
Research Article
J Bacteriol Mycol. 2015; 2(2): 1015.
Antifungal Activities of Camellia Sinensis Crude Extract on Selected Pathogenic and Mycotoxic Fungi
Cheruiyot SE¹*, Muturi M¹ and Bii C²
¹Department of Medical Laboratory Sciences, School of Medicine, Kenyatta University, Kenya
²Department of Infectious Diseases, Centre for microbiology Research, Kenya Medical Research Institute, Kenya
*Corresponding author: Sigei Erolls Cheruiyot, Department of Medical Laboratory Sciences, School of Medicine, Kenyatta University,
Received: May 13, 2015; Accepted: September 10, 2015; Published: October 01, 2015
Abstract
The objective of this study was undertaken to evaluate the in vitro antifungal activity, Minimum inhibitory concentration (MICs) and fungicidal/ fungi static property of Camellia Sinensis crude extracts. The aqueous extracts of Camellia Sinensis were investigated against seven fungal species; Green and black tea crude (100mgmL-1) extracts were evaluated for antifungal activities. Quantitative bioassay was done using disc diffusion method and MIC done using broth dilution methods. The fungal isolates used for bioactivity testing were yeasts. Green tea crude extract showed stronger inhibitory effect against the fungal strains tested than black tea crude extract. There was a significant difference in zone of inhibitions (T=4.09, P<0.05). Zone of inhibition exhibited by green tea crude extracts (11.92±0.00mm) were higher than black tea (8.14±0.56mm). The pattern of activity by tea crude extracts against ATCC standard fungal strains and clinical isolates strains were similar. Candidafamata, Candidalusitaniae, Candidatropicalis ATCC 750 and dermatophyte, Trichophytonmentangrophyte were inhibited by green tea crude extract (IZD=15mm). Clinical isolates of Candida albicans (strain 4 and strain 5); Cryptococcus neoformans (strain 3, 5 and 12), showed susceptibility to Camellia Sinensis green crude extracts. The MIC of crude extracts against fungal isolates tested ranged from 50 mg mL-1 to 1.6 mg mL-1. Hot green tea crude extract (mean MIC 12.25mg mL-1) had a higher MIC on clinical fungal isolates than cold green tea crude extract (Mean MIC 12.167 mg mL-1).The studies on Camellia Sinensis have shown remarkable antifungal activity and highlighted its significance as potential health products.
Keywords: Camellia Sinensis; Crude tea extracts; Green tea; Black tea; Fungal species
Introduction
Human fungal infections pose serious medical issues. Tea is one of the most consumed drinks worldwide where green tea (Camellia Sinensis) comprises of about over 20% of the total tea consumption [1]. Recently, several studies have shown that green tea consumption can protect against diseases that are associated with free radical damage including atherosclerosis, coronary heart disease and cancer [1-3]. The Kenyan tea germ plasm has been observed to be diverse in its polyphenol composition and contents and therefore provides raw material for production of different types of tea products including health drinks [4]. However, the state of research on tea regarding its pharmacological properties in relation to fungi is limited and the majority of work has been conducted on green tea with very little on black and white tea. Beneficial effects of tea have been attributed to the strong antioxidative activity of the tea phenolic compounds known as catechins [5]. In addition, many scientific research reports have presented data on the antimicrobial activity of different types of tea extracts on various pathogenic microorganisms [6,7]. Green tea elicits strong antibacterial activity including potential to inhibit gram positive cocci; gram negative bacilli [8]. Studies have also shown that tea can inhibit and kill a
wide range of pathogenic bacteria at or slightly below typical concentrations found in brewed tea [8]. Various studies have shown significant suppressive effects of green tea polyphenols against many microorganisms [9]. Black tea, a major source of phenolic, [10] including theaflavins and thearubigins, [11] has also been shown to have antimicrobial properties both in vivo and in vitro [12]. This study attempts to facilitate to establish the potentiality of Camellia Sinensis plant product as novel modalities in the line of new drug discoveries.
Material and Methods
Samples of Camellia Sinensis
Processed Commercial Camellia Sinensis (black and Green tea) produced and packed by James Finlay (K) Ltd were purchased off shelf in retail outlet at the factory in Kericho County, Kenya.
Test fungal organisms
The standard test fungi of American Type Culture Collection (ATCC) was sourced from Kenya Medical Research Institute (KEMRI) and included: Cryptococcus neoformans ATCC 66031, Candida albicans ATCC 90028, Candida krusei ATCC 6258, Candida glabrata ATCC 24433, Candida tropicalis ATCC 750, and Candida parapsilosis ATCC22019 as standard organisms. Clinical isolates included: Cryptococcus neoformans, Candida albicans, Candida famata, Candida lusitaniae, Trichophytonmentangrophytes, Microsporum gypseum. Mycotoxigenic fungi included: environmental pathogenic isolates Fusarium moniliforme, Aspergillus flavus, Aspergillus niger and Penicillium chrysogeneum. The selection of test strains was based on their significance as opportunistic pathogens and their resistance to conventional drugs.
Preparation of McFarland standard
McFarland standard is used as a reference to adjust the turbidity of fungal suspension so that fungal organisms will be within a given range. Exactly 0.5 McFarland equivalent turbidity standards was prepared by adding 0.6 ml of 1% barium chloride solution (BaCl2.2H20) to 99.4 ml of 1% sulphuric acid (H2SO4) and mixed thoroughly. A small volume of the turbid solution was transferred to cap tube of the same type that was used to prepare the test and control inocula. It was then stored in the dark at room temperature (25°C). Exactly 0.5 McFarland gives an equivalent approximate density of fungi 1.5 x 108 Colony Forming Units per ml (CFU) mL-1 [13].
Crude extraction of Camellia Sinensis (Teas)
The prepared soluble granules of both black and green tea samples sealed in silver lined sachets stored at room temperature were obtained. Cold aqueous crude extracts were done by soaking weighed amount of dry soluble granules of tea (10 grammes) in 100 mL of sterile distilled water and shaken for half an hour in an electric shaker. The extracts were filtered using Whatman No.1 filter paper to exclude any suspending granules. Crude extract, supernatant, was then transferred to sterile screw cap bottles, labeled and stored under refrigerated condition (40C) until use. For hot aqueous crude extraction, 10 grammes of dry soluble granules of tea samples were extracted with 100 mL of boiling water and filtered using sterile Whatman filter paper No.1 to give a solution that contains 100 mg/ ml [6]. Crude extract, filtrate, was then transferred to sterile screw cap bottles, labeled and stored under refrigerated condition (40C) until use. Only fresh extracts was used in the experiment, as marked chemical changes occurred when tea was allowed to stand [14].
Preparation of tea extracts stock and working solutions
A twofold dilutions were obtained (100 mg/ml, 50 mg/ml, 25 mg/ml, 12.5 mg/ml, 6.25 mg/ml, 3.125 mg/ml, 1.5625 mg/ml) concentrations. Antifungal activities of the above concentrations were determined.
Preparations of antifungal compounds stock and working solutions
The antifungal compounds were removed from storage (-200C) and allowed to come to room temperature. Each 250 μg of antifungal compound (Fluconazole) was weighed and dissolved in sterile distilled water to make a final 10 mL solution. The stock solutions of azole group of compounds (Fluconazole) used was usually kept at -200C until used. Doubling dilutions of stock solutions were made to obtain working solution.
Antimicrobial assay
The antimicrobial activities of the extracts were evaluated by the disc diffusion method [15]. The use of agar disc diffusion method to screen for antimicrobial activities of the crude tea extracts was done according to the National committee of clinical and laboratory standards [16] now CLSI. Dilutions of several concentrations of the crude tea extracts and azole group of compounds, Fluconazole, were then made in a test tube using sterile distilled water. Positive and negative standard controls were used. Blank sterile paper discs measuring 6mm were impregnated with 20 μL of test concentration of crude tea extract. The discs were air dried and aseptically transferred into respective inoculated plates [17]. The activities of the tea crude extracts were established by the presence zones of inhibition which were measured in mm. Fluconazole discs containing (25 μg) were used as antifungal reference standards. Similarly the sterile distilled water was set as negative controls.
Extracts with activity was serially diluted and re-tested to determine the minimum inhibitory concentrations (MIC). All the assays were carried out in triplicates, average result calculated and recorded against corresponding concentrations as described [18]. Assays were subjected to quality control procedures recommended by clinical laboratory standard institute (CLSI). Fluconazole disc was prepared as described by [19]. Minimum inhibitory concentrations were determined by Broth micro dilution method for the active crude extracts against test fungal organisms. The procedures were done as recommended by the National Committee for Clinical Laboratory Standards (NCCL) now Clinical Laboratory Standard Institute (CLSI) [20]. The MIC was recorded as the lowest extract concentration demonstrating no visible growth as compared to the control broth turbidity [21]. Wells that were not inoculated were set to act as control.
Results
The antifungal activities of green and black tea (Camellia Sinensis) crude extracts having a concentration of 100 mg/ml of extracting solvent (sterile distilled water) on selected yeasts and mould are presented in the Table 1 below. Their inhibitory effects against selected pathogenic and mycotoxic fungi were then compared.
Cold extract
Hot extract
FUNGI
Black tea
Green tea
Black tea
Green tea
Candida albicans ATCC 90028
6
6
6
6
Candida lusitaniae
8
18
10
20
Candida parapsilosis ATCC 22019
6
15
6
16
Candida glabrata ATCC 24433
6
6
6
6
Candida famata
12
22
18
22
Candida tropicalis ATCC 750
6
22
8
20
Candida krusei ATCC 6258
6
14
6
12
Cryptococcus neoformans
ATCC 66031
6
17
6
17
Microsporugypseum
(clinical isolate)
9
17
10
15
Aspergillus niger(clinical isolate)
6
6
6
6
Fusarium monilliforme
(clinical isolate)
12
6
13
6
Penicillium chrysogenium
( Clinical isolate)
6
6
6
6
Trychophyton mentagrophytes
(Clinical isolate.)
8
16
19
18
Table 1: Zones of inhibitions (mm) by crude tea extracts on the selected pathogenic and mycotoxigenic fungi.
The samples were analyzed using paired simple T-test to establish the differences in zones of inhibition caused by black tea crude extracts from green tea crude extracts. The results revealed that there was a significant difference in zones of inhibitions (T = 4.09, P < 0.05). Zones of inhibition caused by green tea crude extracts (11.92 ± 0.99 mm) were higher than inhibition by black tea crude extracts (8.14 ± 0.56 mm).
Zones of inhibition for clinical isolates at different extraction modes
The result showed that at different treatments of green tea crude extract, there was a significant difference in inhibition of these clinical isolates (F = 4.1, df = 2, P = 0.026). The Cold green tea crude extract (mean zone of inhibition 12.17 ± 1.20 mm) was lower than hot green tea crude extract (12.25 ± 1.24 mm). The green tea crude extracts inhibitions on the clinical isolates were as shown in Figure 1 below. Candida albicans strain 4 and strain 5 among all the clinical isolates showed greatest susceptibility to antifungal activity of green tea extract with inhibition zone diameter of 18 mm. Among the Cryptococcus neoformans clinical isolates tested, C. neoformans strain 3, strain 5 and strain 12 showed susceptibility to antifungal activity of green tea extracts (cold and hot) with inhibition zone diameter = 10.0 mm each. This is moderately active as considered highest at 18.0 mm and least 6.0 mm.
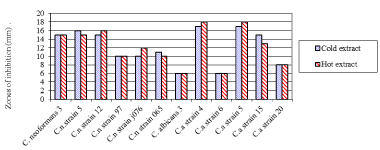
Figure 1: Zones of inhibition of fungal clinical isolates by green teaat different
extraction modes.
Key: Cn: Cryptococcus Neoformans; Ca: Candidaalbicans
Growth inhibition of fungal clinical isolates by black tea crude extracts at different extraction treatments were not significantly different (P > 0.05). The black tea crude extracts inhibition on the clinical isolates was as shown in (Figure 2).
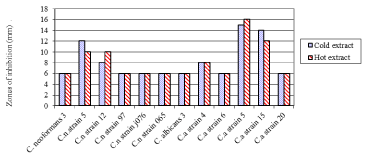
Figure 2: Zones of inhibition of fungal clinical isolates by black tea at different
extraction modes.
Key: Cn: Cryptococcus Neoformans; Ca: Candidaalbicans
Minimum inhibitory concentration to the standard fungal isolates
Minimum Inhibitory Concentrations (MIC) of tea crude extracts to the fungal strains were established. Tested at 15 mm diameter of inhibitory zone diameter, the MIC of green cold and green hot tea crude extracts was recorded in g/ml. Black hot tea crude extract was only tested against C. famata strain which was the only which showed inhibition activity (Table 2).
Tea crude extract
Fungal test strain
Green
cold (mg/ml)
Green
hot
(mg/ml)
Black
hot (mg/ml)
C. lusitaniae
25.00
25.00
-
C. famata
6.250
1.600
50.00
C. parapsilosis ATCC 22019
50.00
50.00
-
C. tropicalis ATCC 750
3.125
3.125
-
C. neoformansATCC 66031
6.250
3.125
-
M. gypseum
6.250
6.250
-
T. mentagrophytes
3.125
3.125
-
NB: The black cold crude extracts had no inhibitory activity
KEY: No Inhibition activity
Table 2: Zones of inhibition of fungal clinical isolates by black tea at different extraction modes. Key: Cn: Cryptococcus Neoformans; Ca: Candidaalbicans
The MIC of the Camellia Sinensis crude extracts which had inhibition diameters of 15 mm and above (significance activity) was determined. The results show that cold green tea crude extract, at 3.125 mg/ml inhibited growth of C. tropicalis ATCC 750. A concentration of 6.25 mg/ml inhibited growth of 50% of the fungal isolates tested; this therefore gives the MIC50 of the test fungi when using cold green tea crude extract. Hot green tea at 1.6 mg/ml inhibited the growth of C. famata. A concentration of 3.125 mg/ml inhibited growth of a 50% of the tested fungi; this gives the MIC50 of the test fungi when using hot green tea crude extract. Cold green tea crude extract, at 25 mg/ml was adequate to inhibit growth of C. lusitaniae, while at a concentration of 25 mg/ml inhibited growth of 90% of the fungal isolates tested. At this concentration of cold green tea crude extract, only C. parapsilosis ATCC 22019 was not inhibited. Similarly, hot green tea crude extract at 25 mg/ml, inhibited growth of C. lusitaniae. This concentration of 25 mg/ml also inhibited growth of 90% of the tested fungi. At this concentration of hot green tea crude extract only C. parapsilosis ATCC 22019 was not inhibited. The MIC90 of Hot green tea crude extract includes for C. famata, C. neoformans ATCC 66031, C. tropicalis ATCC 750, T. mentagrophytes, M. gypseum and C. lusitaniae respectively was 1.6mg/ml, 3.125mg/ml, 3.125mg/ml, 3.125 mg/ml, 6.25mg/ml, 25mg/ml, 50mg/ml.
MIC for tea crude extracts to the fungal clinical isolates
When tea crude extracts were tested against fungal clinical isolates, the green tea crude extract mean 0.0165 ± 0.0139 mm had less zones of inhibition than black tea crude extract mean 0.0518 ± 0.0279 mm. The MIC of the Camellia Sinensis crude extracts which had inhibition diameters of 15 mm and above (significance activity) was determined. Green and black tea extracts had the least minimum inhibition concentration of 0.78 mg/ml against Cryptococcus neoformans 065 and Candida albicansstrain15and the highest MIC against Candida albicans strain 5 for green tea and C. neoformans5 against black tea. Green tea crude extract had highest MIC on C. albicans strain 4 and strain 5 than other clinical isolates as shown in (Figure 3).
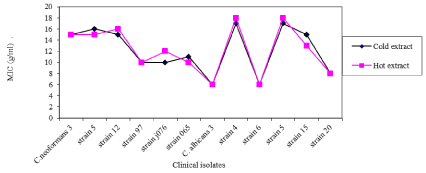
Figure 3: MIC of cold green, hot green tea crude extracts on fungal clinical
isolates.
Cold black tea crude extracts (mean MIC 8.25 mg mL-1) was found to have greater MIC of fungi clinical isolates than hot black tea crude extracts (Mean MIC 8.167 mg mL-1). Black tea crude extract had the highest MIC on C. albicans strain 5 and strain 15 more than it had on other fungal clinical isolates.
Levels at which hot green tea were fungi static to the organisms and the levels to which they were fungicidal to the organisms tested showed that at minimal concentration of 1.5 mg/ml. C. neoformans ATCC 66031 was made static and could not grow. When the concentration of hot green tea reached 25 mg/ml, all the tested fungi including C. famata were static in growth and C. tropicalis ATCC 750 and C. parapsilosis ATCC 22019 were already not able to survive at this concentration. Similarly, minimum fungicidal concentration (MFC) of hot green tea and mixture tea was tested on C. tropicalis ATCC 750, C. neoformans ATCC 66031, C. lusitaniae, C. famata and C. parapsilosis ATCC 22019 fungi.
Discussion
In the present study, the crude extracts of Camellia Sinensis (green and black) produced inhibitory activity against pathogenic and mycotoxigenic fungi. The water crude extraction yielded enough extracts for the experimental study and is the most commonly used and cost effective method of tea preparation. The results obtained in this study indicate a significant difference in antifungal activity of antimycotic activity of Camellia Sinensis green and black crude extracts. For all the yeasts tested, C. famata was the most sensitive fungus to all the crude extracts (Table 1). In cold extraction mode, cold green tea crude extracts showed greater antifungal activity as compared to cold black tea crude extracts. Yeasts C. famata, C. tropicalis ATCC 750, C. lusitaniae, C. neoformans ATCC 66031, C. parapsilosis ATCC 22019 and moulds Microsporum gypseumand Trichophytonmentagrophytes showed greater inhibition zone diameter of = 15 mm. C. krusei ATCC 6258 was moderately active with IZD of 14 mm while yeasts C. albicans ATCC 90028, C. glabrata ATCC 24433 and mould P. chrysogeneum and F. moniliforme were resistant with IZD 6 mm. This conform to earlier studies that extracts of green tea have been reported to be more effective in inhibiting bacterial growth than black tea [22]. The yeasts C. albicans ATCC 90028, C. parapsilosis ATCC 22019, C. glabrata ATCC 24433, C. tropicalis ATCC 750, C. krusei ATCC 6258, C. neoformans ATCC 66031 and mould A. niger as well as P. chrysogeneum were resistant to cold black tea extract with IZD 6 mm.
However, C. lusitaniae, C. famata, M. gypseum, F. moniliforme and T. mentagrophytes were moderately inhibited (Table 1). [23] (1991) reported that 2.5% of black tea extract completely inhibited the growth of T. mentagrophytes and T. rubrum; however, even at 10% concentration, this extract did not inhibit the growth of C. albicans or Cryptococcus neoformans. Similarly, for hot water crude extraction mode, the hot green tea crude extract showed greater inhibitory effects. From the study, C. famata, C. lusitaniae, C. tropicalis ATCC 750 and T. mentagrophytes were more sensitive to the extract with IZD =15 mm. However, yeasts C. albicans ATCC 90028, C. glabrata ATCC 24433 and mould A. niger, F. moniliforme and P. chrysogeneum were resistant with IZD 6 mm. C. parapsilosis ATCC 22019, C. neoformans ATCC 66031, C. krusei ATCC 6258 and M. gypseum were moderately active with IZD of = 10 mm. The black tea hot crude extract showed the least number of fungal test organisms having activity. The results from the present study revealed that there was a significant difference in zones of inhibitions (T=4.09, P<0.05).
Candida albicans strain 4 and strain 5 among all the clinical isolates had greatest susceptibility to antifungal activity of green tea extract with IZD of 18mm (Figure 1). C. neoformans strain 3, strain 5 and strain 12, showed susceptibility to antifungal activity of green tea extracts (cold and hot) with IZD = 10.0 mm each. This is moderately active as considered highest at 18.0mm and least 6.0 mm. These findings are in line with [24] (2010) which indicates that the lowest activity was at 7.0 mm and the highest was at 18.0 mm in diameter. The standard drug inhibition zone diameter was between 18 and 22 mm which compared well with that of the Camellia Sinensis crude extract. When the antifungal activities of antimycotics of these Camellia Sinensis crude extract (green and black) were compared to that of control, green tea crude extract at a concentration of 100 mg mL-1 was found to have almost comparable activity to standard Fluconazole against yeast C. famata and C. tropicalis ATCC 750 and mould T. mentagrophyte. Similarly, black tea crude extract at concentration of 100 mg mL-1 showed comparable activity against the fungi yeast C. famata and mould T. mentagrophyte to that of Fluconazole.
Although different treatments of crude extraction (both cold and hot water extracts) of Camellia Sinensis produced inhibitory actions, hot water extracts showed more inhibitory effects than cold water extracts. This suggests that active ingredients in tea were better extracted with hot water. A considerable amount of higher yields of hot water than cold water extraction of green tea has been reported [25,26]. The green tea has shown higher antimicrobial activity than black tea. This difference in results is probably due to presence of different contents of active substances in the teas [27]. Several studies have shown that the antimicrobial property is due to presence of polyphenols. Specific antioxidant polyphenols called catechins play an important role in green tea’s inhibition of microbial growth. Antimicrobial activities of tea extracts are very selective. This difference in their inhibitory activity depends upon the concentration and type of the extracts. These effects may also differ depending on microbe fungal species so that they may be either growth inhibitory or stimulatory [27].Green tea and black tea crude extracts tested in current study have also shown varying activities against fungal organisms. Studies of the antibacterial activity of catechins (polyphenol) against phyto-pathogenic bacteria showed similar results to those against C. albicans [28].
Catechins have been documented as known to have an affinity for proteins; this is clearly indicated by a decrease in antibacterial activity of tea [6]. The mode of action by the Camellia Sinensis involves inducing rapid leakage of small molecules entrapped in the intra liposomal space and aggregation of the liposomes [29], [30] examined the mechanism of the effects of green tea catechins on T. mentagrophytes using electron microscopy and suggested that catechins attacked the cell membrane and caused lysis of the conidia and hyphae. Catechins dimer theaflavins and its gallates play a part in black tea [6] for antifungal activity. Recent studies have documented evidence to have emerged, however, to suggest that these molecules have the capacity to modulate the physical structure of cell membranes. Thus, a number of membrane dependent cellular processes, such as cell signaling and cell cycle [31], arachidonic acid metabolism and cell proliferation [32], and apoptosis and mitochondrial functionality [33] may be influenced by interaction of catechins with cellular phospholipid palisade [34].
The least susceptible, resistant fungal strains of clinical isolate Cryptococcus neoformans strain 3, strain 97, strain j076 and strain 065 (Figure 2) was most probably as a result of the existence of mucopolysaccharide capsule. The polysaccharide capsular material in some of the pathogenic microorganisms is responsible for virulence and antimicrobial resistance [35]. The Candida species such as Candida albicans ATCC 90028, Candida glabrata ATCC 24433, Candida parapsilosis ATCC 22019 that showed less susceptibility to antimycotics of Camellia Sinensis crude extract could be due to their outer membrane consisting of chitin binding proteins thus able to regulate the access of antifungal properties into the underlying structures. Candida species expresses multidrug efflux transporter (MET), which mediates the efflux of a broad range of compounds including antifungal agents [36]. But in this study, we found contradicting results among the Candida strains clinical isolates (Figure 1 & 2). C. albicans strain 3, strain 6 and strain 20 showed least or no activity whereas strain 4, strain 5 and strain 15 had activity. The disparity in findings tends to be probably due to differences in strains of fungi used and susceptibility to antifungal drugs.
The MIC values of the crude extracts of Camellia Sinensis which had inhibition zone diameter of 15 mm and above was determined so as to demonstrate the potency of the extracts against the selected strains of fungi. The least the MIC, the better the Camellia Sinensis crude extract against the isolate in question. The cold green tea crude extract had the least minimum inhibition of 3.125 mg mL-1 against yeast C. tropicalis ATCC 750 and mould T. mentagrophyte and the highest MIC against C. parapsilosis ATCC 22019 of 50 mg mL-1. The MIC50 of the test fungi using cold green tea crude extract was 6.25 mg mL-1, while at concentration of 25 mg mL-1, it was the least minimum inhibition concentration that inhibited 90% of the fungal isolates tested, MIC90. The hot green tea crude extract had the least MIC of 1.6 mg mL-1 against C. famata and the highest MIC of 50 mg mL-1 against C. parapsilosis ATCC 22019. At a concentration of 3.125 mg mL-1, 50% of the fungal isolates tested were inhibited, giving MIC50, while a concentration of 25 mg mL-1 inhibited 90% of the test fungal isolates giving MIC90.
Fungi
Hot Green tea crudeextract (mg/ml)
C. tropicalis ATCC 750
3.12
C. neoformans ATCC 66031
1.50
C. lusitaniae
1.56
C. famata
25.00
C. parapsilosis ATCC22019
6.25
Table 3: Minimum Fungicidal Concentration of hot green tea crude extract.
From Figure 3, hot green tea crude extract (mean MIC 12.25 mg mL-1) had a higher MIC on of fungi clinical isolates than cold green tea crude extract (Mean MIC 12.167 mg mL-1). Green tea crude extract had greatest MIC on C. albicans strain 4 and strain 5 and C. neoformans strain 12 than other clinical isolates. Contrary to green tea crude extracts on clinical isolates strains, Cold black tea crude extracts (mean MIC 8.25 mg mL-1) was found to have greater MIC of fungi clinical isolates than hot black tea crude extracts (Mean MIC 8.167 mg mL-1). Black tea crude extract thus had highest MIC on C. albicans strain 5 and C. neoformans strain 5 more than other fungal clinical isolates (Figure 4). Generally, the MIC of the Camellia Sinensis crude extracts were as high as 50 mg mL-1 as compared to the standard drugs which is 0.5 mg mL-1 for yeasts and 1.0 mg mL-1 for dermatophytes at 95% confidence interval (P=0.05 level of significance). Although this was significantly lower than that of Fluconazole (P<0.01), the extracts are promising since they are crude extracts compared to pure compound of Fluconazole. This is a clear indication that the active ingredient is present in low quantities which requires the use of large amounts of aqueous crude extracts so as to achieve the desired therapeutic effects.
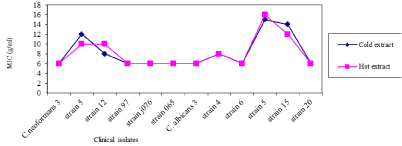
Figure 4: MIC of cold black tea, hot black tea crude extracts on fungal clinical
isolates.
The observed difference in inhibitory activities of green and black tea crude extracts could be attributed to the facts that plants differ phytochemically and the extraction procedures also affect/alter their composition (cold and hot water extraction). Absence of bioactivity does not warrant dismissal of possible ethno botanical utilization of the Camellia Sinensis, however, it tends to suggest that the aqueous crude extracts are acting indirectly in a way where active ingredient exists as a precursor requiring activation in vivo. The Minimum Fungicidal Concentration (MFC) of hot green tea crude extract was tested on C. tropicalis ATCC 750, C. neoformans ATCC 66031, C. lusitaniae, C. famata and C. parapsilosis ATCC 22019 fungi. The findings revealed that green tea hot crude extract at 25 mg mL-1 was effective enough to kill C. tropicalis ATCC 750 and C. parapsilosis ATCC 22019 alone. At a concentration of 50 mg/ml green hot tea was fungicidal for Cryptococcus neoformans ATCC 66031 and C. famata. At 100 mg/ml concentration of hot green tea all the tested fungi including C. lusitaniae were killed by the tea extract. Levels at which hot green tea were fungi static to the organisms and the levels to which they were fungicidal to the organisms tested showed that at minimal concentration of 1.5 mg/ml C. neoformans ATCC 66031 was made static and could not grow. When the concentration of hot green tea reached 25 mg/ml, all the tested fungi including C. famata were static in growth and C. tropicalis ATCC 750 and C. parapsilosis ATCC 22019 were already not able to survive at this concentration.
Similarly, minimum fungi static concentration (MFC) of hot green tea and mixture tea was tested on C. tropicalis ATCC 750, C. neoformans ATCC 66031, C. lusitaniae, C. famata and C. parapsilosis ATCC 22019 fungi. The mechanistic aspect of fungicidal brought about by tea crude extracts is suggestive to be due to catechins and gallates. The bioactive ingredients in crude tea extracts binds to ergosterol, one of the cell membrane sterols, and damages the cell membrane directly, leading to fungicidal activity against fungi. Catechins controls and regulate expression of the gene(s) coding for Cytochrome P450. The scientific physiochemical studies suggest that fungicidal activities of galloylated tea catechins at the cell membrane level may be due to their specific perturbations of ordered structure of chitin binding proteins, a nitrogen containing polysaccharide constituting fungal cell wall. The numerous varying effects of catechins on fungal cell walls in comparison to the membrane of human cells may be as a result of differences in membranous structures of the respective walls. These observations suggest that antifungal activity of antimycotic effect has a linkage to the interactions of catechins in the aqueous crude extracts with oxygen, genes, cell membranes and enzymes. This aspect significantly calls for further studies. This predominantly in vitro information has potential use for Mycotic disease prevention in humans.
Conclusion and Recommendations
In the present study, the crude extracts of Camellia Sinensis produced inhibitory actions against the fungal test strains. Hot aqueous green tea crude extracts showed greater antifungal activity than the black hot tea crude extracts. This showed that bioactive ingredients in Camellia Sinensis are better extracted in hot aqueous mode. Recommendations include; that the Plant based crude extracts represents unlimited sources of modern therapies therefore; a continued and regular exploration of Camellia Sinensis for antifungal agent is required. For potential antifungal beneficial effects, the hot green tea should be consumed in preference to black tea. Assayed antifungal were tested in vitro, but practically in human aspect both antifungal and polyphenolic compounds of Camellia Sinensis undergo metabolic processes in the body; there is no information on the interaction of the related metabolites. This needs further studies.
Acknowledgment
The technical assistance of the staff of the Medical Mycology Department of the Kenya Medical Research Institute (KEMRI), Nairobi is appreciated.
Conflict of Interest Statement
Authors declare that the present work was done by the authors and there is no any financial support from any agency and there are no conflicts of interest.
References
- Agarwal R, Katiyar SK, Zaidi SI, Mukhtar H. Inhibition of skin tumor promoter-caused induction of epidermal ornithine decarboxylase in SENCAR mice by polyphenolic fraction isolated from green tea and its individual epicatechin derivatives. Cancer Research. 1992; 52: 3582–3588.
- Arakawa H, Maeda M, Okubo S, Shimamura T. Role of hydrogen peroxide in bactericidal action of catechin. Biological and Pharmaceutical Bulletin. 2004; 27: 277–281.
- Bandyopadhyah D, Chatterjee TK, Dasgupta A, Lourduraja J, Dastidar SG. In vitro and in vivo antimicrobial action of tea: The commonest beverage of Asia. Biology and Pharmaceutical Bulletin. 2005; 28: 2125–2127.
- Bii C, Korir RK, Rugutt J, and Mutai C. The potential use of Prunus africana for the control, treatment and management of common fungal and bacterial infections. J. Med. Plants. Res. 2010; 4: 995-998.
- Caturla N, Vera-Semper E, Villalaín J, Reyes Mateo C, Microl V. The relationship between the antioxidant and the antibacterial properties of galloylatedcatechins and the structure of phospholipid model membranes. Free Radical Biology and Medicine. 2003; 34: 648–662.
- Chou CC, Lin LL, Chung KT. Antimicrobial activity of tea as affected by the degree of fermentation and manufacturing season, International Journal of Food Microbiology. 1999; 48: 125 –130.
- Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-trastransformed cells: Structure-activity relationship and mechanisms involved. Cancer Res. 1999; 59: 4610-4617.
- Elgayyar M, Draughon FA, Golden DA, Mount JR. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. Journal of Food Proteins. 2001; 64: 1019-1024.
- Esimone CO, Iroha IR, Ibezim EC, Okeh CO, Okpana EM. In vitro evaluation of the interaction between tea extracts and penicillin G against staphyococcussaures. African Journal of Biotechnology. 2006; 5: 1082–1086.
- Ferraro MJ. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard. 8thEdition. National Committee for Clinical Laboratory Standards (NCCLS). 2003.
- Fukai K, Ishigami T, Hara Y. Antibacterial activity of tea polyphenols against phytopathogenic bacteria. Agricultural and Biological Chemistry. 1991; 55: 1895–1897.
- Hamilton–Miller JMT. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrobial Agents Chemotherapy. 1995; 39: 2375-2377.
- Hamilton–Miller JMT. Microbial properties of tea infusions. Schubert R, Spiro M, editors. In: Chemical and biological properties of tea infusions. Frankfurt, Germany. 1997; 63-74.
- Hirasawa M, Takada K, Otake S. Inhibition of acid production in dental plaque bacteria by green tea catechins, Caries Res. 2003; 40: 265–270.
- Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerging Infectious Diseases. 2001; 7: 337-341.
- Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. BiochemicaetBiophysicaActa. 1993; 1147: 132 – 136.
- Isogai E, Isogai H, Hirose K, Hayashi S, Oguma K. In vivo synergy between green tea extract and levofloxacin against enterohemorrhagicEscherichia coli 0157 inflection. Current Microbiology. 2001; 42: 248 – 251.
- Katiyar SK, Mukhtar H. Tea in chemoprevention of cancer: epidemiologic and experimental studies. Int J Oncol. 1996; 8: 221-238.
- Kigondu EVM, Phytochemical and anti-parasitic activity studies of some selected Kenya medicinal plants. M.Sc. Thesis, Jomo Kenyatta University of Agriculture and Technology. Kenya. 2007.
- Klevay MJ, Ebinger A, Diekema DJ, Messer SA, Hollis R, Pfaller MA, et al. Disk diffusion testing of Candida inoculated directly from CHROMagarCandidamedium may decrease time to results reporting. Journal of Clinical Microbiology. 2005; 43: 3497-3499.
- Kohlmeier L, Weterings C, Steck S, Kok FJ. Tea and cancer prevention: an evaluation of the epidemiologic literature. Nutr Cancer. 1997; 27: 1-13.
- Lin SD, Liu EH, Mau JL. Effect of different brewing methods on antioxidant properties of steaming green tea. LWT - Food Science and Technology. 2008; 41: 1616–1623.
- Lorene L, Kennedy F, Kane J. Identification Key Guide to medical mycology, Department of Botany, University of Alberta, Edmonton. 2002.
- Luczaj W, Skrzydlewska E. Antioxidative properties of black tea. Preventive Medicine. 2005; 40: 910 – 918.
- Magoma GN, Wachira FN, Obanda M, Imbaya M, Agong SG. The use of catechins as biochemical markers in diversity studies of tea (Camellia Sinensis). Genetic Resources and Crop Evaluation. 2000; 47: 107–114.
- Manzocco L, Anse M, Nicoli MC. Antioxidant properties of tea extracts as affected by processing. Lebensmittel–Wissenschaft und –Technologie. 1998; 31: 694–698.
- Marchetti O, Moreillon P, Glauser, MP. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrobial Agents and Chemotherapy. 2000; 44: 2373–2381.
- Michael JP, Chan EC, Noel RK, Merna FP. Microbiology. 5th edn. Tata McGraw-Hill, New Delhi, India. 2003; 627-748.
- Muanza DN, BW Kim, KL, Euler L, Williams. Antibacterial and antifungal activity of nine medical plants from Zaire. Int. J. Pharmacog. 1994; 32: 337–345
- National Clinical and Laboratory Standards Institute Performance Standards for antimicrobial susceptibility testing, seventeenth informational supplement Wayne PA. 2007; M100-S17.
- Okubo S, Toda M, Hara Y, Shimamura T. Antifungal and antifungicidal activities of tea extract and catechin against Trichophyton. Japanese Journal of Bacteriology. 1991; 46: 509–514.
- Ramarathnam N, Osawa T, Ochi H, Kawakishi S. The contribution of plant food antioxidants to human health. Trends in Food Science and Technology. 1995; 6: 75 -82.
- Rechner AR, Wagner E, Van Buren L, Van De put F, Wiseman S, Rice-Evans CA, et al. Black tea represents a major source of dietary phenolics among regular tea drinkers. Free Radical Research. 2002; 36: 1127-1135.
- Robinson EE, Maxwell SRJ, Thorpe GHG. An investigation of the antioxidant activity of black tea using enhanced chemiluminescence. Free Radical Research. 1997; 26: 291-302.
- Selitrennikoff CP. Screening for Antifungal Drugs. In: Biotechnology of Filamentous Fungi, Firkeftein DC. Ball (Eds.). Butterworth, London. 1992; 189-217.
- Shetty M, Subbannayya K, Shivananda PG. Antibacterial activity of tea (Camellia sinensis) and coffee (Coffee arabica) with special reference to Salmonella typhimurium. Journal of Communicable Diseases. 1994; 26: 147-1450.
- Spencer JP, Schroeter H, Kuhnle G, Srai SK, Tyrrell RM, Hahn U, et al. Epicatechin and its in vivo metabolite, 3'-O-methyl epicatechin, protect human fibroblasts from oxidative stressinduced cell death involving caspase-3 activation. Biochemical Journal. 2001; 354: 493–500.
- Spiro M. Kinetics and equilibria of tea infusion—part 12. Equilibrium and kinetic study of mineral ion extraction from black Assam Bukial and green Chun Mee teas. Food Chemistry. 1995; 54: 393–396.
- Stein AC, M Sortino, C Avancini, S Zacchino, G von Poser. Ethnoveterinary medicine in the search of antimicrobial agents: Antifungal activity of Pterocaulon (Asteraceae). Journal of Ethanopharmacology. 2005; 99: 211-214.
- Su X, Duan J, Jiang Y, Shi J, Kakuda Y. Effects of soaking conditions on the antioxidant potentials of oolong tea. Journal of Food Composition and Analysis. 2006; 19: 348–353.
- Thelle DS. Coffee, tea and coronary heart disease. Curr Opin Lipidol. 1995; 6: 25–27.
- Tiwari RP, Bharti SK, Kaur HD, Dikshit RP, Hoondal GS. Synergistic antimicrobial activity of tea and antibiotics. Indian Journal of Medical Research. 2005; 122: 80-84.
- Toyoshima Y, Okubo S, Toda M. Effect of catechins on the ultrastructure of Trichophytonmentagrophytes. KansenshogakuZasshi. 1993; 68: 295–303.
- Wachira FN, Kamunya S. Kenyan Teas are Rich in Antioxidant. Tea. 2005; 26: 81-89
- Wang ZY, Huang MT, Chang R, Ma W, Ferraro T, Reulh KR, et al. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Research. 1992; 52: 6657-6665.
- World Health Organization. Report on infectious diseases. WHO, Geneva, Switzerland. 2000.
- Yam TS, Shah S, Hamilton-Miller JMT. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis) and tea components. FEMS Microbiology Letters. 1997; 152: 169 -174.
- Zuo a Yuegang, Hao Chen a, Yiwei Deng. Identification and use of potential bacterial organic antifungal volatiles in bio-control.Soil Biology and Biochemistry. 2002; 37: 955-964.