
Research Article
Austin Biomark Diagn. 2016; 3(1): 1025.
Type V Collagen in Hepatic Fibrosis and as Fibrosis Biomarker
Mak KM*
Department of Medical Education and Center for Anatomy and Functional Morphology, Icahn School of Medicine at Mount Sinai, USA
*Corresponding author: Ki Mark Mak, Department of Medical Education and Center for Anatomy and Functional Morphology, Icahn School of Medicine at Mount Sinai, New York, USA
Received: April 25, 2016; Accepted: May 16, 2016; Published: May 17, 2016
Abstract
Type V collagen (COLV) is a regulatory fibril-forming collagen. It is multifunctional in health, disease and fibrosis. In normal human liver, COLV constitutes 10-16% of total hepatic collagen. COLV expression is upregulated in early stages of hepatic fibrosis and its expression continues to rise as fibrosis progresses to end-stage cirrhosis with a 4-7-fold increase. COLV is ubiquitous in the liver extracellular matrix with COLV immunostaining regularly observed in the space of Disse, stroma of portal tracts, wall of central veins, and cytoplasm of hepatic stellate cells. Enhanced COLV immunostaining occurs in perisinusoidal fibrosis, portal fibrosis, central vein fibrosis, and fibrous septa of septal fibrosis and cirrhosis, coincident with increased presence of COLI, III and VI. Elastin is present in the portal stroma, fibrotic central veins and fibrous septa. In normal rat, COLV constitutes 5.5 % of total hepatic collagen; in experimental cirrhosis, COLV increases 4.6-fold. Collagen fibrils of fibrotic liver are haphazardly arranged and thinner, attributable to COLV’s ability to limit the diameter growth of fibrils resulting from heterotypic fibril formation with COLI. COLV binds TGF-β1, matrix metalloproteinase (MMP)-2, and tissue inhibitor of metalloproteinase-1, regulating their availability for fibrogenesis and fibrolysis. Degradation of COLV by MMP-2 and MMP-9 contributes to extracellular matrix remodeling in fibrosis. Protein fingerprint technique has been applied in the measurement of neoepitopes that serve as fibrosis biomarkers. COLV neo-epitope P5CP-1230 is a useful noninvasive biomarker of liver fibrosis progression and resolution in experimental fibrogenesis and a promising indicator of portal hypertension in alcoholic patients with cirrhosis.
Keywords: Type V collagen; Hepatic fibrosis; Protein fingerprints; Neoepitopes; Liver fibrosis biomarkers
Abbreviations
COLV: Type V Collagen; MMP: Matrix Metalloproteinase; ECM: Extracellular Matrix; HSC: Hepatic Stellate Cell; TGF-β: Transforming Growth Factor-β; CCL4: Carbon Tetrachloride; TIMP- 1: Tissue Inhibitor of Metalloproteinase-1; ELISA: Enzyme-Linked Immunosorbent Assay
Introduction
Type V collagen (COLV) is multifunctional in health, disease and fibrosis. Deficiency of COLV is associated with loss of corneal transparency and classic Ehlers-Danlos syndrome. Increased expression of COLV is found in cancer, inflammation, atherosclerosis, and fibrosis of the lung, skin, kidney, adipose tissue, and liver. These aspects of COLV have recently been reviewed [1]. As a major focus, this article updates COLV’s involvement in hepatic fibrosis of humans and experimental animal models. We will discuss the application of protein fingerprint technology in the measurement of neo-epitope peptides that serve as noninvasive biomarkers for diagnosing and monitoring liver fibrogenesis and fibrosis. Finally, this review summarizes the recent achievements in the use neo-epitope P5CP- 1230, which is a COLV formation marker, as a biochemical marker for the assessment of liver fibrosis progression and resolution in experimental fibrosis as well as an indicator of portal hypertension in patients with alcoholic cirrhosis.
COLV
COLV is a regulatory fibril-forming collagen regulating the fibrillogenesis of interstitial fibrillar COLI and III [2], although it has structural and biological properties different from COLI and III. COLV was first isolated from human placentas and organs rich in basement membranes [3,4], and then in human liver [5]. It was later found to be a ubiquitous component of connective tissue matrices. There are at least three different molecular isoforms of COLV: a1(V)2 a2(V), a1(V)3, and a1(V) a2(V) a3(V). These are formed by combinations of three different polypeptide a chains, namely a1(V), a2(V), and a3(V), providing each isoform with a unique chain composition with different functions and tissue distribution [2, 6-8]. The COLV isoform containing the heterotrimeric a1(V)2a 2(V) chains primarily forms heterotypic fibrils with other fibrilforming collagens and is broadly distributed in tissues and is most often referred to as COLV. COLV is also found to a lesser extent in the form of a 1(V)3, referred to as a1(V) homotrimer, which was first observed in cultured Chinese hamster lung cells [9,10]. Uniquely, the a1(V) homotrimer occurs as microfilaments of 5-10 nm in diameter in the skin dermis [11]. The functionally distinct a1(V)a2(V)a3(V) heterotrimer, referred to as COLV isoform containing the a3(V) chain, has been described in the placenta and other tissues: skin, synovial membrane and uterus [12,13].
COLV in Disease and Fibrosis
Deficiency of COLV associated with mutations in the a1(V) or a2(V) genes–COL5A1 and COL5A2— in humans is a cause of classic Ehlers-Danlos syndrome [14,15]. Loss of corneal transparency has been described in COLV-knockout mice [16]. COLV overexpression is found in the timorous skin of mice, cancer of the colon and mammary glands, hepatocellular carcinoma, granulation tissue, inflammation, atherosclerosis and fibrosis of the lung, skin, kidney, adipose tissue, and liver—reviewed in [1]. The present discussion will focus on fibrosis of the liver, updating COLV’s role in the histogenesis of hepatic fibrosis and its value as a noninvasive biochemical marker of hepatic fibrogenesis and fibrosis.
Hepatic Fibrosis
Hepatic COLV content
COLV is a regular component of the extracellular matrix (ECM) proteins of liver tissue. It comprises 16.3% [17] or 10.6% [18] of the total hepatic collagen of the normal human liver. The amount of COLV is about 45% relative to that of COLI or COLIII (0.9 mg/g of liver tissue vs. 2.0 mg/g of liver tissue), the most abundant collagens in the liver. The level of COLV rises about 7-fold in cirrhosis [17,19] and about 4-fold in alcoholic cirrhosis [18], coincident with increases in COLI, III, and IV and a disproportional elevation of COLI. In the human liver, COLV has been reported as being present in the heterotrimeric a1(V)2 a2(V) isoform [18,20]. Interestingly, expression of pro- a2(V) collagen mRNA occurs in human liver at 15 and 17 weeks of gestation [21].
COLV immunohistochemistry of human liver
Immunofluorescent localization of COLV expression in human liver was first reported in the eighties [17,22,23]. In normal liver, COLV was seen as fine interwoven fibrillar materials in the interstitium of portal tracts. Portal vasculatures showed staining for COLV, predominately in the intima, while portal basement membranes were not stained. A weak but uniform COLV immunofluorescence was detected in the sinusoidal lining of the lobules. In liver fibrosis, enhanced COLV immunofluorescence was noted in foci of parenchymal fibrosis with a fine fibrillar network enmeshing the hepatocytes. The thickened wall of central veins (terminal hepatic venules) was strongly stained for COLV. The fibrotic bands of cirrhotic livers showed intense COLV immunofluorescence. No assessment was made of COLV staining of liver cells.
COLV in progressive stages of liver fibrosis
Little work, however, has characterized the lobular distribution of COLV in progressive stages of liver fibrosis and its codistribution with COLI, III and VI, the latter being filamentous [2,24,25]. Because fibrosis of the liver is prevalent in elderly cadavers, even when liver disease is not indicated as the cause of death [26], we have examined the lobular distribution of COLV in elderly cadaveric livers with progressive stages of fibrosis by the immunoperoxidase method [1,27]. In the liver showing nearly normal histology with minimal fibrotic changes, COLV immunostaining is generally uniform along the sinusoidal borders of the lobules—from periportal to pericentral— in most of the specimens. In the lobules, there are perisinusoidal located hepatic stellate cells (HSCs) that contain COLV immunedeposits, which could be a source of COLV deposition in the space of Disse. Figure 1 illustrates the localization of COLV immunostained fibrils to the collagenous matrix of the perisnusiodal space of Disse. COLV is a regular component of the stroma of portal tracts. In portal tract fibrosis, the fibrotic stroma displays prominent COLV staining (Figure 2A and B). Normal central veins are surrounded by a thin rim, which is vividly stained for COLV (Figure 3A). Central vein fibrosis is characterized by a thickening of the vein wall, > 9 μm in thickness [26], which is conspicuously stained for COLV (Figure 3B). COLV immune-deposits are greatly increased in foci of severe perisinusoidal/pericellular fibrosis, and significantly, the fibrotic lesions also show increased staining for COLI, III and VI (Figure 4), demonstrating codistribution of these collagen types, as previously described [1,27]. The fibrous matrices of developing septa and bridging septa of septal fibrosis and the fibrotic bands of cirrhosis revealed an abundant COLV staining, in coincidence with COLI, III and VI (Figure 5). Moreover, as shown in (Figure 6), elastin— detected by resorcin fuchsin stain—is rich in the fibrous septa. Elastin has also been found in the stroma of normal and fibrotic portal tracts and the wall of fibrotic central veins, but it is not detectable in the space of Disse [28]. These immunohistological data point to a role for COLV in integrating fibrillar COL1/III and filamentous COLVI (as well as elastin when present) in the histogenesis of fibrotic lesions and promotion of hepatic fibrosis progression. (Figure 7) schematizes the distribution of COLV fibrils along with other protein fibers in the ECM, forming an elaborate tissue scaffolds, reminiscent of the matrices of fibrous septa.
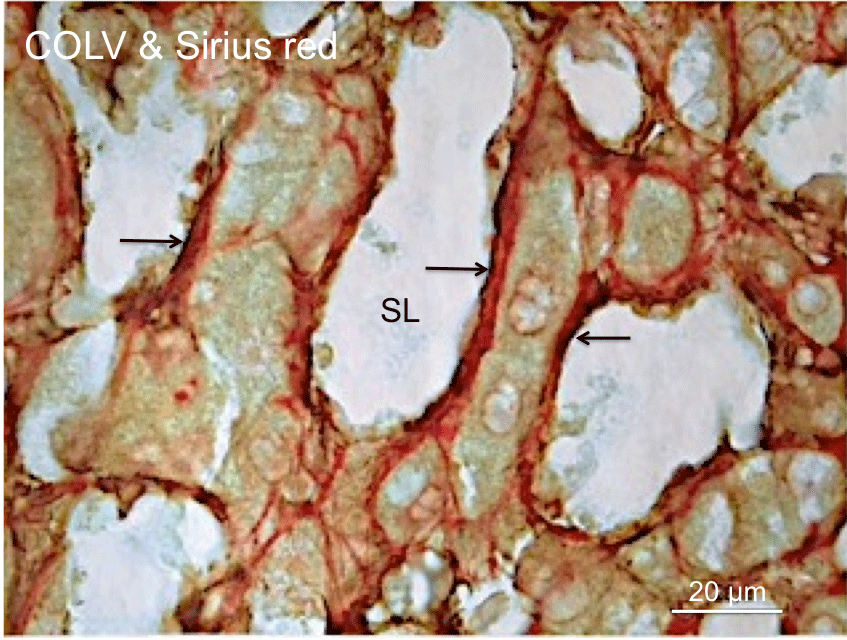
Figure 1: Localization of COLV to collagenous matrix of the space of Disse.
This cadaveric liver section was immunoperoxidase stained for COLV and
subsequently stained with Sirius red for collagens. These staining processes
demonstrate the distribution of COLV immunostained fibrils (arrows)
along the perisinisoidal borders and its localization to the collagenous
matrix (red) of the perisinusoidal space of Disse. SL, Sinusoid Lumen.
For immunohistochemistry, embalmed cadaveric liver tissue was fixed in
formalin and embedded in paraffin. Deparaffinized liver sections were treated
sequentially with a rabbit polyclonal COLV antibody (Novus Biologicals,
Littleton, CO), anti-rabbit polymer-Horse Radish Peroxidase (HRP) (Dako,
Carpinteria, CA), and the chromogen diaminobenzidine tetrahydrochloride
to yield a brown reaction product, with buffer washes between steps. The
specificity of the COLV immunoreaction has been described [1].
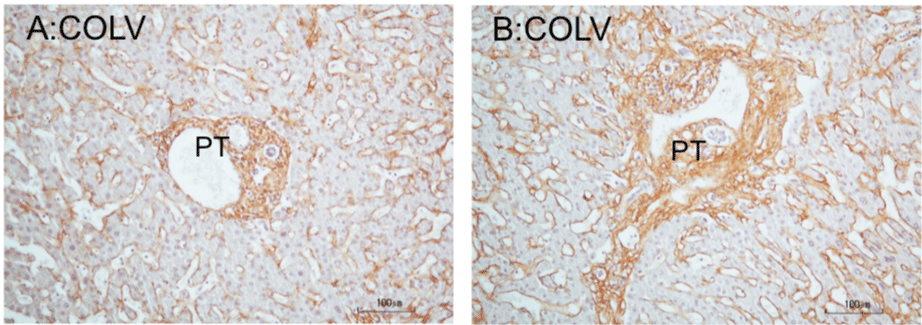
Figure 2: COLV immunoperoxidase staining of portal tracts of cadaveric liver. A: Histological normal portal tract. The tract shows a round and smooth border. The
portal matrix is regularly stained for COLV. Note presence of COLV immunostain in the perisinusoidal borders of the liver lobule. B: Portal tract fibrosis. The fibrotic
tract shows a stellate border with short extensions into the parenchyma, representing beginning septa formation. The tract is also somewhat enlarged. The stroma
displays a robust COLV immunostain. Hematoxylin counterstained.
*PT: Portal Tract.
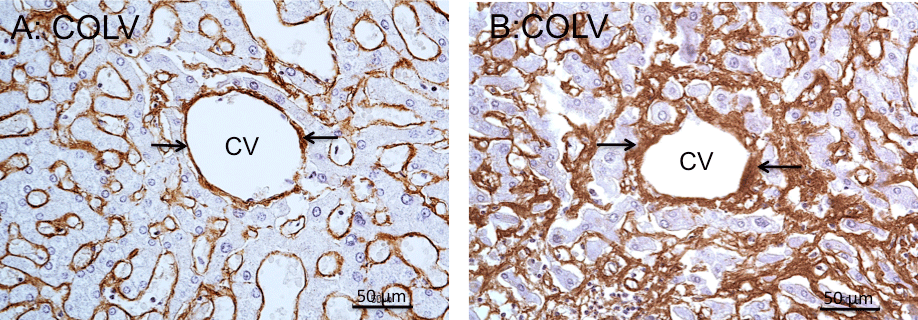
Figure 3: COLV immunoperoxidase staining of central veins of cadaveric liver. A: Normal central vein. The rim surrounding the vein is thin and is regularly stained
for COLV (arrows). Note presence of COLV immunostain in the perisinusoidal borders of the lobule. B: Central vein fibrosis. Positive COLV immunostaining
marks the thickening of the fibrotic vein wall (arrows), > 9μm in thickness, along with fibrous extensions into the perivenous parenchyma. Note increased COLV
immunostaining of the perisinusoidal borders, characterizing perisinusidal fibrosis. Hematoxylin counterstained.
*CV: Central Vein.
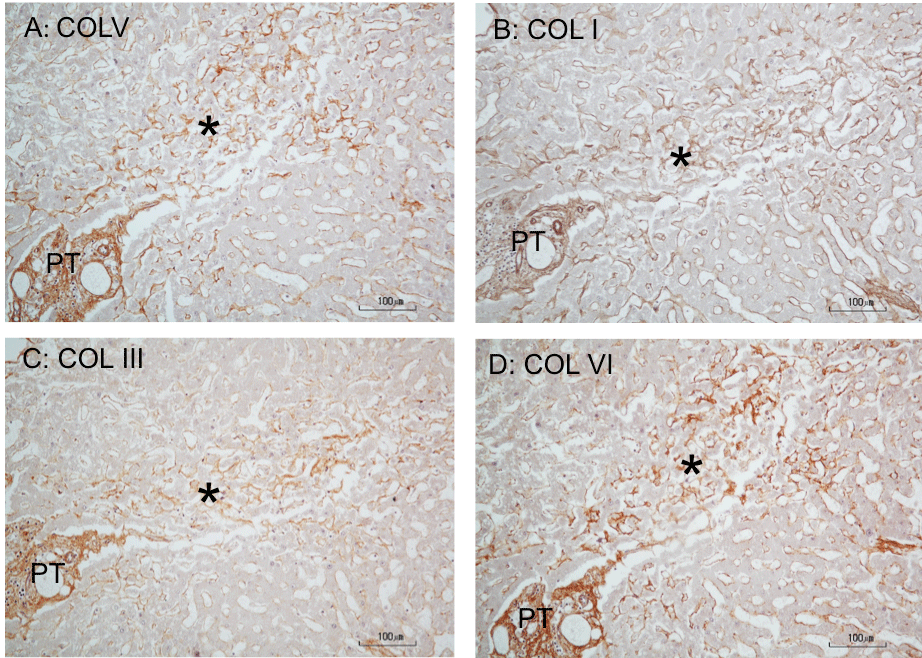
Figure 4: Immunoperoxidase staining for collagens in perisinusoidal fibrosis of cadaveric liver. A, B, C, and D are serially cut consecutive sections (5-μm thick)
stained for COLV, I, III, and VI, respectively. COLI and III polyclonal antibodies were from Rockland Immunochemicals, Albertsville, PA), and COLV and VI
polyclonal antibodies were from Novus Biologicals, Littleton, CO). A: The star marks a perisinusoidal fibrosis in the periportal and mid-lobular parenchyma, revealing
an increased COLV immunostaining compared to the lighter staining reaction surrounding the lesion. The increased COLV staining is coincident with an enhanced
staining for COLI, COLIII, or COLVI in the same fibrotic area (B, C and D), demonstrating codistribution of these collagen types. Hematoxylin counterstained.
*PT: Portal Tract.
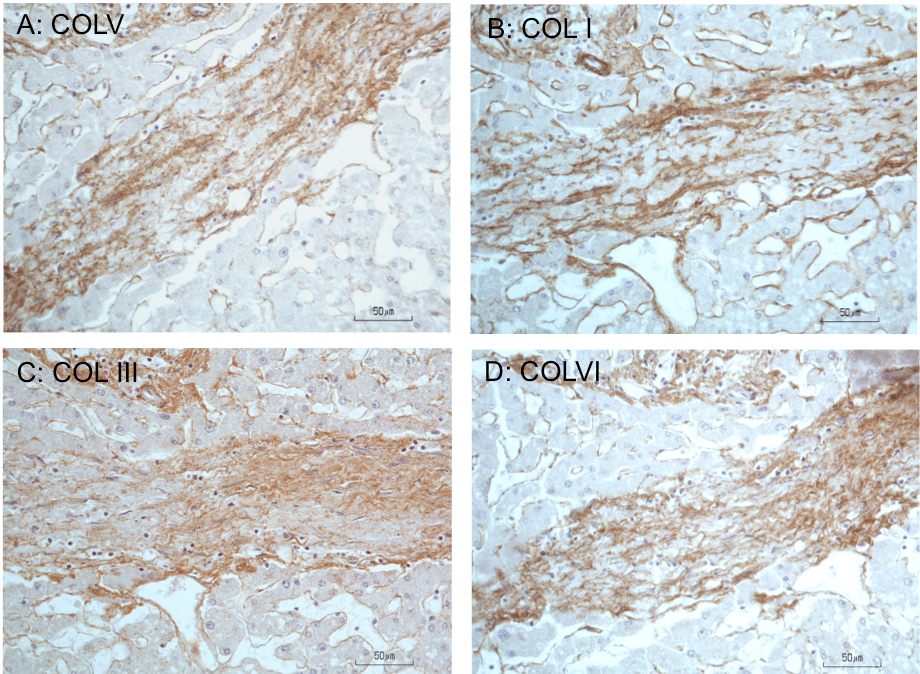
Figure 5: Immunoperoxidase staining for collagens in fibrous septum of cadaveric liver. A, B, C, and D are serially cut consecutive sections (5-μm thick) of a fibrous
septum from a liver with bridging fibrosis and stained for COLV, I, III, and VI, respectively. Compared to the lighter staining reaction in the surrounding lobular
parenchyma, the matrix of the septum shows increased COLV immunostaining coincident with increased staining for COLI, III, or VI, demonstrating codistribution
of these collagen types. Hematoxylin counterstained. (From [25]—Austin Biomarkers and Diagnosis, open access journal; [27]).
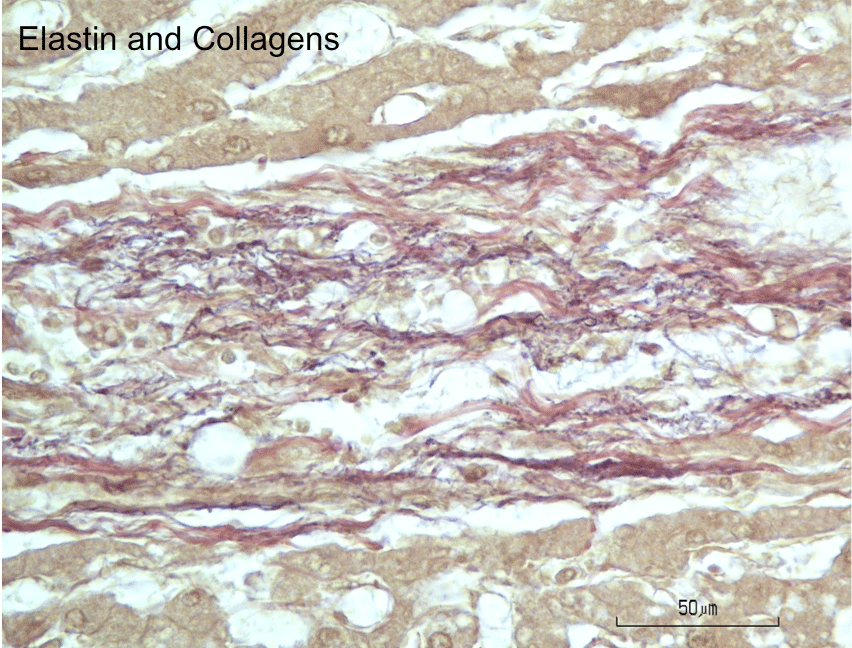
Figure 6: Histochemical staining for elastin and collagen fibers in fibrous
septum of cadaveric liver. Elastic fibers appear purplish-black with resorcin
fuchsin stain and are discernable as fine fibers intertwined with the coarser
collagen fibers stained red with van Gieson’s stain. Note absence of elastin
stain in the lobular parenchyma.
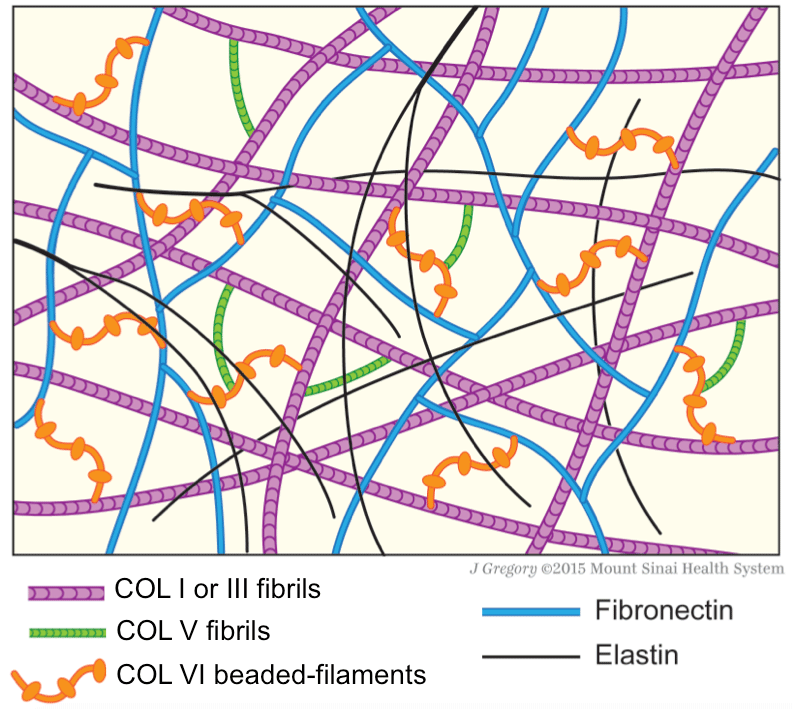
Figure 7: Schematic drawing of protein fiber distribution in extracellular
matrix. COLV, which is fibrillar, links beaded-filamentous COLVI, and in turns,
both collagens make connections with COLI or COLIII fibrils. Fibronectin has
structural connection with COLVI, but not with COLV. Elastin appears as thin
branching fibers and forms an irregular network throughout the matrix, but has
no apparent structural connection with other fibers. Together these structural
proteins form tissue scaffolds rendering structural integrity of the connective
tissue matrix and providing supports to cells (not depicted in drawing).
Moreover, COLV and COLVI bind matrix fibrogenic-related molecules and
control their availability for fibrogenesis and fibrolysis [1,25].
COLV in diagnostic histopathology
The value of COLV expression in staging fibrosis was evaluated. To that end, the expression profiles of different collagen types in biopsies of patients with hepatitis-related liver fibrosis were examined by immunoperoxidase staining [29]. COLV, along with COLIII, IV and VI, trichrome (stains primarily COLI) was tested at various stages of the fibrosis. It was found that COLV and COLVI have the strongest expression in early fibrosis compared to COLIII, IV and trichrome stain, with COLIV being the weakest. However, all these collagen stains showed significant increases at late stage of fibrosis. It was concluded that COLV and VI maybe helpful in identifying early fibrosis when the trichrome stain is weak or negative. During the transition from early stage to late stage of fibrosis, trichrome staining and COLIV demonstrated the steepest increases and they appear to be the most useful discriminators between early and late fibrosis stages.
In the clinic, it was felt to be a challenge in differentiating the fibrous tissue of Glisson’s capsule from the septal fibrosis of cirrhotic liver on needle biopsies that are often fragmented [30]. The distinction between capsular fibrous tissue and septal fibrosis is critical to avoid over staging of liver fibrosis. Using a panel of immunostains for COLIII, IV, V, VI, vitronectin, and laminin, as well as trichrome stain in needle biopsies of cirrhotic livers, it was found that both the capsule and septal fibrous tissues are strongly stained for COLV and trichrome, while the stains for COLIII, IV and COLVI and laminin are weak to negative in the capsule but strong in the septal tissue. It was proposed that if a fibrotic area—in needle biopsies—stains weakly or negatively for COLIII, IV and VI, but strongly for COLV and trichrome, it suggests the area is fibrous capsule rather than septal fibrosis. Therefore, COLV could be useful to help identify the fibrous tissue that is likely to be derived from the liver capsule and not true bridging fibrosis/cirrhosis, avoiding potential over staging of liver fibrosis.
COLV in Experimental Hepatic Fibrosis
Compared to other fibrillar collagen types, there have been fewer studies that examined the involvement of COLV in experimental liver fibrosis. Nonetheless, these investigations in experimental animals have generated useful and relevant information on the role for COLV in liver fibrosis. It is worth noting that the concentration of total collagen content in the rat liver is about 6 times less than that of the human liver [17]. Moreover, COLV level in the rat liver is about 18 times lower than that of the human liver—0.05 mg per g of tissue compared to 0.9 mg per g of tissue of normal human liver.
Upregulation of COLV in liver fibrogenesis induced by CCl4
In normal rat liver, COLV is a minor component of hepatic collagens, constituting about 5.5% of total hepatic liver collagen (0.05 mg/g of tissue vs. 0.91 mg/g of tissue) [17]. This amount of COLV represents 12.5% relative to COLI (0.05 mg/g tissue vs. 0.40 mg/gm tissue), the most abundant collagen type in the liver tissue. Liver fibrosis caused by CCl4 treatment for 7-10 weeks had increased expression of the a1(V) and a2(V) isoforms of COLV, analyzed of pepsin extracted liver tissues by sodium dodecyl sulfatepolyacrylamide gel electrophoresis [20]. Treatment with CCl4 elevated COLV 2.7 fold in fibrosis and 4.6-fold in cirrhosis compared to controls. In comparison, the increase of COLI in fibrosis was 1.8-fold and in cirrhosis, 1.6-fold. Based on these data, the increase of COLV may represent a more sensitive indicator for development of cirrhosis than the increase of COLI. By immunohistochemistry, COLV staining in the control liver was seen along the sinusoidal lining of the liver lobules, some of which showed colocalization with COLI [20]. In CCl4-cirrhotic liver, COLV staining was prominent at the border of the fibrous septa, while COLI staining are largely in the matrix of the septa. Codistribution of COLV and COLI/III in the space of Disse of the snow monkey has been demonstrated by immunoelectron microscopy [31]. In corroboration with the in vivo data, Takai et al. [20] showed that HSC culture with an activated phenotype express a1(V) and a2(V) genes, which are translated into the corresponding proteins for secretion into the culture media, consistent with the immunohostochemical observation of human perisinusoidal HSCs being a source of COLV deposition in the space of Disse—supra vide.
Thinning of collagen fibrils in liver fibrosis
Scott et al. [32] examined the ultrastructure of collagen fibrils in the rat models of liver fibrosis induced by bile duct ligation or thioacetamide treatment. Morphometry of uranyl acetate-stained collagen fibrils revealed that the fibrils are quarter-staggered with a normal a-e banding pattern—characteristic of COLI—but are thinner in diameter (25-35 nm) in the cirrhotic liver than those in control rats (30-50 nm). Moreover, the chemical contents of collagens and several species of proteoglycans were elevated 5 to 10-fold in cirrhosis. Consequently, the fibrils that are thinner—hence with larger surface area—in the fibrotic liver along with the increased collagen content was associated with higher levels of fibril surfaceassociated proteoglycans than those thicker fibrils found in the normal liver. Strikingly, the fibrils in the fibrotic liver are not disposed in parallel ordered sheets as in normal ECM of adult tendon, cornea and skin, but are haphazardly arranged, as seen in developing young tendon [32]. Similarly, Moriya et al. [33] observed collagen fibrils that are thinner in mice after acute CCl4 treatment (41 nm vs. 50 nm in control) and attributed the thinning to an increased production of COLV, in accordance with COLV’s role in limiting the diameter growth of collagen fibrils—discussed below.
COLV production by hepatic stellate cells: Roles of TGF- β1 and COLV in fibrillogenesis
The COLV a1(V)-N-propeptide binds TGF-β1, MMP-2 and tissue inhibitor of metalloproteinase (TIMP)-1 [34], all of which are critical mediators of collagen production or degradation. COLV could serve as a reservoir, regulating the availability of these molecules in the events of fibrogenesis in a manner similar to that described for the filamentous COLVI [25]. However, TGF-β activity is also determined by its binding to the latent TGF-β-binding protein and their binding molecules such as fibronectin in the ECM. Indeed, Moriya et al. [33] showed that TGF-β1 elicits a 2.5-fold increase of Col5a1 mRNA in mouse HSC culture, the response of which is the strongest of the 20 type I/III collagen assembly-related molecules examined. The upregulation of Col5a1 mRNA expression in HSCs is followed by deposition of COLV in the culture matrix, which mediates COLI and III fibrillar assembly with resultant collagen network formation in the matrix. This TGF-β1 effect is inhibited by knockdown of a1(V) mRNA levels using Col5a1 siRNA. Moreover, soluble COLV when added to HSC culture was found to stimulate formation of COLI fibrils but to a lesser extent than when adding TGF-β1. In vivo analysis of collagen fibrils after acute liver injury by CCl4 revealed a codistribution of COLV and COLI fibrils in the liver lobules. The subpopulation of fibrils with a diameter of < 35 nm increased as determined by electron microscopy. The diameter of COLI is inversely proportional to the COLV /COLI ratio—infra vide. The study concluded that both TGF-β1 and COLV participate in initiating HSC fibrogenic response to liver damage.
Regulation of collagen fibril diameter via heterotypic fibril formation
The thinning of collagen fibrils observed in liver fibrogenesis is thought to be due to the formation COLV/COLI heterotypic fibrils consequent to the interaction of COLV with COLI as alluded to above [33]. Actually, it was hypothesized earlier that COLV forms heterotypic fibrils by co-assembling with COLI and influences the diameter growth of the collagen fibrils, contributing to the smaller fibril diameter. This view was tested independently by two groups of investigators using an in vitro self-assembly system in which fibril reconstitution was initiated by mixing soluble COLV and COLI in various proportions [35,36]. Both studies demonstrated that as the ratio of COLV to COLI increased, the diameter of the heterotypic COLI/V fibrils became smaller. The reconstituted heterotypic collagen fibrils displayed the typical 67-nm cross-striated periodicity (D-periodic banding pattern) and had the same characteristics as those seen in vivo with respect to masking of the COLV epitopes [1,36]. Notably, the propeptide NH2 domain of the COLV molecule was required for the full observed effect, and in its absence, little diameter reducing activity occurred. These results define a role for COLV in modulating fibrillogenesis by limiting the growth of collagen fibrils into thicker ones, as was also documented in vivo in the cornea [37]. Nonetheless, it is uncertain whether this model of corneal fibrillogenesis applies in parenchymal organs with very different tissue matrix composition/ morphology and metabolic functions than those of the cornea, such as liver, lungs, pancreas, adipose tissue, aortas, and kidneys.
Enzymatic degradation of COLV by MMPs in liver ECM remodeling
COLV is made and deposited in the ECM where it becomes linked to fibrillar COLI/ III and filamentous COLVI. Together with fibronectin and elastin (in some anatomical sites), these proteins form tissue scaffolds, providing structural and functional supports to connective tissue matrices [1]. However, the ECM is constantly remodeled even in normal conditions with a balanced production and degradation of matrix proteins so as to achieve homeostasis for proper functioning of the tissue. An imbalanced remodeling of ECM will cause pathological changes. As native COLV is resistant to digestion by mammalian interstitial collagenases that degrade COLI and III, advances have been made in identifying and characterizing several metalloproteinases—MMP-2, MMP-8, MMP-9—from rabbit and human pulmonary alveolar macrophages, rabbit synovial cells, human neutrophils [38-41] that degrade both COLV and denatured COLI. In the human liver, Kobayashi et al. [42] found a COLV degrading metalloproteinase. Later studies described the cleavage of denatured COLV as well as denatured COLI by MMP-2 (gelatinase A secreted by activated HSCs) and native COLV and denatured COLI by MMP-9 (gelatinase B secreted by Kupffer cells) [43,44]. These two MMPs have been shown to be the most highly upregulated proteases in liver fibrotic tissues. As the COLV content is substantially elevated in liver fibrosis [17, 18], these gelatinases likely participate in hepatic ECM remodeling, facilitating turnover of COLV and potentiating degradation of denatured COLI during hepatic fibrogenesis.
Hepatic Fibrosis Biomarkers
Despite advances made in the understanding of COLV’s involvement in liver fibrogenesis and progression of fibrosis to cirrhosis, the issue of COLV serving as a noninvasive liver fibrosis biomarker has yet to be addressed. In a number of authoritative reviews of biomarkers for liver fibrosis of different etiologies, COLV has not been included in the discussion as a fibrosis marker [45-48]. This may have been overlooked due to the lesser abundance of COLV relative to COLI, III, and IV in the liver ECM.
Neo-epitopes and protein fingerprints
Protein fingerprints technique based on the measurement of neoepitopes generated in the process of fibrogenesis and fibrolysis has been applied in the development of liver fibrosis biomarkers, which will be discussed. It is known that a highly regulated equilibrium between the synthesis and degradation of ECM proteins—particularly the collagen types—is required to achieve tissue homeostasis. A disruption of this equilibrium will upset the homeostasis, regarded as the basis of pathological processes, fibrosis included. The degradation products of ECM proteins can be measured in biological fluids, and such measurements can give an indication of disease activity and progression. MMPs and cysteine proteases function in the degradation of collagens and proteogylcans of the ECM, respectively, resulting in the generation of specific cleavage peptide fragments, called neo-epitopes [49]. These are post-translational modifications of proteins formed by processes such as cleavage, citrullination, nitrosylation, glycosylation and isomerization, with resultant changes in configuration and properties [50]. Because the neo-epitopes are generated locally in the pathologically affected areas, involving a specific disease, they may carry a unique disease protein fingerprint— or biochemical marker. Measurement of these neo-epitopes has been given the term “protein fingerprint technology” [51-55]. Therefore, compared to serological assays of the intact total protein, quantifying neo-epitopes is likely to provide a more sensitive indicator of the pathological changes. In particular, these neo-epitopes are small enough to be released into the circulation or urine, allowing for their detection by antibodies raised specifically against the neo-epitopes. This is commonly measured by enzyme-linked immunosorbent assay (ELISA) [1]. Measurement of these neo-epitopes in the serum may indicate the degree of remodeling of ECM proteins that are involved in the pathogenesis of liver fibrosis. Notably, neo-epitopes have successfully been used as noninvasive serum markers in osteoporosis and arthritis [49], both of which are characterized by extensive ECM remodeling.
COLV neo-epitopes as hepatic fibrosis biomarker
COLV constitutes a minor component of the total hepatic collagen, 10-16%, but it rapidly proliferates in the events of active fibrogenesis in response to experimental liver injury [20]. To that effect, neo-epitopes derived from the first ten amino acids of the C-terminal propeptide of the a2(V) chain, which is a formationspecific region, could be used to monitor the turnover of COLV in liver fibrogenesis [56]. Specifically, a COLV fragment containing the sequence TAALGDIMGH between amino acid position 1230’ and 1239’ located in the first ten amino acids of the C-terminal propeptide of the a2(V) chain has been generated. This COLV neo-epitope is designated as CO5-1230 or preferably P5CP and is classified as COLV formation marker [57]. It was used as an immunogen to raise a monoclonal antibody, which was used to quantify the levels COLV in the serum by ELISA. Accordingly, COLV was evaluated as a biomarker of liver fibrosis in two rat models of experimental fibrosis, namely CCl4 inhalation treatment and bile duct ligation. It was found that the serum levels of P5CP from the fibrotic liver increase in association with the extent of collagen deposition in the liver—assessed by Sirius red stain for collagens—during the progression of hepatic fibrosis from early stage to end-stage cirrhosis. It was proposed that the neoepitope P5CP, which is a COLV formation marker, maybe of value as a disease-specific diagnostic biomarker of liver fibrosis.
In another study by Leeming et al. [52], hepatic collagen in CCl4- treated rats was quantified by histomorphometry of collagens stained by Sirius red and the values were expressed as four quartiles Q1, Q2, Q3 and Q4, representing early, moderate, severe fibrosis and cirrhosis, respectively. Serum neo-epitope P5CP levels were significantly elevated in all collagen quartiles in CCl4-treated rats compared to controls. When evaluated as a single COLV formation marker, P5CP cannot distinguish early, moderate, or severe fibrosis; however, when P5CP was used in conjunction with C6M (also named CO6-MMP), which is a COLVI degradation marker, the combination of the two markers showed a higher and better correlation with hepatic collagen than any of the single markers, including COLI, III or IV. The combination of scores generally enabled separation of early fibrosis, severe fibrosis, and cirrhosis from the respective controls. Moreover, the P5CP and C6M combined scores differentiate early and moderate fibrosis, as well as severe fibrosis and cirrhosis, but not moderate fibrosis from severe fibrosis. Hence, the combined use of COLV and COLVI biomarkers is the most reliable indicator of both early- and late-stage fibrosis (i.e. severe fibrosis and cirrhosis).
To further validate P5CP neo-epitope as a formation marker of COLV, this system was tested by monitoring changes in serum levels of P5CP during liver fibrosis progression and regression in a wellestablished rat model of reversible liver fibrosis using CCl4 [58]. Rats were given CCl4 by intraperitoneal injection for 4, 6 and 8 weeks, once a week, and then left to regress for a period of 14, 20, and 26 weeks. The measurements indicate that P5CP progressively elevated from 5.2 ng/ml before the treatment to 10.8 ng/ml at 8 weeks. Upon the withdrawal of CCl4 and during the regression phase, P5CP levels were normalized to levels of the control. The changes in P5CP levels correlated with the histopathology of collagen deposition during the fibrosis progression and regression, determined by Sirius red stain for collagens. Therefore, not only is the neo-epitope marker valuable in monitoring fibrosis progression, it also has a potential for assessing fibrotic regression and resolution in association with therapeutic efficacy of fibrosis with antifibrotic agents. P5CP-1230 as a biochemical marker for monitoring both fibrosis progression and regression appears to be a unique property of the neo-epitope, when compared to COLIII neo-epitope CO3-610, which is a MMP- 9 generated degradation product of COLIII [59]. Using a similar fibrosis progression and regression model induced by CCl4 as described above in the P5CP study, it was found that the levels of COLIII correlated significantly with the degree of fibrosis during the progression phase, but were not correlated with total collagen during the regression. Liver MMP-9 expression was enhanced in the rats with fibrosis. CO3-610 seems to be produced only under the CCl4 stimulus, indicating that CO3-160 is a potential marker of progression rather than regression, contrasting the ability of P5CP- 1230 in the monitoring of fibrosis resolution.
Portal hypertension occurring in end-stage cirrhosis is associated with complications and mortality. Measurement of hepatic venous pressure gradient (HVPG) is an important and an invasive diagnostic and prognostic marker in hypertensive patients with cirrhosis. To that end, Pro-C5, also previously known as P5CP-1230, was tested as a noninvasive biochemical maker in plasma of patients to estimate the degree of portal hypertension [60]. Ninety-four patients with alcoholic cirrhosis and fourteen controls were studied. Plasma Pro-C5 level was significantly correlated with the degree of portal hypertension. Pro-C5 was able to detect significant clinical portal hypertension and was related to Child-Turcotte score. Furthermore, plasma Pro-C5 reflects liver function and systemic hemodynamic parameters. Therefore, circulating Pro-C5 represents a promising new tool for noninvasive evaluation of portal hypertension and hepatic dysfunction in patients with cirrhosis.
In line with this human investigation, the COLIII degradation marker CO3-610 has been evaluated to assess the development portal hypertension in CCl4-fibrosis rat model [61]. The data demonstrated a significant relationship between serum neo-epitope CO3-610 and portal hypertension as well as increases in hepatic collagen content. Because the amino sequence of COLIII is identical in rats and humans, the neo-epitope CO3-610 could be tested as noninvasive biomarker of fibrosis in patients with chronic liver disease.
Thus far, with respect to use of COLV neo-epitopes as biomarker for evaluating liver fibrosis-related disorders, there has been just one report focusing on human investigation. Implementation of the animal (preclinical) models to translational medicine requires further study, for the etiologies of hepatic fibrosis in humans are multiple and are more variable than the experimental rat models of fibrosis. While the exploitation of noninvasive serum biochemical markers in assessing hepatic fibrogenesis and fibrosis may minimize the need for invasive liver biopsies, it is not likely to replace biopsies, which are the only tool for the evaluation of liver histopathology of patients [45,47,57,62].
Conclusions and Future Directions
COLV is ubiquitous in the liver ECM. Enhanced COLV expression occurs in early stages of hepatic fibrosis and the expression continues to rise as fibrosis progresses to end-stage cirrhosis. However, the significance of these observations has minimally been recognized and the findings are hardly utilized in diagnostic histopathology in the clinic. Since COLV contributes to the structural organization of tissue matrix scaffolds, and since COLV co-distributes with COLI, III and VI as well as elastin in the fibrotic lesions of the liver, data are needed to understand the structural interactions between COLV and these structural proteins in the liver ECM. HSCs express COLV, which could be a source of COLV deposits in the space of Disse. Expression of COLV by fibrogenic myofibroblasts and portal fibroblasts has not been described. Thus far there have been only two studies attempted to analyze the production and secretion of COLV by HSC culture. The data appear to be in line with COLV’s regulatory role of fibrillogenesis. Along this line, it is critical to determine whether the cellular production of COLV is regulated differently from that of COLI, III and VI. Equally important is to assess whether the presence of COLV— being a regulatory fibril-forming collagen—is required for sustaining and/or perpetuating the deposition of COLI, III and VI in the liver ECM in vivo and hence fibrosis progression or resolution. COLV binds TGF-β1, MMP-2 and TIMP-1, and regulates their availability for fibrogenesis and fibrolysis; this is an area of research that deserves further investigation. As ECM remodeling is an integral process of fibrosis, data are needed to understand the enzymatic degradation and turnover of COLV during hepatic fibrogenesis. COLV deficient animal models could be helpful in defining COLV’s specific role in hepatic fibrogenesis and fibrosis, but no COLV knockouts are suitable for this line of investigation. Immunohistochemistry in conjunction with immunoelectron microscopy or in situ hybridization for gene expression analysis are valuable tools for assessing COLV expression and its interactions with other ECM proteins in normal and diseased human liver biopsies (of optimal quality).
An exciting area of research is the application of protein fingerprint technology in the measurement of COLV neo-epitopes that have been found to be a useful noninvasive biomarker for assessing the progression and resolution of fibrosis in experimental models of liver fibrosis as well as an indicator for noninvasive evaluation of portal hypertension in alcoholic patients with cirrhosis. Although the application of COLV to translational medicine needs validation, it is concluded nonetheless that as a biomarker, COLV may have diagnostic significance to hepatic fibrosis either used alone or in combination with other biochemical markers such as COLVI.
Acknowledgement
The author wishes to thank Jill Gregory (CMI, FAMI Manager, Academic Medical Illustration, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System) for her contribution to the illustration (Figure 7) and acknowledge the support by the Research Fund of the Center for Anatomy and Functional Morphology at Icahn School of Medicine at Mount Sinai.
References
- Mak KM, Png CY, Lee DJ. Type V Collagen in Health, Disease, and Fibrosis. Anat Rec (Hoboken). 2016; 299: 613-629.
- Birk DE, Bruckner P. Collagens, suprastructures and collagen fibril assembly. In: Mecahm RP, editor. The Extracellular Matrix: An Overview. New York: Springer-Verlag. 2011; 1: 77-115.
- Burgeson RE, El Adli FA, Kaitila II, Hollister DW. Fetal membrane collagens: identification of two new collagen alpha chains. Proc Natl Acad Sci USA. 1976; 73: 2579-2583.
- Chung E, Rhodes RK, Miller EJ. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976; 71: 1167-1174.
- Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979; 76: 710-719.
- Broek DL, Madri J, Eikenberry EF, Brodsky B. Characterization of the tissue form of type V collagen from chick bone. J Biol Chem. 1985; 260: 555-562.
- Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990; 10: 1-10.
- Fichard A, Kleman JP, Ruggiero F. Another look at collagen V and XI molecules. Matrix Biol. 1995; 14: 515-531.
- Haralson MA, Mitchell WM, Rhodes RK, Kresina TF, Gay R, Miller EJ. Chinese hamster lung cells synthesize and confine to the cellular domain a collagen composed solely of B chains. Proc Natl Acad Sci USA. 1980; 77: 5206-5210.
- Haralson MA, Mitchell WM, Rhodes RK, Kresina TF, Miller EJ. Evidence that the collagen in the culture medium of Chinese hamster lung cells contains components related at the primary structural level to the al(V) collagen chain. Arch Biochem Biophys. 1984; 229: 509-518.
- Bonod-Bidaud C, Roulet M, Hansen U, Elsheikh A, Malbouyres M, Ricard-Blum S, et al. In vivo evidence for a bridging role of a collagen V subtype at the epidermis-dermis interface. J Invest Dermatol. 2012; 132: 1841-1849.
- Niyibizi C, Fietzek PP, van der Rest M. Human placenta type V collagens. Evidence for the existence of an alpha 1(V) alpha 2(V) alpha 3(V) collagen molecule. J Biol Chem. 1984; 259: 14170-14174.
- Huang G, Ge G, Wang D, Gopalakrishnan B, Butz DH, Colman RJ, et al. a3(V) collagen is critical for glucose homeostasis in mice due to effects in pancreatic islets and peripheral tissues. J Clin Invest. 2011; 121: 769-783.
- Malfait F, Wenstrup RJ, De Paepe A. Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. 2010; 12: 597-605.
- Symoens S, Syx D, Malfait F, Callewaert B, De Backer J, Vanakker O, et al. Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum Mutat. 2012; 33: 1485-1493.
- Sun M, Chen S, Adams SM, Florer JB, Liu H, Kao WWY, et al. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci. 2011; 124: 4096-4105.
- Rojkind M, Ponce-Noyola P. The extracellular matrix of the liver. Coll Relat Res. 1982; 2: 151-175.
- Aycock RS, Seyer JM. Collagens of normal and cirrhotic human liver. Connect Tissue Res. 1989; 23: 19-31.
- Rojkind M, Perez-Tamayo R. Liver fibrosis. International Review of Connective Tissue Research. Academic Press. 1983; 10: 333-382.
- Takai KK, Hattori S, Irie S. Type V collagen distribution in liver is reconstructed in coculture system of hepatocytes and stellate cells; the possible functions of type V collagen in liver under normal and pathological conditions. Cell Struct Function. 2001; 26: 289-302.
- Lui VCH, Kong RYC, Nicholis J, Cheung ANY, Cheah KSE. The mRNAs for the three chains of human collagen type Xl are widely distributed but not necessarily co-expressed: implications for homotrimeric, heterotrimeric and heterotypic collagen molecules. Biochem J. 1995; 311: 511-516.
- Biempica L, Morecki R, Wu CH, Giambrone MA, Rojkind M. Immunocytochemical localization of type B collagen: a component of basement membrane in human liver. Am J Pathol. 1980; 98: 591-602.
- Schuppan D, Becker J, Boehm H, Hahn EG. Immunofluorescent localization of type-V collagen as a fibrillar component of the interstitial connective tissue of human oral mucosa, artery and liver. Cell Tissue Res. 1986; 243: 535-543.
- Knupp C, Squire JM. Molecular packing in network-forming collagens. Adv Protein Chem. 2005; 70: 375-403.
- Mak KM, Sehgal P, Harris CK. Type VI collagen: Its biology and value as a biomarker of hepatic fibrosis. Austin Biomark Diagn. 2014; 1: 9.
- Mak KM, Kwong AJ, Chu E, Hoo NM. Hepatic steatosis, fibrosis, and cancer in elderly cadavers. Anat Rec (Hoboken). 2012; 295: 40-50.
- Mak KM. Codistribution of collagens V and VI with collagens I and III in hepatic fibrosis of elderly cadavers. FASEB J. 2015; 29: 544.4.
- Mak KM, Chu E, Lau KHV, Kwong AJ. Liver fibrosis in elderly cadavers: Localization of collagen types I, III and IV, a-smooth muscle actin, and elastic fibersis. Anat Rec. 2012; 295: 1159-1167.
- Chen W, Jonathan JB, Yearsley MM, Ferrell LD, Frankel WL. Different collagen types show distinct rates of increase from early to late stages of hepatitis C–related liver fibrosis. Human Pathol. 2014; 45:160-165.
- Chen W, Rock JB, Yearsley MM, Hanje AJ, Frankel WL. Collagen immunostains can distinguish capsular fibrous tissue from septal fibrosis and may help stage liver fibrosis. Appl Immunohistochem Mol Morphol. 2014; 22: 735-740.
- Adachi E, Hayashi T, Hashimoto PH. A comparison of the immunofluorescent localization of collagen types I, III, and V with the distribution of reticular fibers on the same liver sections of the snow monkey (Macaca fuscata). Cell Tissue Res. 1991; 264: 1-8.
- Scott JE, Bosworth TR, Cribb AM, Gressner AM. The chemical morphology of extracellular matrix in experimental rat liver fibrosis resembles that of normal developing connective tissue. Virchows Arch. 1994; 424: 89-98.
- Moriya K, Bae E, Honda K, Sakai K, Sakaguchi T, Tsujimoto I, et al. A fibronectin-independent mechanism of collagen fibrillogenesis I adult liver remodeling. Gastroenterology. 2011; 140: 1653-1663.
- Symoens S, Renard M, Bonod-Bidaud C, Syx D, Vaganay E, Malfait F, et al. Identification of binding partners interacting with the a1-N-propeptide of type V collagen. Biochem J. 2011; 433: 371-381.
- Adachi E, Hayashi T. In vitro formation of hybrid fibrils of type V collagen and type I collagen. Limited growth of type I collagen into thick fibrils by type V collagen. Connect Tissue Res. 1986; 14: 257-266.
- Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990; 95: 649-657.
- Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001; 32: 223-237.
- Mainardi CL, Hibbs MS, Hasty KA, Seyer JM. Purification of a type V collagen degrading metalloproteinase from rabbit alveolar macrophages. Coll Relat Res. 1984; 4: 479-492.
- Niyibizi C, Chan R, Wu JJ, Eyre D. A 92 kDa gelatinase (MMP-9) cleavage site in native type V collagen. Biochem Biophys Res Commun. 1994; 202: 328-333.
- Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985; 260: 2493-2500.
- Hibbs MS, Hoidal JR, Kang AH. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987; 80: 1644-1650.
- Kobayashi M, Nakatsukasa H, Watanabe A, Yamauchi Y, Fujiwara M, Hashimoto M, et al. Detection of type V collagen-degrading enzyme activity in human liver. Acta Med Okayama. 1986; 40: 179-182.
- Arthur MJ. Collagenase and liver fibrosis. J Hepatol. 1995; 22: 43-48.
- Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim Biophys Acta. 2013; 1832: 876-883.
- Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006; 12: 3682-3694.
- Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin Chim Acta. 2007; 381: 107-113.
- Lieber CS, Weiss DG, Paronetto F. Value of fibrosis markers for staging liver fibrosis in patients with precirrhotic alcoholic liver disease. Alcohol Clin Exp Res. 2008; 32: 1031-1039.
- Fallatah HI. Noninvasive biomarkers of liver fibrosis: An overview. Hindawi Publishing Corp. Advances in Hepatology. 2014; 357287:15.
- Karsdal MA, Henriksen K, Leeming DJ, Mitchell P, Duffin K, Barascuk N, et al. Biochemical markers and the FDA Critical Path: How biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009; 4: 181-202.
- Karsdal MA, Henriksen K, Leeming DJ, Woodworth T, Vassiliadis E, Bay-Jensen AC. Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers--are they the cause or the consequence of the disease? Clin Biochem. 2010; 43: 793-804.
- Karsdal MA, Delvin E, Christiansen C. Protein fingerprints - relying on and understanding the information of serological protein measurements. Clin Biochem. 2011; 44: 1278-1279.
- Leeming DJ, Byrjalsen I, Jiménez W, Christiansen C, Karsdal MA. Protein fingerprinting of the extracellular matrix remodelling in a rat model of liver fibrosis--a serological evaluation. Liver Int. 2013; 33: 439-447.
- Schierwagen R, Leeming DJ, Klein S, Granzow M, Nielsen MJ, Sauerbruch T, et al. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Front Physiol. 2013; 4: 195.
- Vassiliadis E, Barascuk N, Karsdal MA. Atherofibrosis - a unique and common process of the disease pathogenesis of atherosclerosis and fibrosis - lessons for biomarker development. Am J Transl Res. 2013; 5: 1-14.
- Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014; 7: 4.
- Vassiliadis E, Veidal SS, Simonsen H, Larsen DV, Vainer B, Chen X, et al. Immunological detection of the type V collagen propeptide fragment, PVCP-1230, in connective tissue remodeling associated with liver fibrosis. Biomarkers. 2011; 16: 426-433.
- Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012; 7: 105-117.
- Vassiliadis E, Veidal SS, Hansen C, Karsdal MA, Leeming DJ. Circulating levels of a collagen type V propeptide fragment in a carbon tetrachloride reversible model of liver fibrosis. Biomark Insights. 2012; 7: 159-166.
- Vassiliadis E, Larsen DV, Clausen RE, Veidal SS, Barascuk N, Larsen L, et al. Measurement of CO3-610, a potential liver biomarker derived from matrix matalloproteinase-9 degradation of collagen type III, in a rat model of reversible carbon-tetrachloride-induced fibrosis. Biomark Insights. 2011; 6: 49-58.
- Leeming DJ, Veidal SS, Karsdal MA, Nielsen MJ, Trebicka J, Busk T, et al. Pro-C5, a marker of true type V collagen formation and fibrillation, correlates with portal hypertension in patients with alcoholic cirrhosis. Scand J Gastroenterol. 2015; 50: 584-592.
- Segovia-Silvestre T, Reichenbach V, Fernández-Varo G, Vassiliadis E, Barascuk N, Morales-Ruiz M, et al. Circulating CO3-610, a degradation product of collagen III, closely reflects liver collagen and portal pressure in rats with fibrosis. Fibrogenesis Tissue Repair. 2011; 4: 19.
- Burroughs AK, Cholongitas E. Non-invasive tests for liver fibrosis: encouraging or discouraging results? J Hepatol. 2007; 46: 751-755.