
Original Article
J Bacteriol Mycol. 2023; 10(2): 1209.
Developing and Evaluating a Weaned Piglet Model of Enterotoxigenic Escherichia Coli Infection
Tsukahara T1*; Matsui T2; Sawada K2; Nakamura1,5; Imaoka T3; Nakanishi N3; Sato T4; Hamabata T4
1Kyoto Institute of Nutrition & Pathology, Ujitawara, Kyoto, Japan
2Advanced Technology Research Laboratories, Idemitsu Kosan Co. Ltd., Sodegaura, Chiba, Japan
3KYODOKEN Institute, Fushimi, Kyoto, Japan
4Research Institute, National Center for Global Health and Medicine, Shinjuku, Tokyo, Japan
5Laboratory of Veterinary Pathology, Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Ehime, Japan
*Corresponding author: Tsukahara T Kyoto Institute of Nutrition & Pathology, 11-1 Madani, Tachikawa, Ujitawara, Kyoto 610-0231, Japan Tel: +81-774-99-7331; Fax: +81-774-99-7332 Email: tsukahara@kyoto-inp.co.jp
Received: July 25, 2023 Accepted: August 17, 2023 Published: August 24, 2023
Abstract
Enterotoxigenic Escherichia Coli (ETEC) remains the main pathogen associated with post-weaning diarrhea in piglets. Here, we introduce a reliable method to develop an ETEC-infected weaned piglet model. For the first 24 h post-birth, neonatal piglets were separated from dams so that they did not receive colostrum. Afterwards, piglets were returned to the care of their mothers, until weaning (21-day-old). On days 25, 26 and 27 post-birth, piglets were orally challenged with 1011 Colony-Forming Units (CFU) of virulent ETEC strain incubated in colony-forming antigen medium and delivered in chitosan-coated capsules. In all infected piglets, severe diarrhea and typical body weight loss were observed post-infection. To simplify the work, for the newly developed method, the number of challenging ETEC cells was lowered to 1010 CFU/head and the incubation medium was changed to tryptic soy. These modifications influenced neither the diarrheal defecation ratio nor the body weight loss in piglets. To induce experimentally ETEC infection in future work, we recommend that 1010 CFU/head of ETEC cell grown in tryptic soy broth be delivered in chitosan-coated capsules to colostrum restricted piglets, because this methodology stably caused 100% diarrheal defecation and growth reduction.
Keywords: Enterotoxigenic Escherichia coli; Heat-labile toxin; Infection model; Post-weaning diarrhea; Weaned pig
Introduction
Pathogenic Escherichia coli infection remains a major problem in the swine industry. Pathogenic strains of E. coli recovered from intestinal tracts of animals have been categorized as enterotoxigenic, enteropathogenic, enterohemorrhagic, and necrotoxigenic [1]. In particular, Enterotoxigenic E. Coli (ETEC) is the main pathogen associated with post-weaning diarrhea in piglets [2,3]. To date, antimicrobials are mainly used to prudently prevent and/or treat post-weaning porcine diarrhea worldwide. Nonetheless, alternative agents to cure or prevent ETEC infection are being actively evaluated [2]. To discover preventive or therapeutic agents against ETEC infection, researchers generally use experimental infection methods [2,4]. However, piglets experimentally infected with pathogenic E. coli do not always have symptoms. Previous work speculated that lacking symptoms in experimental infection was caused by a low pH in the stomach [5]. For example, in 58 previous studies, the mean diarrheal defecation ratio was merely 68% [4]. Diarrheal defecation is the best parameter to evaluate the effectiveness of agents; therefore, to minimize the number of experimental animals to a humane level, a stable method with 100% diarrheal defecation is deemed necessary.
Recently, Matsumoto et al. [6] reported that MUC4 genotype and/or pig breed-line are the controlling factors of susceptibility to ETEC infection, and that regulation of these factors could induce 100% diarrheal defecation in weaned piglets. Separately, we have reported an improved method to experimentally induce Shiga-toxin-producing E. coli (STEC) infection [7]. The aforementioned method caused the typical pathological symptom of porcine edema disease in almost all piglets [7, 8]. In the present study, to induce 100% diarrheal defecation in weaned piglets, we took a different approach than that of Matsumoto et al [6]. Indeed, we used our own developed STEC method [7] (i.e., colostrum depletion and chitosan capsule inoculation) and applied it to cause ETEC in weaned piglets. In Experiment 1, the maximal challenging dose [1011 colony-forming units (CFU)/head] was prepared using a ETEC strain incubated in a Colony-Forming Antigen (CFA) broth, which was the most suitable medium for the expression of ETEC adhesion factors [9]. In Experiment 2, to simplify the methodology, we used Tryptic Soy (TS), which is a general and basic broth medium that can be purchased from several commercial suppliers; in addition, using it, helped lower the challenging dose to 1010 CFU/head.
Materials and Methods
Challenging Strain
The ETEC strain 8185 isolated from pig diarrhea, kindly donated by Dr. Ikuko Koike from Swine Management Consultation (Kanagawa, Japan), was used in the present study. This strain was positive of several virulence genes (estB, elt1, ast1 and faeG). As phenotypes, we detected heat-liable toxin (LT) and F4 with the commercial kits (VET-RPLA and Toxigenic E. coli Pilli Antisera, Denka Seiken, Tokyo, Japan). The characteristics of this strain are shown in Table S1.
The ETEC strain was cultured either in CFA medium broth (1% of casamino acids, 0.15% of yeast extract, 0.05% of MgSO4, 0.0005% of MnCl2, pH 7.4) (Experiment 1) or in TS medium broth (Difco, Detroit, MI, USA) (Experiment 2), under an aerobic condition at 37°C. Four h post-incubation, ETEC cells in logarithmic growth phase were harvested by centrifugation (1,450×g, 15 min, 4°C). Cell slurries were prepared immediately prior to use.
Pigs
The pregnant sows (Landrace × Large White), used in the present work, which were impregnated by Duroc boars and purchased from a commercial pig farm, were the same as those previously reported [7]. A qualified veterinarian (Dr. Nakanishi) investigated beforehand the pathogenic permeation of the farm used in the present study. No enteropathogens such as hemolytic E. coli, Salmonella sp., Clostridium perfringens, Lawsonia intracellularis, Brachyspira hyodysentariae, porcine epidemic diarrhea virus, rotavirus and parasites were detected in the feces of weaning piglets of this farm. The screening methods for these enteropathogens have been described elsewhere [3]. The health statuses of the introduced sows were checked by the veterinarian, and who also assessed the retrospective statuses of stable deliveries. Sows were vaccinated against atrophic rhinitis and swine erysipelas [ARBP/Swine Erysipelas-combined vaccine (inactivated), Nisseiken, Tokyo, Japan)], Japanese encephalitis, porcine parvovirus infection, Getah virus infection (“Kyotobiken” swine abnormal birth 3 combo live vaccine; Kyotobiken, Kyoto, Japan), and porcine reproductive and respiratory syndrome (Ingelvac PRRS MLV, Boehringer Ingelheim Animal Health Japan, Tokyo, Japan). For all experiments, sows were individually housed in fallowing pens at the KYODOKEN Institute (Fukuchiyama, Kyoto). No LT genes were detected in the feces of any of the sows. Throughout the study, the sows were fed a commercial diet (Bree-Meal Maxim, Feed One, Yokohama, Japan). After delivery and for the first 24 h, neonatal piglets received no colostrum and instead, were fed artificial milk (Meiji Hohoemi, Meiji, Tokyo, Japan), as previously described [7]. Afterwards, all piglets were returned to their own dams and, during the nursing period, received maternal milk only. All piglets were weaned at 21 days of age, put together in experimental groups and transferred to concrete pens with brooders. Throughout the weaning period, the piglets were fed ad libitum a commercial feed for weaned piglets (SDS No. 1; Feed One). The experimental diet was free of intestinal microbiota modifiers, such as antimicrobials and probiotics. The nutrient composition of the aforementioned diet (g/kg), expressed on a dry-matter basis (906g/kg), was as follows: crude protein, 220g; crude fat, 46g; crude fiber, 9g; and crude ash, 63g.
Experiment 1: ETEC Challenge Using the Maximal Dose
Of 10 healthy piglets delivered by one sow, six were used for Experiment 1. After weaning, piglets were divided into two groups (n=3). One group was considered the non-infection control (group C1; mean body weight, 5.3 kg; one male and two females) and the ETEC-infected group (group LT1; mean body weight, 6.0 kg; two males and one female). Four days post-acclimatization, the piglets (25 days old) in LT1 group were orogastrically inoculated the ETEC preparation in chitosan-coated capsules (Sansho, Tokyo, Japan). These capsules were resistant to gastric digestion and hence, viably delivered ETEC to the small intestine [5]. The ETEC capsules containing approximately 1011 CFU were given to piglets for three consecutive days [i.e., days post-infection (dpi) 0, 1 and 2]. The precise numbers of challenging ETEC cells were 7.2×1010, 7.8×1010, and 1.0×1011 CFU/head on dpi 0, 1, 2, respectively. Separately, the same type of capsules but filled with saline were given to the C1 piglets. Individual body weights and feed intake of each group were measured on dpi 0, 3, 5 and 9. In addition, fecal samples from all piglets were simultaneously collected. The fecal samples were stored at -80 °C until DNA extraction.
Experiment 2: ETEC Challenge Using a Lower Dose
Two sows delivered 11 healthy piglets each. Of these, six were used for Experiment 2. After weaning, piglets were divided into two groups. One group was considered the non-infection control (group C2; mean body weight, 6.1 kg; one male and two females) and the ETEC-infected group (group LT2; mean body weight, 3.6 kg; one male and two females). Four days post-acclimatization, the piglets in LT2 group were orogastrically inoculated ETEC in the same manner as in Experiment 1 (i.e., colostrum depletion and chitosan capsule inoculation), with the following exceptions. Unlike in Experiment 1, the ETEC cell numbers were 6.3×109, 9.1×109, and 8.1×109 CFU/head on dpi 0, 1, 2, respectively. Individual body weights and feed intake of each group were measured on dpi 0, 3 and 9. We omitted dpi 5 measurement, because we considered measurement of this point was unnecessary to assess the successively of ETEC infection. As in Experiment 1, fecal samples from all piglets were simultaneously collected. Fecal samples were stored at -80 °C until DNA extraction.
Clinical Observations
Throughout the study, the condition of fresh feces was individually evaluated once daily (dpi 1-9). The criteria used for feces scoring have been previously described [5]. Briefly, the criteria of fecal score were described as follows, 0: normal, 1: loose stool, 2: moderate diarrhea, 3: severe diarrhea.
Dissection and Histopathologic Observation
All piglets in both experiments were euthanized by exsanguination under deep sedation with an intraperitoneal injection of sodium pentobarbital (Somnopentyl, Kyoritsu, Tokyo, Japan; 32.4 mg/kg B.W.). Afterwards, the whole intestines were removed and screened for abnormalities with the naked eye.
In Experiment 1, micro-abnormalities were observed in the intestines of ETEC-infected piglets. The small intestine was dislodged and cut into eight segments of equal length [10]. Segments S2, S5 and S8 from the small intestines were considered as stemming from the 1) jejunum, 2) jejunum and ileum complex and 3) ileum, respectively [10]. The middle portions of ceca were also collected. Each intestinal segment was longitudinally incised and fixed in 10% (v/v) phosphate-buffered formalin. Formalin-fixed intestinal tissues were further cut into cross sections of an approximate length of 10 mm. Each intestinal sub-segment was embedded in paraffin wax. To visualize any histopathological abnormality under a light microscopy, micro-sections 3-μm thick were prepared and stained with hematoxylin and eosin. The histopathologic study in Experiment 2 was omitted because remarkable abnormality was not detected in Experiment 1.
Detection of Gene elt1
DNA was extracted from fecal samples of pigs on dpi 0, 3, 5 and 9 (Experiment 1) and dpi 0, 3 and 9 (Experiment 2), respectively. The DNA extraction method was the same as that previously described [11]. The excretion of ETEC was measured in the feces by Taqman quantitative real-time PCR for detection of gene elt1, as described elsewhere [12].
Validation of Methods
To validate Experiment 2, an additional experiment was carried out.
Four colostrum-restricted piglets delivered from one sow were readied. The experimental design was the same as that of Experiment 2, and it was as follows. After weaning, piglets were divided into two groups. One group was considered the non-infection control (group C3; mean body weight: 7.2 kg; one male and one female) and the ETEC-infected group (group LT3; mean body weight: 6.1 kg; one male and one female). Four days post-acclimatization, the piglets in group LT3 were orogastrically inoculated ETEC in the same manner as in Experiment 2 (i.e., colostrum depletion and chitosan capsule inoculation). The ETEC cell numbers were 1.4×1010, 9.9×109, and 1.4×1010 CFU/head on dpi 0, 1, 2, respectively. Individual body weights and the feed intake of each group were measured on dpi 0, 3 and 9. Fecal samples from all piglets were simultaneously collected. Fecal samples were stored at -80 °C until DNA extraction. Clinical observation and detection of gene elt1 in the feces are described in above sections.
Statistical Analyses
Depending on the results of the F-test, either the Student’s or Welch’s t-test was used to analyze differences in body weight gain between groups in Experiments 1 and 2. The Mann–Whitney U-test was used to analyze the differences in ETEC numbers in the feces and clinical and histopathological scores between groups. In all statistical analyses, differences between means were considered significant if P<0.05. These body, clinical and pathological parameters were analyzed using STATCEL (OMS, Saitama, Japan), an add-in application for Microsoft Excel© (Seattle, WA, USA). Values are given as the means ± the standard deviations.
Results
Experiment 1
In general, and unlike control (C1) piglets, ETEC-infected (LT1) piglets experienced significant reductions in their body weights from dpi 0 to 5 (Table 1) (P<0.05). In addition, reductions in body weights caused “no calculation” in the feed conversion ratio during this period. Nonetheless, from dpi 5 onwards, reductions in body weight stopped and LT1 piglets gained weight and thus, body weight gain differences between the groups became unclear. However, the feed conversion ratio of LT1 group improved at a later infection stage (dpi 5-9). At first, LT1 group was not experiencing substantial diarrheal defecation in unison with body weight reductions; however, between dpi 4 and 9 it reached 100% (Table 1), before decreasing in severity between dpi 6 and 9. In total, LT1 piglets had diarrhea for an average of 5.3 days. Regarding the ETEC-infected group, as expected, no cells were detected in feces of any of the piglets on dpi 0 (prior to the ETEC challenge). However, following inoculation, ETEC cells were readily detected in the feces of LT1 piglets in dpi 3, with a mean number of 7.68 log CFU/g. Unexpectedly, no ETEC cells were detected in the feces of LT1 piglets on dpi 5 onwards. Visual inspection with the naked eye detected no abnormalities in the small and large intestine samples. However, some abnormalities were detected after histopathological observation was conducted under a light microscope (Table 2, Figure 1). In particular, the scores of lymphocyte infiltration and abnormalities in the crypts increased significantly with ETEC infection.
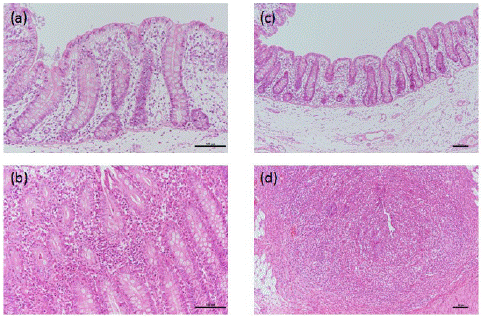
Figure 1: Representative photomicrographs of the cecum of ETEC infected piglets. (a) Normal mucosa was observed in C1 piglets, ×200; (b) Lymphocyte infiltration was observed in the lamina propria of LT1 piglets, ×200; (c) Normal mucosa was observed in C1 piglets, ×100; (d) Crypt disappearance was observed in the mucosa of LT1 piglets, ×100.
Experiment 2
As in Experiment 1, in Experiment 2, reductions of body weights of piglets were also observed between dpi 0-3 and dpi 3-9 (Table 3). Hence, the mean body weight gains significantly differed between control (C2) and ETEC-infected (LT2) piglets during these periods. Diarrheal defecation ratio occurred not only in an early stage (dpi 1-3), but also in a later stage (dpi 4-9). In C2 piglets and throughout the study, no ETEC cells detected in their feces. As for the ETEC-infected group, as expected, no ETEC cells were detected in feces of any of the piglets on dpi 0. Following the ETEC challenge, ETEC cells were detected in the feces of LT2 piglets, with a mean number of 6.11 log CFU/g. However, no ETEC cells were detected in the feces of LT2 piglets on dpi 9. As in Experiment 1, no abnormalities were observed by the naked eye in small and large intestinal samples of any of the piglets.
Validation of Experiment 2
We validated the repeatability of Experiment 2 (Table S2). Although each group (non-infection control and ETEC-infected) had only two piglets, diarrheal episodes (0 v.s. 100%) and low body weight gains (433 v.s. 192 g/d in dpi 3-9) in ETEC-infected pigs were evident. In addition, on dpi 3, ETEC cells were detected only in ETEC-infected pigs (6.33 log CFU/g).
Discussion
In the present study, our previously reported method of piglet STEC infection model [7] was applied to ETEC infection. The STEC infection method encompassed two unique techniques, namely “colostrum depletion piglets” and “STEC inoculation within chitosan-coated capsules”. (1) It is well known that the ingestion of colostrum, especially during the first 24 h of life, is crucial for newborn piglets because it contains several immune factors that can be transferred to them [13]. Therefore, colostrum remains one of the most crucial factors for a healthy development of piglets [13]. For instance, the mortality of piglets ingesting less than 100 g of colostrum during the first 24 h is over 60%, whereas that of piglets ingesting more than 200 g is merely 10% or less [14]. Although sows used in the present work were negative for elt1 gene, the existing evidence on the importance of colostrum was applied to our infection method. For example, we hypothesized that immunoglobulins or lymphoid cells (present in the colostrum) specific to ubiquitous E. coli might decrease the successfulness of ETEC infection [7]. (2) In addition, to effectively deliver ETEC to the small intestine, chitosan-coated capsules were used as these are resistant to gastric digestions [5].
Since about 76% previous work used CFA medium to incubate the ETEC strains [4], we firstly selected CFA to incubate ETEC in Experiment 1. While ETEC cells were detected in fecal samples of LT1 piglets in dpi 3, no ETEC cells were observed on dpi 5 onwards, which implied that they gradually decreased in the intestine, which agrees with Matsumoto et al. [6], who reported that ETEC numbers markedly decreased on dpi 7. Nonetheless, the reason for the decrease in ETEC numbers in the intestines at a later stage remained unclear. Furthermore, while the maximal dose (1011 CFU/head) used induced ETEC infection, it may well have been an unneeded overdose, because dosages in previous studies were ranged from 106 to 1010 CFU/head [4]. In addition, from a health standpoint, it is very likely that diarrheal defecation deteriorated the growth performance of piglets (Table 1). Apart from diarrheal defecation, body weight gain can also be a good parameter to evaluate a therapeutic agent. While no macro-abnormalities were detected in the intestines, micro-abnormalities such as lymphocyte infiltration and abnormalities in the crypt were observed particularly in the large intestines (Table 2). Inflammatory infiltration such as lymphocyte infiltration and reduced crypt depth including abnormalities in the crypt have been also caused by non-infectious diarrhea such as antibiotic-induced diarrhea [15]. Thus, we theorized that histopathological abnormalities observed in Experiment 1 may have been caused by the diarrheal defecation, but not by the ETEC infection, because this E. coli adheres to and colonizes the small intestine [16]. During the evaluation of whether or not the ETEC infection model was accomplished, histopathological analysis was excluded from the parameters. Previous work suggested that histological analysis was unnecessary to evaluate an ETEC infection model [17]. Therefore, in Experiment 2, no histopathological analyses were carried out.
A lower dose of ETEC (1010 CFU/head) also successfully induced severe diarrhea and growth deterioration in Experiment 2. A change of medium from CFA to TS broth did not seemingly influence the growth of the ETEC cells, as per an unaltered diarrheal defecation ratio in the piglets. TS is very convenient as it can be purchased from many suppliers and has already been previously used by a research group to induce an experimental infection of ETEC [4].
When comparing the results between Experiments 1 and 2, piglets infected with 1010 CFU/head of ETEC had a more severe diarrheal condition than those infected with 1011 CFU (duration of diarrhea, LT2: 7.3 vs. LT1: 5.3 days). Unlike in high-dose infected piglets, a severe reduction in average daily gain was detected in low-dose infected piglets (daily weight gain in dpi0-9, LT1: 66.7 vs. LT2: -11.1 g/d). Experimental condition was different between the experiment, of course, but a hypothesis could be shown this adverse phenomenon. Diarrhea defecation was stopped in 2 of 3 piglets in 1011 CFU infected condition at the dpi 9, whereas all piglets in 1010 CFU infected condition was not cure the diarrhea at this time. Based on these results, it was speculated that excessive infection might have induced a rapid excretion of the pathogenic ETEC from the intestine. The continuous body weight reduction observed in low-dose infected piglets supported this hypothesis. Nonetheless, as the number of piglets used in the present work was limited, to fully validate these results, our observations need further investigation.
When comparing the usefulness of MUC4 genotyping [6] and our newly developed method, some merits and drawbacks can be noted. For example, while MUC4 genotyping (i.e., DNA extraction and RFLP genotyping) is relatively easy to carry out, this technique has shown that some piglets develop resistance against ETEC infection [6]. Based on this evidence, we believe that using MUC4 genotype selection to assess ETEC infection, in the same manner as Matsumoto et al. did [6], would be futile to analyze piglets showing resistance to ETEC. By contrast, we acknowledge that our method was complicated by logistics and required specialized techniques such as colostrum restriction and capsule inoculation. However, for the analysis, we were able to use all piglets delivered from the sows. Taking into account the aforementioned merits and drawbacks, we concluded that our method could be particularly useful if suckling piglets are to be used to assess ETEC infection.
It must be noted that the present work had limitations. First, the low number of animals used to evaluate the newly developed experimental model might have subtracted some robustness from the study. However, the experimental animals were examined three times (Ex1, Ex2 and validation), and those infected with ETEC had diarrhea and a typical reduction in growth performance. Moreover, the present method has already been used to evaluate the effectiveness of antimicrobial-alternative agents against ETEC infection in 3-5 piglets in the three other, unrelated experiments (Hamabata et al., unpublished data). Therefore, it can be inferred that, based on the consecutive successes using the newly developed model, our experimental methods (i.e., colostrum depletion and chitosan capsule inoculation) are some of the most effective experimentally-infecting methods to evaluate anti-ETEC infection compounds. Second, for the newly developed method, we did not determine the optimal number of ETEC cells to cause diarrhea defecation and body weight reduction. Therefore, this ambiguity needs further investigation. Third, we assessed the successfulness of ETEC infection using only one strain. It goes without saying that the level of pathogenicity partly depends on the strain, and that diarrheal defecation and body weight reduction may not be observed if a different strain is used. Therefore, we recommend that, for future research and whenever possible, a highly pathogenic ETEC be used. Fourth, in the present work we selected the challenging strain in the feces by PCR, which directly detected the virulence gene. However, the PCR methodology is unable to assess the viability of microbes during their travel throughout the gastrointestinal track. Thus, the viability of ETEC strain used in the present study was unclear. Nonetheless, continuous diarrheal defecation and body weight reduction in piglets seemed to indirectly confirm that the challenging strain had a good viability.
Conclusion
An experimental infection of ETEC, using our previously described method to cause STEC infection [7,8], successfully caused incidental diarrhea in piglets inoculated not only with 1011 CFU/head (maximal challenging dose) of ETEC cells grown in CFA medium, but also with 1010 CFU/head (giving 1/10 doses in capsules was easier than 1011 CFU/head) of ETEC cells grown in TS broth. We recommend the newly developed method (1010 CFU/head of ETEC cells grown in TS broth in chitosan-coated capsules) to effectively induce experimental ETEC infection in colostrum-restricted piglets because, in the present work, it caused 100% diarrheal defecation and weight reduction in piglets.
Author Statements
Acknowledgements
This work was supported by a Grant-in-Aid from the ‘Development of fundamental technologies for production of high-value materials using transgenic plants’ project managed by the Ministry of Economy, Trade and Industry, of the Government of Japan. The authors would like to thank Messrs. Sachio Chomei and Kei-ichi Ohya of the KYODOKEN Institute for providing care for the animals. We also thank Messrs. Keizo Nakayama and Motohiro Nishikawa of the Kyoto Institute of Nutrition & Pathology for conducting the histopathological study.
Declaration of Interest
The authors declare there is no conflict of interests.
References
- DebRoy C, Maddox CW. Identification of virulence attributes of gastrointestinal Escherichia coli isolates of veterinary significance. Anim Health Res Rev. 2001; 2: 129-40.
- Luise D, Lauridsen C, Bosi P, Trevisi P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J Anim Sci Biotechnol. 2019; 10: 53.
- Ushida K, Kishimoto A, Piao SJ, Itoh M, Shiga A, Nakanishi N, et al. An epidemiological survey on pigs showing symptoms of infectious enteric diseases and dyspepsia in Japan. Anim Sci J. 2009; 80: 556-61.
- Porter CK, Riddle MS, Tribble DR, Louis Bougeois AL, McKenzie R, Isidean SD, et al. A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine. 2011; 29: 5869-85.
- Tsukahara T, Nakanishi N, Nakayama K, Matsubara N, Ushida K. Experimental infection of enterotoxemic Escherichia coli associated with porcine edema disease and its pathogenic characteristics in the intestine. J Vet Med Sci. 2005; 67: 1167-71.
- Matsumoto H, Miyagawa M, Takahashi S, Shima R, Oosumi T. Improvement of the enterotoxigenic Escherichia coli infection model for post-weaning diarrhea by controlling for bacterial adhesion, pig breed and MUC4 genotype. Vet Sci. 2020; 7: 106.
- Sato T, Hamabata T, Takita E, Matsui T, Sawada K, Imaoka T et al. Improved porcine model for Shiga toxin-producing Escherichia coli infection by deprivation of colostrum feeding in newborn piglets. Anim Sci J. 2017; 88: 826-31.
- Hamabata T, Sato T, Takita E, Matsui T, Kawabata T, Imaoka T, et al. Shiga toxin 2eB-transgenic lettuce vaccine: N-glycosylation is important for protecting against porcine edema disease. J Vet Med Sci. 2021; 83: 1708-14.
- Evans DG, Evans DJ Jr, Clegg S, Pauley JA. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect Immun. 1979; 25: 738-48.
- Tsukahara T, Kishino E, Inoue R, Nakanishi N, Nakayama K, Ito T, et al. Correlation between villous height and the disaccharidase activity in the small intestine of piglets from nursing to growing. Anim Sci J. 2013; 84: 54-9.
- Tsukahara T, Inoue R, Nakayama K, Inatomi T. Inclusion of Bacillus amyloliquefaciens strain TOA5001 in the diet of broilers suppresses the symptoms of coccidiosis by modulating intestinal microbiota. Anim Sci J. 2018; 89: 679-87.
- Ram S, Vajpayee P, Shanker R. Rapid culture-independent quantitative detection of enterotoxigenic Escherichia coli in surface waters by real-time PCR with molecular beacon. Environ Sci Technol. 2008; 42: 4577-82.
- Inoue R, Tsukahara T. Composition and physiological functions of the porcine colostrum. Anim Sci J. 2021; 92: e13618.
- Quesnel H, Farmer C, Devillers N. Colostrum intake: influence on piglet performance and factors of variation. Livest Sci. 2012; 146: 105-14.
- Tsukahara T, Iwasaki Y, Nakayama K, Ushida K. Microscopic structure of the large intestinal mucosa in piglets during an antibiotic-associated diarrhea. J Vet Med Sci. 2003; 65: 301-6.
- Moon HW, Isaacson RE, Pohlenz J. Mechanisms of association of enteropathogenic Escherichia coli with intestinal epithelium. Am J Clin Nutr. 1979; 32: 119-27.
- Boeckman JX, Sprayberry S, Korn AM, Suchodolski JS, Paulk C, Genovese K, et al. Effect of chronic and acute enterotoxigenic E. coli challenge on growth performance, intestinal inflammation, microbiome, and metabolome of weaned piglets. Sci Rep. 2022; 12: 5024.
- Fukushima H, Tsunomori Y, Seki R. Duplex real-time SYBR Green PCR assays for detection of 17 species of food- or waterborne pathogens in stools. J Clin Microbiol. 2003; 41: 5134-46.
- Zhang W, Zhao M, Ruesch L, Omot A, Francis D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet Microbiol. 2007; 123: 145-52.