
Research Article
J Bacteriol Mycol. 2016; 3(4): 1038.
Developmental Morphology of Conidiomata in Phyllosticta Caryotae
Murugan M¹*, Arumugam P² and Arunkumar K³
¹Department of Microbial Technology, School of Biological Sciences, Madurai Kamaraj University, India
²Department of Zoology, School of Life Science, Bharathiar University, Coimbatore, Tamil Nadu, India
3Department Of Plant Science, School of Biological science, Central University of Kerala, Kerala, India
*Corresponding author: Murugan M, Department of Microbial Technology, School of Biological Sciences, Madurai Kamaraj University, India
Received: October 31, 2016; Accepted: December 09, 2016; Published: December 12, 2016
Abstract
Coelomycetes are anamorphic Ascomycota that produce conidia within a flask- shaped ostiolate pycnidial conidiomata, acervular conidiomata or on a mass of vegetative hyphae (stroma). Coelomycetes fungi play an important role in terrestrial ecosystems. The objective of this work was to clarify the development of conidiomata in Phyllosticta caryota using light microscopical techniques. Light microscopic observations shows symphogenous type of pycnidial formation. The conidia within young pycnidia were found with the sheath surrounding them. The mature conidium is multinucleate. The conidiogenous cells which are produced initially inside the cavity are referred to as “temporary conidiogenous cells”. Subsequently, the cells lining the cavity elongate to form a palisade of permanent conidiogenous cells, which then start producing conidia. During dehiscence of the conidioma, these inner hyaline layers in the papilla dissolve to form the ostiole and the outer thick walled cells from the beak like structure of the conidioma. The papilla may also exert pressure to rupture the epidermis of the host for releasing the conidia outside.
Keywords: Appendages; Coelomycetes; Conidium ontogeny
Introduction
Traditional classification of Coelomycetes was based on the morphology and was thus subjective, often resulting in artificial generic and species boundaries [1]. In view of the variability and diversity of the coelomycete conidiomata it is no easy task to accommodate them in a system of classification based on conidiomata satisfactorily. The fungi coelomycetes have conidia formed within a cavity lined by fungal or fungal/host tissue. The conidia-bearing structure (conidioma) is classified into five types according to exterior morphology: pycnidial, pycnothyrial, acervular, cuplate, and eustromatic [2] (Watanabe et al. 1998). Many researchers have described pycnidial-type conidiomatal development [3-8] (Mercer 1913). These facts suggest that the morphogenesis of conidioma has taxonomic value. In view of the variability and diversity in the form of the Coelomycete conidiomata it is no easy task to accommodate them in a system of classification based on conidiomata satisfactorily. Development is divided into three stages: primordia, cavity formation, and conidiogenesis, with each pycnidial fungus having a determinate mode in each stage.
The genus Phyllosticta (Teleomorph: Guignardia Viala & Ravaz was established by Persoon in 1818). Many species of Phyllosticta have been known as the causal fungi of leaf spot diseases of various plants [9]. The genus - Phyllosticta is also known as the endophytic fungus in a wide range of host plants, including Ericaceae [10-14]. Plant diseases caused by species of Phyllosticta are reported worldwide. Saccardo [15,16] revised the generic concept of Phyllosticta. The revision of Saccardo was based on perithecia which are subepidermal, lenticular, thin membranous, ostiolate, punctiform, areolate on leaf or rarely on stem, with small, ovoid to oblong, aseptate, hyaline to greenish, conidia. However, most subsequent investigators described the species based on the concept as “All leaf inhabiting pycnidial fungi with hyaline, one celled conidia should be classified in Phyllosticta, whereas morphologically comparable fungi on stem in Phoma” [9]. The genus concept however was reconstructed by van der Aa and collaborators [17].
However, there is still controversy for the species concept. The species of teleomorph Guignardia and its anamorph are host species, with a host range confined to species of a single family, from the results of inoculation tests [18-21], whereas some are endophytic Phyllosticta cultures from 67 plant species in 54 genera of 38 families. All these cultures were identified as a single species, Phyllosticta capitalensis Henn, based on morphology and sequence analysis of ribosomal DNA internal transcribed spacer regions. Baayen et al. [10], revealed that Guignardia citricarpa Kiely (anamorph: P.citricarpa (McAlpine) Aa was different from nonpathogenic species Guignardia mangiferae. Roy isolated from citrus, based on cultural characteristics, growth rate, and thickness of mucoid sheath surrounding the conidial wall. Also, they are different in nucleotide sequence data, although the size of their conidia was same. On the other hand, delimitation of the species of Phyllosticta relationship is not yet available. Hence, in this study, we applied Van der Aa and Vanev species concept [17,9] wherein the species epithet was given on the basis of fungus morphology on diseased leaf of host plant, cultural morphology on diseased leaf of host plant, cultural characterististics, and connection with teleomorph species.
Cryptosporiopsis radicicola produces only excipular covering conidioma-like tissue with adhesive amorphous material and setae. Synnematous conidiomata with abundant macroconidia dominate the colony of Cryptosporiopsis ericae. Cryptosporiopsis rhizophila is different in its globose to subglobose conidiomata, consisting of loosely aggregated vegetative hyphae developing macroconidial conidiophores. Cryptosporiopsis grisea, being the only teleomorph connected species, differs from the others in its distinct columnar surface [22].
Therefore, as more and more data on this effect becomes available, the distinction between Hyphomycetes and Coelomycetes may be abandoned because of the presence of intermediary stages between hyphomycetes, acervular, stromatic and cupulate conidiomata. In this investigation developmental stages of the conidioma in the Phyllosticta caryotae state of the fungus were investigated. This forms part of a programme of work on developmental morphology and taxonomy of Coelomycetes which is in progress in this department.
Materials and Methods
Culture characters and identification
A culture of Phyllosticta caryotae Chen was isolated from leaf of Citrus sinensis from kodaikanal, India. It was grown on Oat Meal Agar (OMA) and Potato Dextrose Agar (PDA) in Petri dishes at room temperature (28°C). Cultures were incubated at 24°C in continuous light, and cultural morphology was examined after 7 days. Spore size was determined by measuring the length and width of 30 to 40 arbitrarily selected conidia from a conidial suspension. The isolates were identified initially by comparing morphological and cultural characteristics (i.e., size of conidia, thickness and length of apical appendages).
Germination study
The initial stages of the development of conidiomatal primordia were studied by slide cultures [23]. For germination studies, conidia were collected aseptically from the teased out conidiomata in 1% glucose solution and allowed to germinate in cavity slides were kept at room temperature (28°C) and were examined every 5hrs for 36hrs to study germination.
Light microscope
To study the development of conidiomata, selected conidiomata with agar were trimmed into 2 mm square blocks and fixed in 2% glutaraldehyde in 0.1M phosphate buffer (pH 7.2) for 2hrs at room temperature (27°C) and 1h at 4°C and post fixed for 12hrs in 1% osmium tetroxide. Specimen were dehydrated through an ascending series of acetone (30-100%) at room temperature, each changes at 30min intervals, followed by 2-3 changes in fresh Spurr in the ratio of 3:1 (Acetone : Spurr) for 6hrs, followed by two changes with absolute Spurr mixture for 24hrs each lasting for 8hrs and polymerised in fresh spurr at 70°C for 8hrs. The samples infiltrated with the resin were transferred to a vacuum chamber for 1h for complete removal of air bubbles. Thin sections (0.5μm) were cut from these blocks and stained with 1% aqueous toluidine blue to study the development of conidiomata and conidiogenesis under the light microscope.
Results
Description of the fungus
Conidiomata pycnidial, pycnidia black, spherical to subspherical, 75-120 μm in diam. sometimes 200 μm diam. with a small ostiole. In vertical section, the pycnidial wall 3-4 layered, the outer layer brown in colour, becoming pale brown to hyaline towards inside. Conidiogenous cells lining the pycnidial cavity, 1-2 celled, short, erect, 5-12 x 2.5 μm, aseptate. Conidia formed singly at the apex of the conidiogenous cell, hyaline, aseptate, 7-13 x 5-10 μm. The appendage arises from the apex of the conidium while the conidium is undergoing development and still attached to the conidiogenous cell (Figure 1).
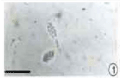
Figure 1: Mature conidia. Conidia enveloped with a thick sheath (bar
=12.5μm).
Structure of conidia
The germination study showed that the germ tube is a stout structure observed at the basal region of conidium. Light microscopic studies by Punithalingam [24] showed that conidium is enveloped by a sheath which remains intact, while the outermost layer gradually gelatinizes with aging of the conidium. When observed with light microscope, the conidia within young pycnidia were found with the sheath surrounding them. The mature conidium is multinucleate (Figure 2).
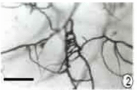
Figure 2: Compound meristogenous methods of primordial formation (bar
=25μm).
Development of pycnidial primordia
The pycnidial primordium is initiated by the interweaving of hyphal branches to form a network, which is at first, loosely interwoven. This type of pycnidia initiation is termed as symphogenous. By repeated divisions, the primordial become pseudoparenchymatous (Figure 3). The earliest stage of pycnidial development seen in sectioned material consisting of small pseudoparenchymatous mass of tissue, the pycnidial primordium. The pycnidial primordium is almost spherical consists of a compact cluster of thick walled cells. During further development, the primordium considerably increased in size by continued transverse and longitudinal divisions of the cells.
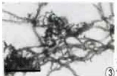
Figure 3: Symphogenous methods of primordial formation scale (bar =25μm).
Formation of pycnidial cavity
As a prerequisite for the formation of pycnidial cavity, the cells at the periphery and centre of primordium were arranged as follows. The cells along the outer layer of the primordium became tangentially arranged which subsequently formed the conidiomatal wall. The inner cells of the primordium were thin walled and hyaline whereas the peripheral cells were thick walled and pigmented (Figures 4,5).
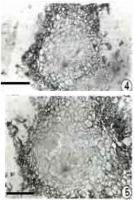
Figure 4&5: Sections of young primordial showing the formation of cavity
(bar =25μm).
Formation of pycnidial cavity and sporogenous tissue
The central cells in the primordium showed signs of schizogenous activity to form the cavity. Further development stages showed the formation of the conidium simultaneously with the cavity formation. The cells lining the cavity commence conidial production by a budding-like process. Eventually, these conidiogenous cells along with conidia become detached from the wall of the conidiomata and remain floating inside the conidiomatal cavity (Figures 7-10). The conidiogenous cells which are produced initially inside the cavity are referred to as “temporary conidiogenous cells” by Punithalingam [25]. These temporary conidiogenous cells occasionally bear an annellation and were normally blastic. As the temporary conidiogenous cells and conidium are detached inside the cavity, some of the conidiogenous cells might disorganize or dissolve to form a mucilaginous matrix which was clearly visible inside the cavity (Figure 6). Several sections of the conidiomata showed the presence of the mucilaginous matrix along with the detached conidia. Subsequently, the cells lining the cavity elongate to form a palisade of permanent conidiogenous cells, which then start producing conidia. Consequently, the conidioma becomes considerably larger in size by proliferation of outer pseudoparenchyma while the inner cells become arranged tangentially into several layers (Figures 11,12).
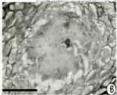
Figure 6: Sections of young primordium showing mucilaginous matrix (bar
=50μm).
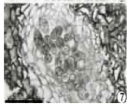
Figure 7: Vertical sections of conidiomata showing mature conidia (Note the
well developed conidiomatal wall (bar =50 μm).
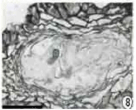
Figure 8: Section of young primordium showing conidial formation (bar =50
μm).
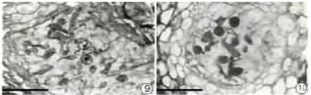
Figure 9&10: Vertical section of young primordium showing the dissolving
central cells (bar =50 μm).

Figure 11: Vertical sections showing young conidiomata with the initially
formed conidia (bar =25 μm).

Figure 12: Vertical section of mature conidiomata showing the permanent
conidiogenous cells and conidia (Note the developed wall layers) (bar =50
μm).
Formation of ostiole
When the conidioma becomes fully mature, a papilla-like projection appears at the apex. In vertical section, the papillate region showed thick walled, dark cells towards the outerside and thin walled, hyaline, elongated cells towards the inner side. During dehiscence of the conidioma, these inner hyaline layers in the papilla dissolve to form the ostiole and the outer thick walled cells from the beak like structure of the conidioma. The papilla may also exert pressure to rupture the epidermis of the host for releasing the conidia outside.
Discussion
The origin and structure of the apical conidial appendage of Phyllosticta spp. had already been fully described by Punithalingam and Woodhams [26]. The cultural characters and developmental studies of the Phyllosticta state of Guignardia mangiferae Roy along with the connection between the ascogenous state has also been thoroughly investigated [27,28]. Conidiomata developmental morphology of Pestalotiopsis disseminate is known to produce only acervular conidiomata on its host whereas in artificial culture media, pycnidial conidiomata were formed [29]. The morphology of conidia, appressoria and cultural characters of the ex-neo type culture was provided [30]. This work deals with different stages of development of the conidiomata within the natural host and also in artificial culture media. It was observed that the Phyllosticta caryotae investigated here produces temporary and permanent conidiogenous cells similar to those of Ascochyta spp [25] and Macrophomina phaseolina [24]. Normally the temporary conidiogenous cells produce a single conidium after which they get sloughed off from the wall layers. In the present fungus, the temporary conidiogenous cells occasionally show one annellation indicating that it is capable of producing more than one conidium. Probably after producing one or two conidia, the temporary conidiogenous cells may become detached from the wall layers. Another interesting finding is the presence of the thick sheath around the conidium. This sheath was also observed covering the conidiogenous cell and the conidium initial. The presence of the sheath around the conidium confirms observation made by light microscope. Both transverse and longitudinal sections of the old conidia failed to reveal a sheath surrounding the conidia suggesting that the sheath gradually gelatinizes and disappears as the conidium becomes old.
The dual conidiation process was found as a general feature of Ascochyta species in culture [25] and also in Macrophomina phaseolina [24]. Punithalingam [25] reported that some of the observations made with Ascochyta in culture were in conformity with the interpretations of Archer [3] during his investigation of cavity formation and ostiole development in some members of Sphaeropsidales. Other coelomycete species studied by the authors, namely, Botryodiplodia theobromae Pat., Ciliochorella mangiferae Syd., Coleophoma cylindrospora (Desm.) Hohn., Pestalotiopsis palmarum (Cooke) Stey. And Urohendersonia pongamia Nag Raj & Ponnappa show this type of dual conidiation process in culture. In the present investigation Phyllosticta caryotae also was found to produce temporary and permanent conidiogenous cells. But Robillarda depazeoides (Welw. et Curr.) Sacc. was found to produce only one type of conidiogenous cells during conidiation in culture. Therefore the present study reveals that necessary data collected based on the developmental morphology of conidiomata for many more coelomycetes will be useful for segregating genera into different groups [31-33].
Acknowledgements
We thank Dr. J. Muthumary, Centre for Advanced studies in Botany, University of Madras, Chennai for the facilities provided. We thank to the U.G.C. for financial assistance.
References
- Wijayawardene NN, Mckenzie EHC, Chukeatirote E, Yong Wang Y, Hyde KD. Coelomycetes Cryptogamie. Mycologie. 2012; 33: 215-244.
- Hawksworth DL, Kirk PM, Sutton BC, Pegler DN. Ainsworth and Bisby's Dictionary of the fungi. 8th edn. CAB International, Wallingford, U.K. 1995; 104-105.
- Archer WA. Morphological characters of some Sphaeropsidales in culture. Annual Mycology. 1926; 24: 1-84.
- Chippindale HG. The development in culture of Ascochyta gossypfi Syd. Transactions of the British Mycological Society. 1929; 14: 201-215.
- Dodge BO. Development of the asexual fructifications of Chaetomellaraphigera and Pezizella lythri. Mycologia. 1930; 22: 169-174.
- Harris H A. Morphology studies of Septoria lycopersici Speg. Phytopathology. 1935; 25: 790-799.
- Mass J L, Pollack FG, Uecker FA. Morphology and development of Pilidiella quercicoa. Mycologia. 1979; 71: 92-102.
- Punithalingam E. Development of the pycnidium in Septoria. Transactions of the British Mycology Society. 1966; 49: 19-25.
- Van der Aa HA, Vanev SG. A revision of the species described in Phyllosticta. Centraalbureau voor Schimmelcultures, Utrecht. 2002.
- Baayen RP, Bonants PJM, Verkley G, Carrol GC, van der Aa HA, de Weerdt M, et al. Nonpathogenic isolates of the citrus black spot fungus, Guignardia citricarpa, identified as a cosmopolitan endophyte of woody plants, G.mangiferae (Phyllosticta capitalensis). Phytopathology. 2002; 92: 464-477.
- Okane I, Nakagiri A. Identity of Guignardia sp. Inhabiting Ericaceous plants. Canadian Journals of Botany. 2001; 79: 101-109.
- Okane I, Lumyong S, Nakagiri A. Extensive host range of an endophytic fungus Guignardia endophyllicolo (anamorph: Phyllosticta capitalensis). Mycoscience. 2003; 44: 353-363.
- Petrini O. Taxonomy of endophytic fungi of aerial plant tissues. In: Fokkema NJ, Heuvel JVD edn. Microbiology of the phyllosphere. Cambridge University Press, New York. 1986; 175-187.
- Petrini L, Petrini O, Leuchtmann A, Carrol G C. Conifer inhabiting species of Phyllosticta. Sysodia. 1991; 43: 148-169.
- Saccardo PA. Fungi Veneti novi vel critici, series 7. Michelia. 1878; 1: 133-161.
- Saccardo PA. Sylloge Fungorum omnium 7. Michelia. 1884; 1: 133-161.
- Van der Aa HA. Studies in Phyllosticta. Studies in Mycology. 1973; 5: 1-110.
- Luttrell ES. Black rot of muscadine grapes. Phytopathology. 1946; 36: 905-924.
- Luttrell ES. Physiologic specialization in Guignardia bidwellii cause of black rot of Vitis and parthenocissus species. Phytopathology. 1948; 38: 716-723.
- Reusser FA. Uber einige Arten der Gattung Guignardia Viala et Ravaz. Phytopathology. 1964; 51: 205-240.
- Stewart VB. The leaf blotch disease of hoyse-chestnut. Phytopathology. 1916; 6: 5-19.
- Wang W. Conidiomatal ultrastructure and cultural characteristics of root-inhabiting species of Cryptosporiopsis. Mycology. 2011; 2: 237-247.
- Riddell RB. Permanent stained mycological preparations obtained by slide cultures. Mycologia. 1950; 42: 265-270.
- Punithalingam E. Conidiation and appendage formation in Macrophomina paseolina (Tassi) Goid. Nova Hedwigia. 1982; 36: 249-290.
- Punithalingam E. Graminicolous Ascochyta species. Mycology. 1979; 142: 1-214.
- Punithalingam E, Woodhams JE. The conidial appendage in Phyllosticta spp. Nova Hedwigia. 1982; 36: 151-198.
- Punithalingam E. Studies on Sphaeropsidales in culture II. Mycology. 1974; 136: 1-63.
- Muthumary J, Jayachandra JA, Bhagavathy Preetha M. Development of conidiomata in the Phyllosticta state of Guignardia mangiferae Roy and observations on the fine structure of the conidium. Mycotaxon. 1993; 47: 147-155.
- Murugan M, Arumugam P. Studies on Conidiomata developmental morphology of Pestalotiopsis disseminate. 2014.
- Liua F, Hyde KV, Caia L. Neotypification of Colletotrichum coccodes, the causal agent of potato black dot disease and tomato anthracnose. Mycology. 2011; 2: 248-254.
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. Journals of Ultrastrastructure Research. 1969; 26: 31-43.
- Kempton FE. Origin and development of the pycnidium. Botanical Gazette. 1919; 68: 233-261.
- Reynolds ES. The use of lead citrate at high pH as an electron opaque strain in electron microscopy. Journal of cell Biology. 1963; 17: 208-212.