
Review Article
Austin Immunol. 2018; 3(1): 1015.
The Detection and Identification of Mixed Strains of Fusobacterium necrophorum in Foot Rot Lesions of Sheep in Kashmir, India
Farooq S1*, Wani SA2, Hassan MN3, Aalamgeer S2, Kashoo ZA2, Magray SN2 and Bhat MA4
1KVK-Kupwara, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir (SKUAST-K), India
2Division of Veterinary Microbiology and Immunology, SKUAST-K, India
3KVK-Nyoma, SKUAST-K, India
4Division of Veterinary Microbiology and Immunology, Sher-e-Kashmir University of Agricultural Sciences and Technology of Jammu (SKUAST-J), India
*Corresponding author: Shaheen Farooq, Subject Matter Specialist (Animal Science), Krishi Vigyan Kendra, Kupwara, India
Received: March 01, 2018; Accepted: March 21, 2018; Published: March 28, 2018
Abstract
The objective of this study was to determine the prevalence and identification of lktA variant strains of F. necrophorum in the foot rot lesions of sheep. The detection of F. necrophorum was carried out by Polymerase Chain Reaction (PCR) targeting the leukotoxin (lktA) gene fragment and identification of lktA variant strains was done by PCR–Single Stranded Conformational Polymorphism (PCR-SSCP) and gene sequencing of the 450 swabs collected from foot rot lesions of sheep, 117 were positive for F. necrophorum of the 50 swabs collected from apparently asymptomatic sheep, only one was positive for F. necrophorum. The overall prevalence of F. necrophorum in foot rot affected sheep in Kashmir valley was 26%, with the highest prevalence of 34.8% recorded in samples from the districts of Kulgam and Pulwama, and the lowest (20%) in Baramulla district. PCR-SSCP of lktA gene fragment revealed the presence of three lktA variants, designated as JKS-F1/ F2/ F3, while two samples (1.70%) showed the presence of multiple lktA variant strains of F. necrophorum on a single foot rot affected sheep hoof. The JKS-F3 was the most frequent (75.4%), followed by JKS-F2 (14.4%) and JKS-F1 (8.4%) lktA variant, respectively. Of the three lktA variants identified, JKS-F3 was detected in 74 (86.0%) out of 86 samples taken from foot rot affected sheep with lesion score 4. The data suggest that JKS-F3 is the predominant and most adapted lktA variant of F. necrophorum and is associated with severe foot rot in sheep, hence a significant variant contributing to the severity and duration of the disease. This appears to be the first report on the presence of more than one lktA variant of F. necrophorum in a foot rot lesion of sheep.
Keywords: Fusobacterium necrophorum; Ovine foot rot; PCR-SSCP; lktA gene
Introduction
Foot rot is a contagious bacterial infection of the feet of sheep that causes lameness, and significant production and economic losses worldwide. The disease is characterized by an exudative inflammation, followed by necrosis of the epidermal tissue of the inter digital skin and hoof matrix, resulting in separation of the hoof from the underlying soft tissue. The affected animal’s exhibit lameness, loss of body weight, reduced wool and meat production [1]. The infectious syndrome is caused by the synergistic action of several bacterial species, particularly Dichelobacter nodosus, and Fusobacterium necrophorum [2]. Both the anaerobes are found together at a significantly higher rate in severe foot rot lesions in sheep in which D. nodosus drives the pathogenesis of foot rot from initiation of Interdigital Dermatitis (ID) to Severe Foot Rot (SFR) and F. necrophorum contributing to the severity and duration of SFR [3,4].
F. necrophorum is a Gram negative, anaerobic, pleomorphic and non-spore-forming bacterium, which is more sensitive to oxygen than D. nodosus [5]. It is a normal resident of manurecontaminated environments. The F. necrophorum has two subspecies; F. necrophorum subsp. necrophorum (formally biovar A) and F. necrophorum subsp. funduliforme (formally biovar B). The subsp. necrophorum is more pathogenic due to a higher lipopolysaccharide content and higher production of leukotoxin [6]. Among a variety of virulence factors, the best described and major virulence factor is leukotoxin, encoded by lktA gene. The lktA gene has been used for the detection of F. necrophorum and to determine variation among its strains [7].
In India foot rot is endemic in the states of Jammu and Kashmir, Andhra Pradesh, Tamil Nadu, Uttar Pradesh and Himachal Pradesh [8,9]. Recently, its severity has increased and attained significant importance in sheep husbandry practices [10,11]. There is very little knowledge about the lktA variant(s) associated with severe foot rot in sheep (lesion score 4) and no study has described the most predominant lktA variant(s) of F. necrophorum in ovine foot rot lesions. The aim of the present investigation was to study the prevalence, isolation and identification of predominant lktA variant of F. necrophorum that is linked to severe foot rot in sheep.
Materials and Methods
Survey
The present survey was conducted from 2012 to 2016 to determine the prevalence of F. necrophorum in foot rot-affected sheep of Kashmir valley. A two-stage simple random sampling procedure was followed. A complete list of all villages falling under the administrative control of these districts were obtained from district offices of respective districts and 5% of villages from each district were selected through a Simple Random Sampling (SRS) procedure. Subsequently, a complete list of household flocks in the selected villages was prepared and again simple random sampling (second stage sampling) was followed to select household flocks. Again 5% of household flocks were selected. These household flocks were finally visited to determine the prevalence of F. necrophorum in foot rot affected sheep.
Collection of clinical samples
A total of 500 swab samples (450 from symptomatic and 50 from healthy asymptomatic sheep) were collected from ninety nine foot rot affected flocks across the valley as detailed in (Table 1). The samples constituted duplicate swab samples of exudates of foot rot lesions with a lesion score of 2 (ID) to 4 (SFR i.e under running of the hard horn of the hoof) collected at the active lesion that developed between the horn of the hoof and the sensitive underlying tissue. One sample from each case was inoculated onto media for isolation and other used for DNA extraction for direct detection of F. necrophorum. Only one foot per sheep was sampled. The swabs used for DNA extraction were transported to laboratory in thioglycolate broth (Hi Media, Mumbai, India). The sampling was done by a single trained researcher to minimize the variation.
S. No.
No. of flocks inspected
No. of samples collected from footrot affected sheep
Samples positive for F.n*
No. of samples collected from healthy sheep
Samples positive for F.n
Distribution of lktA variants in positive samples
Distribution of lktA variants in SFR with LS*=4
Prevalence of F.n (%)
JKS*-F1/F2/F3/MSI*
JKS*-
F1/F2/F3/MSI
1
3
21
5
2
-
0 2 3 0
0 1 3 0
23.8
2
33
150
40
15
-
4 7 27 2
1 2 24 2
26.7
3
6
23
8
3
-
1 1 6 0
0 0 5 0
34.8
4
8
36
9
4
-
1 1 7 0
0 0 4 0
25
5
16
90
19
10
01
3 3 14 0
1 2 11 0
21.1
6
5
23
8
2
-
0 1 7 0
0 0 5 0
34.8
7
4
28
9
3
-
0 1 8 0
0 1 7 0
32.1
8
5
24
7
3
-
0 0 7 0
0 0 6 0
29.1
9
9
25
5
4
-
0 1 4 0
0 1 4 0
20
10
10
30
7
4
-
1 0 6 0
0 1 5 0
23.3
99
450
117
50
01
10 17 89 2
2 8 74 2
26
*Fusobacterium necrophorum (F.n)
*Accession numbers of the strains JKSF1/F2/ F3 are JF911484.1, JX104210.1 & JX104211.1, respectively
*MSI= Multiple Strain Infection
* LS= Lesion score
Table 1: Depicting samples and lktA variant distribution of Fusobacterium necrophorum vis-à-vis Severe Foot Rot (SFR) in sheep.
Extraction of DNA
The swab samples in thioglycolate broth were vortexed briefly to suspend swabbed material and swabs were then removed and broth subjected to centrifugation at 5000×g for 10 min. The supernatant was discarded and suspensions of the pelleted material were prepared in 1.5 ml micro centrifuge tubes in 150 µl of sterile Phosphate Buffered Saline (PBS) by gentle vortexing. The samples were boiled for 5 min, cooled on ice for 10 min and centrifuged at 10,000×g for 1 min. Two microlitres of the supernatant were used as the template for each PCR reaction.
Detection of Fusobacterium necrophorum
All the samples were subjected to the lktA gene specific PCR for detection of F. necrophorum as detailed by [12]. The PCR conditions consisted of initial denaturation at 94oC for 4min, followed by 35 cycles of 94oC for 30s, 60oC for 30s and 72oC for 40s. This was followed by final extension of 5 min at 72oC. The DNA from F. n. subsp. necrophorum strain, which was isolated by the Division of Veterinary Microbiology and Immunology and confirmed by sequencing of its lktA gene fragment, served as positive control.
Isolation and identification of Fusobacterium necrophorum from clinical samples
The fifty swab samples positive for F. necrophorum by PCR were inoculated on Brain-Heart-Infusion-Blood-Agar (BHIBA, Difco) containing 10% defibrinated sheep blood, 0.5% yeast extract, 0.01% magnesium sulphate and antimicrobials (vancomycin and neomycin) @ 5µg and 100 µg/ml of media, respectively. After inoculation, the plates were placed immediately in a 3.5 liter anaerobic jar (Oxoid, UK) with requisite number of AnaeroGaspack (Becton and Dickinson, USA). After 48-72 h of incubation at 37°C, suspected colonies which appeared flat, irregular, grayish and surrounded by zone of ß-haemolysis were subcultured on the same medium until they were free from contaminating bacteria. Confirmation of the isolates as F. necrophorum was done by demonstration of the typical cellular morphology in Gram-stained smears, a biochemical test for lipolytic activity of isolates on egg yolk agar and detection of the lktA gene fragment by PCR as described above.
Analysis of strain variation of Fusobacterium necrophorum isolates through Single Strand Conformational Polymorphism (SSCP)
The lktA gene fragment of F. necrophorum was amplified by PCR as described earlier. A 5µl aliquot of each amplicon was mixed with 15µl loading dye (98% formamide, 10mM EDTA, 0.025% bromophenol blue and 0.025% xylene cyanol), and after denaturation at 95°C for 5min, instantly, samples were snap-cooled on ice for 15 min. Electrophoresis was performed in an SE 600 Ruby electrophoresis unit(GE Healthcare) using a12% polyacrylamide gel (37.5:1) at 300 V for 18 h at 4°C in 0.5 X TBE buffer, and gels were silver stained as per the protocol of [13].
Cloning of variant lktA amplicons
Representative amplicons with unique SSCP patterns in gel were purified using the MinElute PCR Purification Kit (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. The PCR products were ligated into the p-Drive Cloning Vector (Qiagen) according to the manufacturer’s recommended protocol. The ligation mixture was used to transform DH5a Escherichia coli cells by electroporation. Five positive colonies for each transformation were picked up and transferred into Luria Bertani (LB) broth (Hi Media, India) then incubated with shaking (200rpm) overnight at 37°C in a rotary shaking incubator (JEIO TECH, Korea).
DNA sequencing and analysis
The plasmid DNA from selected colonies were extracted using a QIAprep® Miniprep Kit (Qiagen, Hilden, Germany) and the concentration of the DNA adjusted to 200 ng/ml. The DNA was sequenced commercially by Macrogen Inc., Korea, using M13 universal primers. Sequence alignments and translation were performed using Basic Local Alignment Search Tool (BLAST), Fast PCR (Primer Digital Ltd, Helsinki, Finland) and Clustal X (Genome Net, Japan) and the phylogenetic tree was constructed using software MEGA5.
Results
Prevalence and lktA variant distribution of Fusobacterium necrophorum in ovine foot rot
Out of 450 swab samples collected from foot rot affected sheep, 117 (26.0%) were detected positive as revealed by 400 base pair (bp) amplicon characteristic of the lktA gene fragment of F. necrophorum (Figure 1). Among 50 swabs collected from clinically healthy sheep only one sample carried F. necrophorum. Of the 118 F. necrophorum positive samples, 89 (75.4%) samples carried JKS-F3 lktA variant, while JKS-F1 & F2 variants were observed in 10 (8.4%) and 17 (14.4%) samples, respectively. Out of 117 positive samples, 86 (73.5%) were collected from affected sheep with lesion score 4, while rest of positive swabs (31) belonged to lesion scores of 2 and 3, respectively. Among samples with lesion score 4, 74 (86%) samples carried JKS-F3 lktA variant of F. necrophorum (Table 1).
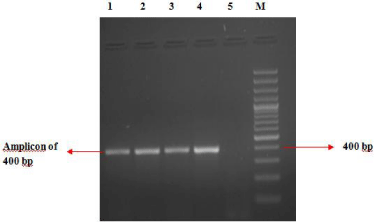
Figure 1: Detection of lktA gene fragment of Fusobacterium necrophorum by
Polymerase Chain Reaction (PCR).
Lane 1 to 3 = Test samples; Lane 4 =Positive control; Lane 5 =Negative
control; Lane M = 100 bp DNA ladder
Isolation of Fusobacterium necrophorum from clinical samples
Out of fifty samples tested positive for the lktA gene, 41 (82%) yielded the isolates in pure culture. After subsequent subculture on the same medium (BHIBA), pure colonies of F. necrophorum were obtained. The pure colonies of F.necrophorum produced characteristic lipolytic activity on egg yolk agar medium.
Strain variation of Fusobacterium necrophorum
The SSCP analysis of the lktA gene fragment of 118 samples (117 from foot rot affected and one from healthy asymptomatic sheep) found positive for F. necrophorum, revealed four different banding patterns (Figure 2) designated as JKS-F1, JKS-F2, JKS-F3 and JKS-F4, respectively. Three of these patterns revealed two bands each while the fourth banding pattern (JKS-F4) revealed unusually multiple (six) bands. This fourth banding pattern was observed in two samples only. To ascertain the status of this unusual banding pattern the amplified products of these two samples were cloned as described above. All the transformed colonies obtained from these two samples were grown separately, plasmid DNA extracted and subsequently subjected to PCR-SSCP analysis which revealed three banding patterns similar to JKS-F1, JKS-F2 and JKS-F3 indicating mixed strain infection of all the three lktA variants of F. necrophorum on a single infected sheep hoof. The DNA sequences of these representative amplicons have been deposited into the NCBI Gen Bank with the accession numbers depicted in (Table1).

Figure 2: Strains of Fusobacterium necrophorum as observed by PCR –
SSCP of lktA gene fragment.
Lane 1= Multiple strain infection; Lane 2= JKS-F1 strain; Lane 3= JKS-F2
strain; Lane 4= JKS-F3 strain.
Phylogenetic Analysis of lktA gene variants of Fusobacterium necrophorum
A comparison was made among different lktA nucleotide sequences of F. necrophorum recorded from foot rot lesions in Kashmir valley viz JKS-F1, JKS-F2 and JKS-F3, with the nucleotide sequences of already reported lktA variants B, C and D of F. necrophorum [12], F. equinum [14] and F. necrophorum subsp. funduliforme (Fnf) [15]. The neighbor joining tree was constructed using software MEGA5 [16]. The evolutionary history was inferred using the neighbor-joining method [17]. The optimal tree with the sum of branch length = 2.43515723 is shown in (Figure 3). There were a total of 400 positions in the final dataset. The average pair wise distance between sequences is 0.84. The newly identified lktA sequences from ovine foot rot in Kashmir valley were homologous to previously reported lktA sequences such as clones B, C and D of F. necrophorum [12]. The clone B of F. necrophorum showed 99% sequence homology to our JKS-F2, while clones C and D 100% and 99% to our JKS-F3, respectively. The neighbor joining tree of the lktA gene fragment sequence also revealed JKS-F1 to be a genetically distant variant of F. necrophorum, reported so far. A close genetic relationship between variant JKS-F2 with clone B and that of variant JKS-F3 with clone C and D of Fusobacterium species was also revealed. While F. equinium and F. n. subsp. fundiliforme (Fnf) shows that these were at some genetic distance from the rest. The amino acid sequences of three variants of F. necrophorum recorded from the Kashmir valley were analyzed using the Clustal-W (CLUSTAL 2.1 multiple sequence alignment) to generate a sequence alignment report as shown in (Figure 4).
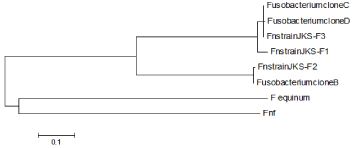
Figure 3: Neighbor-joining tree of lktA sequences. Tree was constructed using
the lktA nucleotide sequences identified from ovine footrot lesions (JKS F1/
F2/F3) and from already recorded sequences of Fusobacterium necrophorum
clones (B-D), F. equinum and F. necrophorum subsp. funduliforme (indicated
as Fnf), respectively.

Figure 4: Alignment of putative amino acid sequence of three lktA variants
(JKSF1/F2 and F3) of Fusobacterium necrophorum using the CLUSTAL 2.1
multiple sequence alignment tool.
Discussion
This paper describes the prevalence of F. necrophorum in foot rot affected sheep. The overall prevalence of F. necrophorum observed in the Kashmir valley was 26%, with the highest prevalence of 34.8% in Pulwama and Kulgam districts each and the lowest prevalence of 20% in district Baramulla. The high prevalence suggests that F. necrophorum is frequently associated with foot rot lesions as compared to clinically healthy sheep in which only one out of fifty swabs were detected positive for F. necrophorum. The highest prevalence of F. necrophorum in ovine foot rot in the districts of Pulwama and Kulgam could be because the pastures around the villages of the districts are favorable for the persistence of the organism, due to the manure contaminated environment (as F. necrophorum is a commensal in the alimentary tract and is shed in faeces), water logging and marshy areas. However, it is notable that a large portion of swabs (74.0%) were negative for F. necrophorum. This would suggest either that PCR is not sensitive enough to detect F. necrophorum at lower cell count and/or due to the presence of PCR inhibitory substances in some swabs soiled with dirt and dust that may act as a barrier to the successful amplification of DNA. Secondly, cotton swabs allow sampling from the surface but not from deeper areas of skin and hoof where anaerobic conditions favour more growth of F. necrophorum.
The current study also reveals three different lktA variants of F. necrophorum in the hooves of sheep affected with foot rot. Out of 450 swab samples collected from the lesions of foot rot affected sheep, only two samples carried all the three lktA variants of F. necrophorum in the same infected ovine hoof. This seems to be first report of its kind. This is in contrast with the findings of [12] who reported single strain infection of F. necrophorum in cattle, sheep and goats in New Zealand. However, mixed serogroup/ strain infections are quite common in case of D. nodosus where up to seven serogroups may be present on a single hoof [18,19]. The JKS-F3 was the most frequent lktA variant strain (75.4.0%) detected and was found to be associated with severe foot rot (86%) lesions in sheep. Its high frequency in ovine foot rot is likely to be due to its greater degree of adaptation to sheep in comparison to other two lktA variants of F. necrophorum. These findings show that mixed strain infections, although observed in only two samples is possible in case of F. necrophorum like D. nodosus in which multiple serogroups are detected on single foot rot affected hoof. It was also observed that in swabs in which JKS-F3 lktA variant was present, the affected sheep had severe foot rot with lesion score mostly 4 in comparison to other foot rot affected sheep in which either of the two other strains were present. These results highlight the higher degree of adaptation and virulence of JKS-F3 lktA variant of F. necrophorum in sheep, contributing to severity and duration of foot rot lesion in comparison to other two lktA variants. The reason for this is unknown and further studies in more flocks and different areas would be useful to confirm these findings.
The three lktA variants of F. necrophorum reported in the current study, isolated from sheep may represent different species of Fusobacterium as suggested by [12] as well. Further studies in this direction may confirm this opinion or otherwise. A similar phenomenon has been previously reported for “F. necrophorum” strains isolated from horses, with the reclassification of F. equinum as a new species based on phylogenetic analysis of the 16S rRNAgene, DNA-DNA hybridization and phenotypic characterization [20].
We have previously reported [19,21] that samples in which both the anaerobes were detected together, were more likely to come from sheep that had severe foot rot with lesion scores mostly 4 in comparison to other foot rot affected sheep in which F. necrophorum was absent or found alone. The present study reveals predominant and adapted lktA variant (JKS-F3) of F. necrophorum that is associated with severe foot rot in sheep, therefore, contributing to the severity and duration of disease. These findings may further contribute towards understanding the pathogenesis of ovine foot rot, in order to develop appropriate treatment and prevention measures. Hence, this lktA variant of F. necrophorum need also to be considered while managing an outbreak, or maintaining a quarantine or future strategies to combat foot rot.
Acknowledgement
The authors wish to thank the National Agricultural Innovation Project (NAIP) of the Indian Council of Agricultural Research (ICAR), New Delhi, Grant No. 4132 for financial assistance.
References
- CM Thorley. A simplified method for the isolation of Bacteroides nodosus from ovine footrot and studies on its colony morphology and serology. J. Appl. Bacteriol. 1976; 40: 301-309.
- FE Dewhirst, BJ Paster, S Lafontaine, JI Rood. Transfer of Kingella indologenes (Snell and Lapage 1976) to the genus Suttonella gen. nov. as Suttonella indologenes comb. nov.; transfer of Bacteroides nodosus (Beveridge 1941) to the genus Dichelobacter gen. nov. as Dichelobacter nodosus comb. nov.; and assignment of the genera Cardiobacterium, Dichelobacter, and Suttonella to Cardiobacteriaceae fam. nov. in the gamma division of Proteobacteria on the basis of 16S rRNA sequence comparisons. Int. J. Syst. Bacteriol. 1990; 40: 426-433.
- LA Witcomb, LE Green, LA Calvo-Bado, CL Russell, EM Smith, RG Thomas, EMH Wellington. First study of pathogen load and localisation of ovine footrot using fluorescence in situ hybridisation (FISH). Veterinary Microbiology. 2015; 176: 321–327.
- G Bennett, JG Hickford, R Sedcole, H Zhou. Dichelobacter nodosus, Fusobacterium necrophorum and the epidemiology of footrot. Anaerobe. 2009; 15: 173-176.
- GS Myers, D Parker, K Al-Hasani, RM Kennan, T Seemann, Q Ren, et al. Genome sequence and identification of candidate vaccine antigens from the animal pathogen Dichelobacter nodosus. Nat Biotech. 2007; 25: 569-575.
- ZL Tan, TG Nagaraja, MM Chengappa. Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism, and control measures. Vet Res Commun. 1996; 20: 113–140.
- TG Nagaraja, SK Narayanan, GC Stewart, MM Chengappa. Fusobacterium necrophorum infections in animals: pathogenesis and pathogenic mechanisms. Anaerobe. 2005; 11: 239-246.
- S Farooq, SA Wani, I Hussain, MA Bhat. Prevalence of ovine foot rot in Kashmir, India and molecular characterization of Dichelobacter nodosus. Indian J Anim Sci. 2010; 80: 826-830.
- D Sreenivasulu, S Vijayalakshmi, D Raniprameela, A Karthik, SA Wani, I Hussain. Prevalence of ovine footrot in the tropical climate of southern India and isolation and characterisation of Dichelobacter nodosus. Rev Sci Tech. 2013; 32: 869–877.
- Hussain I, SA Wani, SD Qureshi, S Farooq, Serological diversity and virulence determination of Dichelobacter nodosus from footrot in India. Mol Cell Probes. 2009; 23: 112-114.
- MA Rather, SA Wani, I Hussain, MA Bhat, ZA Kabli, SN Magray. Determination of prevalence and economic impact of ovine footrot in central Kashmir, India with isolation and molecular characterization of Dichelobacter nodosus. Anaerobe. 2011; 17: 73-77.
- H Zhou, G Bennett, JGH Hickford. Variation in Fusobacterium necrophorum isolates from sheep, goats and cattle infected with footrot. Vet Microbiol. 2009; 135: 363-367.
- L Svensson, UhnooI, M. Grandien, G. Wadell. Molecular epidemiology of rota virus infections in Uppsala, Sweden: Disappearance of a predominant electropherotype. J Med Virol. 1986; 18: 101-111.
- H Zhou, G Bennett, RM Kennan, JI Rood, JGH Hickford. Identification of a leukotoxin sequence from Fusobacterium equinum. Vet Microbiol. 2009; 133: 394-395.
- AM Oelke, TG Nagaraja, MJ Wilkerson, GC Stewart. The leukotoxin operon of Fusobacterium necrophorum is not present in other species of Fusobacterium. Anaerobe. 2005; 11: 123-129.
- K Tamura, J Dudley, M Nei, S Kumar. MEGA5: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0, Mol. Biol. Evot. 2007; 2: 1596-1599.
- N Saitou, M Nei. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987; 4: 406-425.
- H Zhou, JG Hickford. Novel fimbrial subunit genes of Dichelobacter nodosus: recombination in vivo or in vitro?. Vet Microbiol. 2000; 76: 163-174.
- S Farooq, SA Wani, MN Hassan, N Nazir, QJ Nyrah. The detection of Dichelobacter nodosus and Fusobacterium necrophorum from ovine footrot in Kashmir, India. Anaerobe. 2015; 35: 41-43.
- M Dorsch, DN Lovet, GD Bailey. Fusobacterium equinum sp. nov., from the oral cavity of horses. Int J Syst Evol Microbiol. 2001; 51: 1959-1963.
- LA Witcomb LE Green, J Kaler , Ul-Hassan A, LACalvo-Bado , GF Medley, et al. A longitudinal study of the role of Dichelobacter nodosus and Fusobacterium necrophorum load in initiation and severity of footrot in sheep. Prev Vet Med. 2014; 115: 48-55.