
Research Article
J Bacteriol Mycol. 2018; 5(2): 1063.
Interaction of Pseudobombax marginatum Robyns Stem Bark Extract on the Cell Surface of Bacillus cereus and Staphylococcus aureus
Santos ECG1*, Donnici CL2, Camargos ERS3, Araújo-Silva G4, Xavier-Junior FH5, Farias LM6, Corrêa LA2, Luz JRD1, Carvalho MAR6 and Almeida MG4
¹Postgraduate program in Health Sciences, Federal University of Rio Grande do Norte, Brazil
²Department of Chemistry, Federal University of Minas Gerais, Brazil
³Department of Morphology and Microscopy Center, Federal University of Minas Gerais, Brazil
4Department of Clinical and Toxicological Analysis, Federal University of Rio Grande do Norte, Brazil
5Department of Pharmacy, Federal University of Rio Grande do Norte, Brazil
6Department of Microbiology Federal University of Minas Gerais, Brazil
*Corresponding author: Santos ECG, Postgraduate program in Health Sciences, Federal University of Rio Grande do Norte, Brazil
Received: February 20, 2018; Accepted: March 21, 2018; Published: March 28, 2018
Abstract
The search for new drugs has favored the research on medicinal plants as a source of bioactive substances, mainly due to the increased microbial resistance. The aim of this study was to investigate the antibacterial activity of Pseudobombax marginatum against bacteria of clinical interest and its possible mechanism of action. The antibacterial activity of hydroalcoholic extract of Pseudobombax marginatum bark (HAEPMB) and of Butanolic Fraction (BF) was determined by Agar diffusion and dilution methods in which Bacillus cereus was more susceptible, followed by Staphylococcus aureus 29213 and 33591, with minimal inhibitory concentrations (MICs) corresponding to 0.5mg/mL and 1.0mg/mL, respectively. Changes in the bacterial cell ultra structure were shown through SDS-PAGE electrophoresis and Transmission Electron Microscopy, suggesting that P. marginatum interferes in cell surface of B. cereus as its possible mechanism of action. HAEPMB and BF affected the bacterial cell wall with changes in the cytoplasmic membrane and content, causing growth inhibition or cell death. The chemical analysis showed presence of phenolic compounds, among them catechin derivatives, and the activities are attributed to these derivatives.
Keywords: Pseudobombax Marginatum; Malvaceae; Antibacterial Activity; Mechanism of Action; Catechin Derivatives
Abbreviations
HAEPMB: Hydroalcoholic Extract of Pseudobombax Marginatum Bark; BF: Butanolic Fraction; MTT: 3-(4,5-Dimethylthiazol-2-Yl)- 2,5-Diphenyltetrazolium Bromide; 3T3: Mouse Fibroblast Cells; RAEC: Rat Aortic Endothelial Cells; HEK 293: Epithelial Embryonic Human Kidney Cells; VERO E6: African Green Monkey Kidney Cells; DMEM: Dulbecco´S Modified Eagle´S Medium; FBS: Fetal Bovine Serum; DMSO: Dimethylsulfoxide; MIC: Minimal Inhibitory Concentration; MBC: Minimal Bactericidal Concentration; TEM: Transmission Electron Microscopy; SDS-PAGE: Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis; BHI: Brain Heart Infusion; SDS: Sodium Dodecyl Sulfate; CL: Chlorophorm Fraction; EAF: Ethyl Acetate Fraction; DA: Diffusion Agar Method; RT: Retention Time; ND: No Determined; VAN: Vancomycin
Introduction
The Brazilian semiarid region, which occupies 11.5% of the national territory, is estimated to have about 318 species of 42 botanical families that are endemic to Caatinga’s biota. Plants belonging to this biome have peculiar characteristics, as they have adapted to survive in hot and dry climate [1,2]. Most of these plants are used in the popular northeastern medicine to treat several conditions, being an important source for pharmacological and phytochemical studies [3].
Pseudobombax marginatum is a tree that belongs to the Malvaceae family, measuring from 6 to 14 meters in height. It is popularly known as “embiratanha”, “embirat&aTilde;”, “embiraçu”, and “emburuçu”. This tree is exclusively distributed in South America, particularly in Bolivia, Paraguay, Brazil, and Peru. In Brazil it is found in the Southeastern, Center-Western, and Northeastern regions [4-6].
In Brazilian popular medicine the bark of P. marginatum is used for to treat inflammations of urinary tract and of the spinal column and of its inner bark to treat spinal pain [5-7]. Studies the hydroalcoholic extract from the inner bark of P. marginatum proved the anti-inflammatory and antinociceptive activities [8]. Although there are few studies demonstrating antimicrobial activity these plants, the literature showed activity of 70% ethanol extract against Candida species and Gram-positive and Gram-negative bacteria, at high concentrations [9], and the 70% ethanol extract against Gram positive bacteria [10].
Due to the increasing growth of microbial resistance to conventional antimicrobials, several studies have been conducted with medicinal plants in search of new drugs. The discovery of new natural or synthetic compounds has been essential to combat microbial resistance, which has increased whereas the acquisition and development of new antibiotics has decreased [11].
Since the biodiversity of Caatinga is large and poorly studied considering its biome and the use of its native plants in popular medicine, it is relevant to further the study its pharmacological potential, investigating the interactions occasioned by P. marginatum and its mechanism of action on bacteria of clinical interest.
Methods
Microorganisms
Staphylococcus aureus ATCC 25923/ 29213/ 33591, Staphylococcus epidermidis ATCC 12228, Enterococcus faecalis ATCC 19433, Bacillus cereus ATCC 11778, Listeria monocytogenes ATCC 15313, Escherichia coli ATCC 25922, Salmonella Typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 10145, and Shigella sonnei clinical strain, all used in the experiments, were obtained from the Anaerobe and Oral Microbiology Laboratory, Department of Microbiology, ICB/UFMG, Brazil. Stock cultures were kept at 4°C on nutrient agar and in a -80°C freezer in Brain Heart Infusion (BHI) broth supplemented with 10% glycerol.
Plant materials and extract preparation
Stem barks of Pseudobombax marginatum (A. St.-Hil, Juss & Cambess) A. Robyns were collected in Vale do Açu, Rio Grande do Norte, Brazil, in September 2008. These were identified by Dr. Maria Iracema Bezerra Loiola, and a voucher specimen was deposited at the Federal University of Rio Grande do Norte, Brazil, under the reference number 8204. The vegetal material was weighed and dried in an air-circulating stove at a temperature of 40ºC ± 2ºC for seven days. After drying, the bark was manually ground to obtain the vegetable raw material, which was then used to prepare both aqueous and hydroalcoholic extracts (hydroalcoholic extract of Pseudobombax marginatum bark - HAEPMB). The aqueous extract was prepared through decoction and the hydroalcoholic extracts were prepared with different proportions through maceration for five days. HAEPMB at the proportion of 50:50 presented the best activity (data not shown). This extract (225mL) was partitioned with chloroform, ethyl acetate, and n-butanol solvents (3 x 300mL for each solvent). All fractions were concentrated under reduced pressure at 40°C.
Total phenolic content
Total phenolic content of HAEPMB was analyzed through the colorimetric method of Folin-Ciocalteu as previously described [12,13]. Total phenolics were measured spectrophotometrically in 760nm (UV-VISIBLE Shimadzu 1650-PC, Tokyo, Japan) and expressed as µg of acid gallic/mg equivalent of HAEPMB. All analyses were performed in triplicate.
Infrared (IR) and Ultraviolet-Visible (UV-Vis) spectroscopy analysis
Spectra in the IR region were registered with the Thermo scientific Nicolet 380 FT-IR device by ATR, both for liquid and for solid compounds. The spectra in the ultraviolet-visible (UV-Vis) region were registered with the UV-160A Shimadzu spectrophotometer using 10mm optical path quartz cuvettes.
HPLC quantitative analysis
Chromatographic separation was performed by reversed phase HPLC analysis, as previously described [14]. The solution of catechin was used as standard compound, mixed and diluted in methanol at concentration of 1.5-50 µg/mL to obtain the linear range. A Varian HPLC system equipped with a quaternary pump (ProStar 240), an autosampler (ProStar 410), and a photodiode array detector (model 355 PDA UV/Vis) was used for the analysis of major compounds in the HAEPMB and BF (butanolic fraction). The analysis was carried out with a Phenomenex C18 chromatography column (100mm × 4.6mm, 2.6µm/ Torrance, CA, USA). The binary mobile phase consisted of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B), run with a gradient program, at a flow rate of 1.3mL/ min, monitored at 280nm. Identification and quantification were accomplished by external calibration. Results were expressed in mg of catechin/g of the extract.
Cell Viability by MTT assay (Cytotoxicity)
Cell Viability was determined using the MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. The mouse fibroblast cells (3T3), rat aortic endothelial cells (RAEC), epithelial embryonic human kidney cells (HEK 293) and African green monkey kidney cells (VERO E6) were cultured under standard conditions in Dulbecco´s modified Eagle´s medium (DMEM) (Invitrogen, San Diego, CA, USA), supplemented with fetal bovine serum (FBS) at a final concentration of 10%. Cells were maintained in cell culture flasks at 37°C in a humidified atmosphere containing 5% CO2 and were collected by treatment with trypsin. Cells (1 x 105 cells per well) were seeded in medium supplemented with FBS (10%) and cultured for 24h in 96-well microplates to promote adhesion. After 24h, cells were treated with different concentrations of HAEPMB (50, 100, 1000, 2000, and 4000 µg/mL) in triplicate and incubated at 37°C for 24, 48 and 72h. After this period, 100µL of MTT (5mg/mL) was added to each well and the cells were incubated again. After 4h, the culture medium was removed and then 100µL of DMSO was added to each well. The reading was performed at 570nm with a micro plate ELISA reader (Epoch-Biotek, Winooski, VT, USA).
Agar-well diffusion method
This method consisted of seeding homogeneous inoculums on the surface of Mueller Hinton media (Himedia) with the aid of swab. Bacterial inoculums were prepared with a cell density of 0.5 on the McFarland scale. In test plates, wells were made on the agar, which received 20µL of the studied extract. After incubation at 37ºC, for 24h, reading was performed by the presence or absence of inhibitory halo around the wells. For the control of the experiments, standardized disks were used with antimicrobials (gentamicin 10µg and vancomycin 30µg). All tests were performed in triplicate [15,16]. This method was used to screen the bark extract of P. marginatum with a better antibacterial profile (data not shown) and for the tests with the fractions.
Determination of the Minimal Inhibitory Concentration (MIC)
The extracts in study were submitted to tests in order to determine MICs, through the agar dilution method [16,17]. The technique employed consisted of adding increasing concentrations of the extracts in molten Mueller Hinton agar, and then they were plated, always in duplicate. Bacterial inoculums were standardized as previously described. Seeding was performed with the help of a Steers replicator, and then incubated at 37°C for 24h. The value of MIC was considered as the lowest concentration able to inhibit the bacterial growth. Two controls were carried out for each test, with and without adding solvent extractor. Antimicrobials vancomycin (Vancocin®) and gentamicin (Gentamicin®) were used as control.
Determination of the Minimal Bactericidal Concentration (MBC)
The bactericidal activity was determined by broth microdilution as previously described [17]. The two-fold serial dilutions of the HAEPMB were prepared in 96-well microplates with MH broth as diluent, to give concentrations of 0.125-2mg/mL. After incubation at 37°C for 24h, samples from each well were seeded on BHI agar and incubated. The concentration that prevented growth of the bacterium in the test substance-free medium was considered the bactericidal concentration.
Time-kill kinetics
The assay of the bacterial time-kill kinetic for HAEPMB was performed by broth microdilution method, in concentrations ranged from 0.0625-1.0mg/mL and 0.125-2.0mg/mL against B. cereus 11778 and S. aureus 29213, respectively. At predetermined time intervals (0h, 2h, 6h, 14h, 18h and 24h) were performed readings of the growth in an ELISA reader (ELX-800 Epoch, Biotek, USA) at 595nm. The experiment was conducted at 37°C under stirring (200rpm). The time kill curve was plotted as the mean absorbances against time.
Transmission Electron Microscopy (TEM)
B. cereus and S. aureus 29213 treated for 24h and not treated with HAEPM and BF were fixed in a glutaraldehyde solution at 2.5% in cacodylate buffer 0.1mol/L, pH 7,2-7,4, at room temperature for 6h. After three successive washes with cacodylate buffer 0.1mol/L, an osmium tetroxide solution was fixed at 1% in cacodylate buffer for 1h, at room temperature. The material was washed in a buffer solution and immersed in aqueous solution of tannic acid at 1%, for 20 minutes. After washing, it was fixed for the second time with an osmium tetroxide solution for 90 minutes, followed by dehydration in ethanol solutions. Then, the material was infiltrated with a mixture of Epon: acetone (1:1) resin and included in Epon resin. Ultrathin sections were obtained in LEIC UC6 ultra microtome, stained with uranyl acetate and lead citrate, and images were obtained in a Tecnai G2 FEI TEM at 80keV.
Extraction and analysis of protein from the bacterial cell wall by SDS-PAGE
The inoculum from an 18-24h bacterial culture (BHI broth) was submitted to centrifugation (X 16000g /10min), discarding the supernatant. Two mL of HAEPMB and BF (1 X MIC and 2X MIC), 6.4mM SDS, 4µg/mL vancomycin (B. cereus), and 0.5µg/mL vancomycin (S. aureus) were added to each pellet. Then they were incubated for 24h on a rotary incubator shaker (TE-240, Tecnal) at 37°C and 200rpm. The material was transferred to plastic microtubes and centrifuged at 16000g/ 10min. Supernatant was separated to analyze the extraction of proteins through electrophoresis in SDSPAGE. For electrophoresis, the analyzed sample was heated at 100°C for 5min with sample buffer (1:1). Benchmark TM Protein Marker was used as a protein marker. Gel was used at a concentration of 12%, with the gel running in the electrophoresis device Power supply- EPS 301 (Amersham Biosciences) at 120V/ 80A. All gels were silver stained (Silver Stains Plus Kit, Amersham Biosciences) [18]. HAEPMB, BF, BHI broth, and vancomycin were used as control.
Statistical analysis
Results were analyzed using ANOVA followed by Tukey’s Multiple Comparison Test using the GraphPad Instat® software (version 5.0). Results with P‹0.05 were considered statistically significant.
Results and Discussion
Inhibitory potential of Pseudobombax marginatum
HAEPMB had an inhibitory activity against the Gram-positive bacteria studied, except for L. monocytogenes, and was seen as inactive against Gram-negative bacteria, at the concentrations tested (Table 1). B. cereus was the most susceptible bacterium to HAEPMB with MIC of 0.5mg/mL, followed by S. aureus 29213 and 33591, with MIC corresponding to 1.0mg/mL. The HAEPMB showed bactericidal activity at concentrations of 0.5mg/mL for B. cereus and 2.0mg/mL for the strains of S. aureus 29213 and 33591.
Strains
HAEPMB (mg/mL)
S. aureus 29213
1
S. aureus 33591
1
S. aureus 25923
2.5
S. epidermidis 12228
1.5
E. faecalis 29212
3
B. cereus 11778
0.5
L. monocytogenes 15313
>4.0
E. coli 25922
>4.0
S. Typhimurium 14028
>4.0
S. sonnei
>4.0
P. aeruginosa 27853
>4.0
HAEPMB- hydroalcoholic extract of P. marginatum.
Table 1: Minimal Inhibitory Concentrations (MICs) of crude extract of P. marginatum bark against bacterial samples of clinical interest.
The time-kill kinetics study showed that HAEPMB presented bactericidal effect against the test organisms. It can be observed reduction of growth of B. cereus as of 0.5mg/mL and S. aureus at 2mg/ mL when compared to the growth control (Figure 1A and Figure 1B).
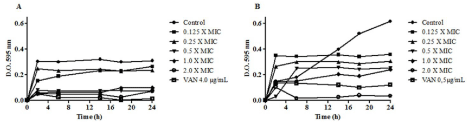
Figure 1: Time-kill kinetic curve of HAEPMB against B. cereus (A) and S. aureus 29213 (B).
Of the factions obtained those of ethyl acetate and of BF were active against all Gram-positive bacteria tested. However, BF showed a better inhibitory activity when tested through the agar dilution method (Table 2), with an inhibitory profile similar to that of HAEPMB for B. cereus and S. aureus strains 29213 and 33591.
Strains
Fractions (mg/mL)
CL
EAF
BF
DA
DA
MIC
DA
MIC
S. aureus 29213
-
16.3±0.3
4
12.0±0.0
1
S. aureus 33951
-
18.0±1.0
4
14.7±0.7
1
S. aureus 25923
-
14.3±1.2
4
14.7±0.3
4
S. epidermidis 12228
-
16.7±0.3
4
14.0±0.6
4
E. faecalis 29212
-
12.7±1.2
>4.0
10.0±1.1
4
B. cereus 11778
-
13.7±0.3
2
12.3±1.2
0.5
DA: Diffusion Agar Method (inhibitory halo-mm); CL: Chlorophorm Fraction; EAF: Ethyl Acetate Fraction; BF: Butanolic Fraction; (-) Not Active; (±) Mean Standard Error.
Table 2: Inhibitory potential of P. marginatum bark fractions against bacterial samples of clinical interest.
Studies with 70% ethanol extract of P. marginatum bark showed antibacterial activity against Bacillus subtilis [10]. In 2013, study [9] observed a weak inhibitory activity of ethanol extract of P. marginatum against bacteria and yeast.
In accordance with the authors [19] the inhibitory activity of P. marginatum may be considered strong and moderate against B. cereus and S. aureus strains 29213 and 33591, respectively, both for its crude extract (HAEPMB) and its BF. Ethyl acetate fractions and BF obtained from HAEPMB had an inhibitory activity similar to the Agar diffusion method; however, BF was shown to be more active by the Agar dilution method.
Bacterial mechanism of action of P. marginatum
The crude extract at concentrations of 0.5 and 1.0 mg/mL, and the BF in concentrations of 0.512 and 1.024 mg/mL were able to extract proteins from the cell surface of B. cereus after 24h exposure, which was also seen with the 4mg/mL vancomycin and 6.4mM SDS controls. In relation to the S. aureus 29213, both the crude extract and the BF were not able to extract proteins from their cell surface, and the same was observed for 0.5mg/ml vancomycin (Figure S1). The test performed with BHI broth, HAEPMB and BF, bacteria-free test, showed that they do not interfere with the results.
The effect of the treatment of HAEPMB and of BF on the bacterial ultra structure was shown with the use of TEM. Figures 2 (A) and 3 (A and B) show the normal aspect of B. cereus and S. aureus 29213 cell structures, respectively. After the treatment with HAEPMB at 0.5mg/mL, a change in the cell wall and cell membrane was verified, with the presence of dense blocks in the cytoplasm and the loss of cytoplasmic material (Figure 1B and Figure1C). This was also observed with B. cereus treated with BF at 0.512mg/mL, except for the presence of dense blocks (1D). Figure 3C shows S. aureus 29213 after being exposed to BF at 0.512mg/mL, in which a change in the cell wall, with the formation of septa, was verified. After the treatment with BF at 2.048mg/mL, the cell structure of S. aureus 29213 was seen to elongate, with regions presenting disruption of the cytoplasmic membrane and cell wall, and coagulated cell-content (Figure 2D).
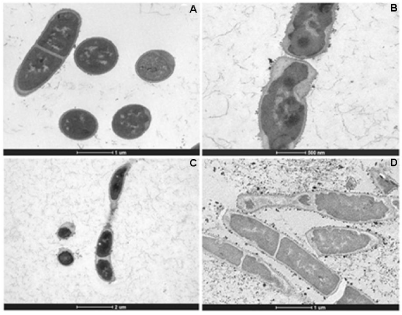
Figure 2: Ultra structure of B. cereus treated with HAEPMB and BF. Control (bars: 10µm and 1µm) (A); B. cereus treated with HAEPMB at 0.5mg/mL (bars: 500nm
and 2µm) (B) and (C); B. cereus treated with BF at 0.512mg/mL (bar: 1µm) (D).
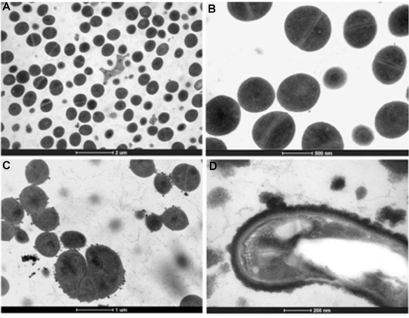
Figure 3: Ultra structure of S. aureus 29213 treated with BF. Control (bars: 2µm and 500nm) (A) and (B); S. aureus treated with BF at 0.512 mg/mL (bar: 1µm) (C);
S. aureus treated with BF at 2.048mg/mL (bar: 200nm) (D).
In this study, HAEPMB and BF were seen to act on the B. cereus cell surface, with the removal of protein material, showing the ability of these substances to extract the protein from its cell surface. The presence of dense blocks was also seen in its cytoplasm, in addition to the loss of cytoplasmic material. BF interfered with the S. aureus 29213 cell wall, forming several septa, which is similarly observed in S. aureus treated with catechins and vancomycin, forming so-called multicellular aggregates [20,21]. It was observed that no material was removed from the cell surface, due to the structural integrity of the S. aureus cell wall. With the concentration of 2,048mg/mL, a change was seen in the morphology of S. aureus with the elongation of the bacterial cell, with fractures in the cytoplasmic membrane and intracellular aggregation of cytoplasmic material (Figure 4D). This activity is thought to be due to the catechin derivatives found in P. marginatum bark.
Cytotoxicity of Pseudobombax marginatum
The assessment of the cytotoxicity of HAEPMB showed no toxicity to the mice fibroblast cells (Figure 4A). No statistically significant differences in any of the tested concentrations were observed compared to the negative control (DMEM). However, with respect to human embryonic kidney cells, HAEPMB was able to maintain its integrity, as demonstrated by a subtle proliferation of these cells at the highest concentration tested (4000µg/mL) (Figure 4C). In relation to rat aortic endothelial cells (Figure 4B) and African green monkey kidney cells (Figure 4D) our results showed a slight cytotoxicity in the highest tested concentrations of approximately 20%. In 2011, study [22] reported a cytotoxicity effect of a methanol extract of Malva silvestris (Malvaceae family) in mice fibroblast cells of approximately 40% at a concentration of 0.05mg/mL, showing a significant deleterious effect. The results shown in this study provide us with a safe use of the hydroalcoholic extract of Pseudobombax marginatum in different normal cell lines without causing severe toxicity effects in vitro.
Chemical Analysis
The spectrum UV-Vis showed a profile similar to what was expected for a polyhydroxylated compound: With bands in the region 205-210nm, a shoulder in the region 240nm, and another less intense band in the region 270-280nm. These bands also suggest the presence of phenolic compounds such as the flavonoids. The concentration of total phenolics, detected through the Folin-Ciocalteu method, was 89.22µg equivalent to gallic acid/mg of HAEPMB. The inhibitory activity of phenolic substances has been known since the antiquity, with a focus on flavonoids that have proven antifungal, antiviral, and antibacterial properties [23]. In 1999, Faizi and Ali [24] attributed the antimicrobial activity of B. ceiba to the shamimin (flavonols C-glycoside) compound, isolated in its leaves.
With the IR analysis, spectra have shown a benzenoid-aromatic nature, with the presence of hydroxyl groups, with no carbonyl or carboxyl group, and with a characteristic band of tetrahydropyran ring, similar to what was seen by Barrow and Searles [25] in 1953. These results suggest a polyhydroxy phenol structure with a tetrahydropyran ring, such as the proanthocyanidins and catechins, when compared to spectra reported in the literature [26]. The presence of bands in 1519 and 1530 cm-1 and lower than 730cm-1 (Figure S2) leads us to suggest that the compound studied would be an analog derivative of catechin.
HPLC analysis of HAEPMB and BF revealed the presence of chromatographic peaks consistent with the pattern shown by the standard catechin (Figure S3 and Figure S4). The peaks were identified by comparison between UV spectra and retention times (RT) of the extracts and the external standard. The major compounds of the extract and fraction were identified as catechin, with the highest concentration observed in the BF (Table 3). With the standard used, it was not possible to identify compounds 1, 2, 7, and 8.
Compound
UV (nm)
RT (min)
mg Eq. catechin (±SE)/
g of HAEPMB
mg Eq. catechin (±SE)/
g of BF
1
229-345
7,17
ND
ND
2
232-346
7,2
-
ND
3
279
7,25
1.69 ± 0.0067
3.04 ± 0.0016
4
279
7,32
3.57 ± 0.0037
-
5
278
7,75
3.17 ± 0.0078
-
6
275
7,96
4.62 ± 0.0062
8.57 ± 0.0083
7
258-385
8,16
ND
ND
8
258-385
8,45
ND
ND
RT: Retention Time; ND: No Determined.
Table 3: Equivalent quantified phenolic compounds in HAEPMB and BF according to the similar catechin standard spectrum.
Antimicrobial activity against bacteria, viruses, yeasts, and filamentous fungi has been attributed to catechin. It may act directly on the microorganism, interfering with its cell wall and membrane, changing its permeability, with the release of intracellular compounds, as well as the suppression and neutralization of its virulence factors, thereby preventing the development of the infection [27]. However, further studies are required to deepen the knowledge of the chemical composition and of the mechanism of action of this compound, in addition to toxicological studies to guarantee its safe use, contributing to its rational use in medical therapy, both in humans and in other animals.
Acknowledgments
The authors would like to thank FAPEMIG (PPM V 356/11) for the financial support and Henry P. Lima for helping to collect the plant P. marginatum, in Vale do Açu, and the researcher Leonardo Rodrigues for reviewing the manuscript. The authors would like to acknowledge the Center of Microscopy at the Federal University of Minas Gerais (https://www.microscopia.ufmg.br) for providing the equipment and technical support for experiments involving electron microscopy.
References
- Novais TS, Costa JFO, David JPL, David JM, Queiroz LP, França F, et al. Atividade bacteriana em alguns extratos de vegetais do semi-árido brasileiro. Rev Bras Farmacogn. 2003; 13: 5-8.
- Oliveira G, Araújo MB, Rangel TF, Alagador D, Diniz-Filho JAF. Conserving the brazilian semiarid (Caatinga) biome under climate change. Biodivers Conserv. 2012; 21: 2913-2926.
- Fontenelle RO, Morais SM, Brito EH, Brilhante RS, Cordeiro RA, Nascimento NR, et al. Antifungal activity of essential oils of Croton species from the Brazilian Caatinga biome. J Appl Microbiol. 2008; 104: 1383-1390.
- Du Bocage AL, Sales MF. The family Bombacaceae Kunth in the state of Pernambuco - Brazil. Acta Bot Bras. 2002; 16: 123-139.
- Maia GN. Caatinga: árvores e arbustos e suas utilidades. S&aTilde;o Paulo: D&Z Computaç&aTilde;o Gráfica e Editora. 2004.
- Roque AA, Rocha RM, Loiola MIB. Use and diversity of medicinal plants from Caatinga in the rural community of Laginhas, Caicó Municipality, Rio Grande do Norte State (Northeast of Brazil). Rev Bras Pl Med. 2010; 12: 31-42.
- Albuquerque UP, Medeiros PM, Almeida AL, Monteiro JM, Neto EMFL, Melo JG, et al. Medicinal plants of the Caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J Ethnopharmacol. 2007; 114: 325-354.
- Paiva DC, Santos CA, Diniz JC, Viana FA, Thomazzi SM, Falc&aTilde;o DA. Anti-inflammatory and antinociceptive effects of hydroalcoholic extract from Pseudobombax marginatum inner bark from caatinga potiguar. J Ethnopharmacol. 2013; 149: 416-421.
- Chaves TP, Santana CP, Véras G, Brand&aTilde;o DO, Felismino DC, Medeiros ACD, et al. Seasonal variation in the production of secondary metabolites and antimicrobial activity of two plant species used in Brazilian traditional medicine. African J Biotechnol. 2013; 12: 847-853.
- Almeida CFCBR, Cabral DLV, Almeida CCBR, Amorim ELC, Araújo JM, Albuquerque UP. Comparative study of the antimicrobial activity of native and exotic plants from the Caatinga and Atlantic Forest selected through an ethnobotanical survey. Pharm Biol. 2012; 50: 201-207.
- Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009; 325: 1089-1093.
- Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002; 50: 6910-6916.
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food. Chem. 2002; 50: 3010-3014.
- Brito NJN, López JA, Nascimento MA, Macêdo JB, Silva GA, Oliveira CN, et al. Antioxidant activity and protective effect of Turnera ulmifolia Linn. var. elegans against carbon tetrachloride-induced oxidative damage in rats. Food Chem Toxicol. 2012; 50: 4340-4347.
- Clinical & Laboratory Standards Institute. Standardization of antimicrobial susceptibility testing by disk-diffusion: approved standard M2-A8. Pennsylvania: CLSI. 2003a.
- Clinical & Laboratory Standards Institute. Performance Standards for antimicrobial susceptibility testing: Twenty-fifth informational supplements approved standard M100-S25. Pennsylvania: CLSI. 2015.
- Clinical & Laboratory Standards Institute. Methodology of antimicrobial susceptibility testing by dilution for aerobic growth bacteria: Approved standard M7-A6. Pennsylvania: CLSI, 2003b.
- Santos ECG, Donnici CL, Camargos ERS, Rezende AA, Andrade EHA, Soares LAL, et al. Effects of Copaifera duckei Dwyer oleoresin on the cell wall and cell division of Bacillus cereus. J Med Microbiol. 2013; 62: 1032-1037.
- Duarte MC, Leme EE, Delarmelina C, Soares AA, Figueira GM, Sartoratto A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J Ethnopharmacol. 2007; 111: 197–201.
- Sakoulas G, Moellering RC. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis. 2008; 46: 360–367.
- Stapleton PD, Shah S, Hamilton-Miller JM, Hara Y, Nagaoka Y, Kumagai A, et al. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int J Antimicrob Ag. 2004; 24: 374–380.
- Razavi SM, Zarrini G, Molavi G, Ghasemi G. Bioactivity of Malva Sylvestris L., a Medicinal Plant from Iran. Iran J Basic Med Sci. 2011; 14: 574-579.
- Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoides. Int J Antimicrob Ag. 2005; 26: 343-356.
- Faizi S, Ali M. Shamimin: A new flavonol c-glycoside from leaves of Bombax ceiba. Planta Med. 1999; 65: 383-385.
- Barrow GM, Searles S. Effect of ring size on the infrared spectra of cyclic ethers. Characteristic frequencies of trimethylene oxides and tetrahydrofurans. J Am Chem Soc. 1953; 75:1175-1177.
- Foo LY. Proanthocyanidins: gross chemical structures by infrared spectra. Phytochemistry. 1981; 20: 1397-1402.
- Taylor PW, Hamilton-Miller JMT, Stapleton PD. Antimicrobial properties of green tea catechins. Food Sci Technol Bull. 2005; 2: 71-81.