001. Error bar: mean plus standard deviation.
Figure 4: SEM images of PSHU-NIPAAm-GRGDS gel cross-sections: A) 3% and B) 5%. A higher concentration resulted in smaller pore size and thicker wall.
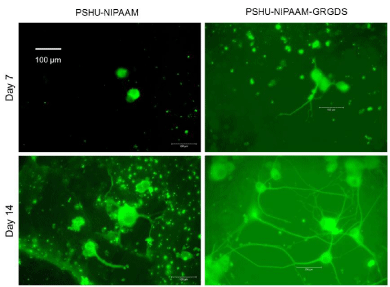
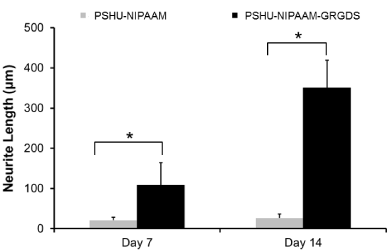
After confirming that PSHU-NIPAAm-GRGDS gel provided favorable environment for PC12 cell growth, studies comparing neurite outgrowth were performed. Since 3% gel resulted in better cell growth, further studies were conducted in 3% gel. Figure 5 shows neurite outgrowth in PSHU-NIPAAm with/without GRGDS peptide sequence. At day 7, no (negligible if any) neurite outgrowth was observed in PSHU-NIPAAm, while some neurites were extended from PC12 cells in PSHU-NIPAAm-GRGDS. Although not extensive, the difference in neurite growth between the gels was obvious. At day 17, the difference in neurite extension appeared much significant. A few and short neurite extension was observed in PSHU-NIPAAm gel indicating that most of the PC12 cells did not differentiate. However, PC12 cells in PSHU-NIPAAm-GRGDS extended much denser and longer neurites. The neurite growth was further compared by quantification of neurite length (Figure 6). In PSHU-NIPAAm, the neurite length showed no statistcal difference between day 7 and day 14, while the neurite length in PSHU-NIPAAm-GRGDS significantly increased from 108 μm to 350μm, all of which indicate that PSHU-NIPAAm-GRGDS provided favorable 3D environment for PC12 cell survival and differntiation.
3D neurite extension in PSHU-NIPAAm and PSHU-NIPAAm- GRGDS gel.
Significantly denser and longer neurite outgrowth was observed in PSHUNIPAAm- GRGDS, while very few (or negligible) neurite were extended in PSHU-NIPAAm.
Figure 5:3D neurite extension in PSHU-NIPAAm and PSHU-NIPAAm- GRGDS gel.
Significantly denser and longer neurite outgrowth was observed in PSHUNIPAAm- GRGDS, while very few (or negligible) neurite were extended in PSHU-NIPAAm.
Figure 6: Time-course neurite elongation in 3D cultures. At all time points, PSHU-NIPAAm-GRGDS showed significantly longer neurite outgrowthcompared with PSHU-NIPAAm. *corresponds to p<0.0001. Error bar: mean plus standard deviation.
Conclusion
Herein, we have demonstrated that PSHU-NIPAAm-GRGDS has great potential as a 3D scaffold for neural tissue engineering. Furthermore, the basic polymer, PSHU, possesses a capacity to be modified with bio molecules to mimic natural ECM. A material that can induce such functionality holds great potential for the application in the field of regenerative medicine. While GRGDS was employed in this work, future work will explore the use of other bioactive cues, which may be better suited for other types of neural cells. Given the positive initial findings presented in this work, we believe PSHU-NIPAAm system to be a platform for myriad regenerative medicine applications.
Acknowledgement
This work was financially supported by a University of Colorado Denver start-up funding.
References
- Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004; 21: 1355-1370.
- Chen S, Pickard JD, Harris NG. Time course of cellular pathology after controlled cortical impact injury. Exp Neurol. 2003; 182: 87-102.
- Sutton RL, Lescaudron L, Stein DG. Unilateral cortical contusion injury in the rat: vascular disruption and temporal development of cortical necrosis. J Neurotrauma. 1993; 10: 135-149.
- Hejcl A, Lesný P, Prádný M, Michálek J, Jendelová P, Stulík J, et al. Biocompatible hydrogels in spinal cord injury repair. Physiol Res. 2008; 57: S121-132.
- Authors Shoichet MS, Tate CC, Baumann MD, LaPlaca MC. Strategies for Regeneration and Repair in the Injured Central Nervous System. Strategies for Regeneration and Repair in the Injured Central Nervous System.
- Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2008; 68: 34-45.
- Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels--review of temperature-sensitive systems. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2004; 58: 409-426.
- Straley KS, Foo CW, Heilshorn SC. Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma. 2010; 27: 1-19.
- Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006; 11: 43-56.
- Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009; 103: 655-663.
- Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998; 8: 51-54.
- Yun D, Famili A, Lee YM, Jenkins PM, Freed CR, Park D. Biomimetic poly(serinol hexamethylene urea) for promotion of neurite outgrowth and guidance. J Biomater Sci Polym Ed. 2014; 25: 354-369.
- Guroff G. PC12 cells as a model of neuronal differentiation. Cell Culture in the Neurosciences: Springer. 1985; 245-472.
- Schimmelpfeng J, Weibezahn KF, Dertinger H. Quantification of NGF-dependent neuronal differentiation of PC-12 cells by means of neurofilament-L mRNA expression and neuronal outgrowth. J Neurosci Methods. 2004; 139: 299-306.
- Tan H, Ramirez CM, Miljkovic N, Li H, Rubin JP, Marra KG. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009; 30: 6844-6853.
- Hubbell JA. Biomaterials in tissue engineering. Biotechnology (N Y). 1995; 13: 565-576.
- Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004; 428: 487-492.
- Hodneland CD, Lee YS, Min DH, Mrksich M. Selective immobilization of proteins to self-assembled monolayers presenting active site-directed capture ligands. Proc Natl Acad Sci U S A. 2002; 99: 5048-5052.
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996; 12: 697-715.
- LeBaron RG, Athanasiou KA. Extracellular matrix cell adhesion peptides: functional applications in orthopedic materials. Tissue Eng. 2000; 6: 85-103.