
Research Article
Austin J Anat. 2018; 5(1): 1076.
Ameliorative Effect of Dark Chocolate on Brain and Heart of Chronic Restraint Stressed Male Albino Rats
Salama RM and El-Sherif NM*
Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Egypt
*Corresponding author: El-Sherif NM, Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Egypt
Received: December 11, 2017; Accepted: January 04, 2018; Published: January 11, 2018
Abstract
Objective: The aim was to investigate for the first time, the ameliorative effect of Dark Chocolate (DC) against oxidative stress and apoptosis in heart and brain frontal cortex of rats subjected to Chronic Restraint Stress (CRS).
Methods: 24 male albino rats were divided into four experimental groups of six rats each: Group I (Control rats), Group II (Control rats treated with DC) Group III (stressed rats) exposed to CRS by placing the rats in restrainer (20 cm × 7 cm plastic tubes) for 2 h/day for 21 days and Group IV (Treated rats).
After 21 days, the rats were sacrificed; blood sample was taken by cardiac puncture for biochemical analysis of Lipid Peroxidase (LPO), Superoxide Dismutase (SOD) & Catalase (CAT). Heart & brain tissues were removed carefully for histological (H&E staining) and immune histochemical analysis of oxidative stress and inflammatory marker (inducible Nitric Oxide Synthase (iNOS) and apoptotic marker (Caspase 3).
Results: Administration of dark chocolate by stressed rats improves their antioxidant status by reducing the indices of lipid peroxidation (LPO, SOD & CAT). Also DC attenuates the restraint stress-induced histopathological changes in brain and heart tissues and resulted in a significant decrease in the mean area % of iNOS and Caspase 3 immunoreactivity.
Conclusion: The results suggest that dark chocolate had antioxidant and antiapoptotic effects, able to block the stress-induced oxidative stress and apoptosis in the heart and brain tissues of rats. These results may be attributed to anti-oxidant flavonoids contained in dark chocolate.
Keywords: Dark chocolate; Chronic restraint stress; iNOS & caspase 3
Introduction
Stress has become an increasingly popular and widely applied term in our everyday languages. People have witnessed the physical damage of stress that can work on the body [1]. Stress disturbs the normal physiological equilibrium which induces emotional and physical strain which has been considered the basic factor in the changes in the different organ systems, including the cardiovascular system [2], gastro-intestinal system [3] and Central Nervous System (CNS) [4,5].
Chronic Restraint Stress (CRS) model is an easy, suitable and most commonly employed in laboratory animals, from the various stress models available for investigation of oxidative stress– related tissue changes, as it effectively mimics chronic exposure to psychological stress in humans that results in increased oxidative stress and resultant tissue damage [6].
It has been suggested that CRS results in the creation of Reactive Oxygen Species (ROS) e.g hydrogen peroxide (H2O2), hydroxyl radical and superoxide anion radical that cause lipid peroxidation, especially in membrane, playing an important role in tissue injury [7] and induce apoptosis [8].
Dark Chocolate (DC) with its high cocoa content, contains a large percentage of antioxidant molecules, mainly flavonoids, that has several beneficial health effects on the brain by inducing widespread stimulation of brain perfusion, provoking angiogenesis, neurogenesis and changes in neuron morphology, mainly in regions involved in learning and memory. Also chocolate induces positive effects on mood and is often consumed under emotional stress [9]. Consumption of dark chocolate are thought to possess cardioprotective properties and lowers cardiovascular mortality due to the contained high levels of polyphenolic flavonoids that exhibit anti-oxidant health benefits preventing cardiovascular disease [10,11]. Also Sentürk and Günay, [12] reported that coronary artery disease, hypertension, heart failure, hyperlipidemia, inflammation and oxidative stress were the favorable effects of flavonoid-rich dark chocolate.
The present study aims to review the effects DC on oxidative stress and apoptosis induced in heart and brain tissues of rats subjected to CRS.
Materials and Methods
Drugs & chemicals
Dark chocolate were purchased from local market, Cairo, Egypt.
All markers were purchased from local distributer (Sigma chemical) Cairo, Egypt.
Induction of chronic restraint stress
Restraint Stress was induced in rats by putting them in 20 cm × 7 cm plastic tubes for 2 h/day for 21 days [13]. There are several 3 mm holes at the both ends of the tubes which allow sufficient air for breathing and the rats were unable to move.
Experimental design
Twenty four adult male albino rats weighing 180 to 200 g were used in this study. All rats were maintained under constant room temperature and standard conditions, with ad libitum access to water and commercial food and were placed in individual plastic cages with soft bedding.
Animals were randomly divided into four experimental groups of six rats each:
Group I: (Control rats): Rats were not subjected to CRS.
Group II: (Control rats treated with DC): DC was administered at a dose of 500 mg/Kg body weight per day, as a solution in water using an intragastric tube, for 21 days to rats which were not subjected to CRS.
Group III: (Stressed rats): Rats were exposed to CRS for 2 h/day for 21 days.
Group IV: (Treated rats): Rats were exposed to CRS for 2 h/day & post-orally treated with DC at a dose of 500 mg/Kg body weight per day, as a solution in water using an intragastric tube, for 21 days.
Following each stress session, rats were returned to home cages and were able to access food and water freely for the reminder of the day.
After the experimental period, blood sample was taken from rats by cardiac puncture for biochemical analysis of Lipid Peroxidase (LPO), Superoxide Dismutase (SOD) & Catalase (CAT). Then all rats were anesthetized and sacrificed by cervical decapitation. Ventricular wall of the heart and frontal cortex of the brain were removed carefully for Histopathological (H& E staining) and immunohistochemical analysis of inducible Nitric Oxide Synthase (iNOS) and caspase 3.
Biochemical analysis
Serum was obtained from cardiac blood samples by centrifugation. The concentration of Lipid Peroxidase (LPO), Superoxide Dismutase (SOD) & Catalase (CAT) in blood are used as an indicator of lipid peroxidation (a biomarker of protective oxidative injury).
Histopathological examination
The heart and brain were removed and fixed in 10% formalin solution and processed routinely by embedding in paraffin and then the heart and frontal brain cortex were sectioned into 5 μm sections and stained with hematoxylin & eosin stain for L/M study.
Immunohistochemistry
Analysis of inducible Nitric Oxide Synthase (iNOS): Kits used: Ready to use target retrieval solution (S1700, Dakocytomation), primary antibody rabbit polyclonal IgG specific for iNOS enzyme in mouse and rat (M-19/Sc 650, Santa Cruz Biotechnology) with no cross reactivity for nNOS or eNOS enzymes and ready to use antibody diluent with background reducing components (S3022, Shireen A. Mazroa et al. 237 Dakocytomation). Universal detection kits (K 0673, Dakocytomation) based on a modified avidin-biotin (LAB) technique in which a biotinylated secondary antibody forms a complex with peroxidase conjugated streptavidin molecule 29. Steps of iNOS immune-staining: Sections of heart and brain tissues were dewaxed in xylol for 20 minutes (two changes) and hydrated in descending grades of alcohol down to distilled water. They were immersed into preheated target retrieval solution to 95- 99°C (without boiling) in water bath for 40 minutes, removed from the bath and allowed to cool for 20 minutes at room temperature. Sections were rinsed 3 times with Phosphate Buffered Saline (PBS). Excess liquid was tapped off the slides. Enough hydrogen peroxide was applied to cover the specimen for 5 minutes, then slides were rinsed gently with PBS and excess liquid was tapped off. Enough amount of primary antibody (dilution 1: 100) was applied on specimens and was incubated for two hours in humidity chamber at room temperature. Slides were rinsed in PBS. Biotinylated link was applied on specimens for 10 minutes and sections were rinsed in PBS. Streptavidin HRP reagent was applied on specimens for 10 minutes and sections were then rinsed in PBS. Freshly prepared DAB substrate chromogen solution (1 drop of DAB chromogen /1 mL of substrate buffer) was removed from 2-8°C storage and applied on specimens for 10 minutes. Slides were rinsed gently in distilled water, immersed in haematoxylin for 1/2 minute and were rinsed in tab water until blue. Slides were dehydrated in ascending grades of alcohol, cleared in xylol, mounted by Canada balsam and covered with a cover slip. Negative control slides were prepared by the same steps except they were incubated with the antibody diluent instead of primary antibody. Positive reaction appeared brown in color [14,15].
Analysis of caspase 3
Immunohistochemical detection of caspase-3 was performed using primary rabbit anti-rat caspase-3 antibody. The avidin–biotin complex technique was used. Serial frozen heart and brain sections (5 μ\m) were allowed to equilibrate to room temperature and exposed to acetone for 10 min before starting the streptavidin–biotin-staining technique (Vectastain Universal Quick Kit; Vector Laboratories). Sections then were preblocked with 10% horse serum/PBS + 0.2% Tween 20 for 20 min at room temperature followed by elimination of excess serum from the session and incubation with specific antibody and isotype-matched control antibody at dilutions (1:500 dilution) [16]. After washing, bound antibodies were detected by a universal biotinylated antibody prediluted in TBS at room temperature for 20 min followed by incubation for 10 min with a peroxidaseconjugated streptavidin. ABC (3-amino-9-ethylcarbazole) was used as colorimetric substrate. Sections were rinsed, counterstained, and mounted in aqueous mounting medium. Analysis of tissue sections was performed by light microscopy.
Positive reaction for caspase 3 was visualized as brown coloration of the cytoplasm of the cardiac and neural cells. Negative controls were done using the same steps except that phosphate buffered saline was applied instead of the primary antibodies [17].
Statistical analysis
Statistical analyses were carried out with the Statistical Package for the Social Sciences for Windows 11.5 (SPSS Inc., Chicago, IL, USA). All values were given as mean ± standard deviation. Differences between groups were statistically analyzed by Kruskal–Wallis test (ANOVA) and chi-square test. Statistical significance was accepted asP < 0.05.
Results
Biochemical results
Dark chocolate administration in (Group IV) ameliorated the changes in antioxidant and oxidative stress indices in stressed rats (Group III). The heart and brain tissue of stressed rats (Group III) had significant greater levels of enzymatic activities of the antioxidants SOD, CAT and LPO (as an index of lipid peroxidation) compared to the control rats (Groups I & II). In parallel, dark chocolate caused a significant reduction in the SOD and CAT, as well as the levels of LPO as compared to stressed rats (Tables 1 and 2). These results demonstrate that dark chocolate antioxidant capability is able to block the stress-induced oxidative stress in the heart and brain tissues of rats.
Parameters
Group I
Group II
Group III
Group IV
SOD (U/min/mg protein)
22.19±3.32
21.76±2.89
40.25±2.14000
29.11±4.12***
CAT (μ mol of H2O2 decomposed/min/mg protein)
13.22±0.18
13.87±0.32
24.13±0.67000
17.35±0.85***
LPO (U/mg protein)
9.11±0.26
9.35±0.45
21.16±1.98000
19.59±1.73***
000Significant increase compared to group I (p < 0.001).
***Significant decrease compared to stressed rats (p < 0.05).
Table 1: Biochemical parameters on heart tissue marker enzymes in all groups.
Parameters
Group I
Group II
Group III
Group IV
SOD (U/min/mg protein
8.36±0.12
8.60±0.15
14.19±1.19000
9.23±6.92***
CAT (μ mol of H2O2 decomposed/min/mg protein)
3.36±0.95
4.11±1.02
7.85±4.28000
5.92±1.65***
LPO (U/mg protein)
1.68±0.59
1.99±0.85
3.27±1.46000
2.61±0.45***
000Significant increase compared to group I (p < 0.001).
***Significant decrease compared to stress rats (p < 0.05).
Table 2: Biochemical parameters on brain tissue marker enzymes in all experimental groups.
Histological and immune histochemical results
There was no significant difference between Group I (Control) and Group II (Control rats treated with DC) in all the outcomes at each time point used in the study; therefore, these two groups were pooled in one group (Control).
Heart
In control rats, the H&E staining cardiac sections showed branching and anastomosing cardiac muscle fibers in different directions. Blood vessels were found between the cardiac muscle fibers. Cardiomyocytes had acidophilic sarcoplasm and central vesicular nuclei. Capillaries were seen in the connective tissue endomysium between the cardiac muscle fibers (Figures 1 and 2).
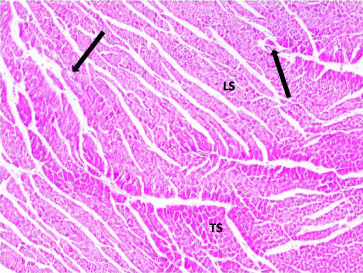
Figure 1: Photomicrograph of a section in the ventricular wall of cardiac
muscle of a control rat (Group I) showing a normal histological architecture of
cardiac myocytes, most of which appear Longitudinally (LS) and Transversely
(TS) cut. Note the presence of blood vessels (arrows) in between the cardiac
myocytes. H&E, × 200.
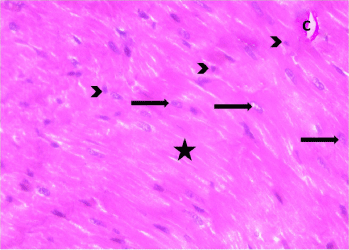
Figure 2: Photomicrograph of a myocardial section from a control rat (Group
II) showing organized cardiac muscle fibers (asterisks) that exhibit acidophilic
sarcoplasm and centrally located vesicular nuclei (arrows). Normal blood
Capillaries (C) and dark nuclei of fibroblasts (arrow heads) are seen within
the endomysium between the cardiac muscle fibers. H&E, × 400.
Myocardial sections from stressed rats (Group III) showed that cardiomyocytes were separated from each other by wide intercellular spaces. Some of the cardiac muscle fibers showed degeneration, cellular infiltration and extravasation of blood. Many fibers exhibited perinuclear cytoplasmic lysis (Figures 3- 5).
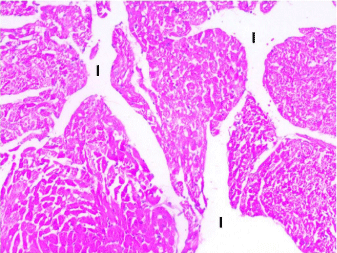
Figure 3: Photomicrograph of a section in the ventricular wall of cardiac
muscle of stressed rat (Group III) showing wide separation of the cardiac
muscle fibers with increase in the interfiber spaces (I). H&E, × 200.
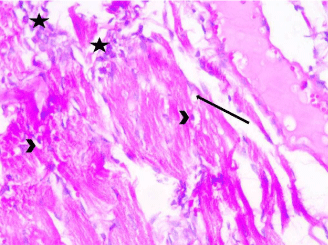
Figure 4: Photomicrograph of a section in the ventricular wall of cardiac
muscle of stressed rat (Group III) showing areas of cellular infiltration (star)
between degenerated cardiac myocytes. Myocardium shows vacuolated
sarcoplasm (arrows). Some muscle fibers exhibit perinuclear cytoplasmic
depletion (myolysis) (arrow head). H&E, × 400.
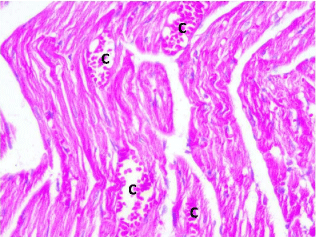
Figure 5: Photomicrograph of a section in the ventricular wall of cardiac
muscle of stressed rat (Group III) showing distorted muscle fibers with dilated
congested blood Capillaries (C) and extravasation. H&E, × 400.
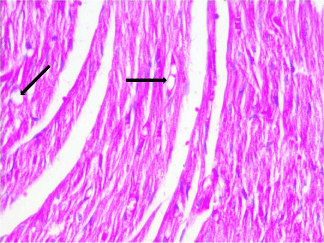
Figure 6: Photomicrograph of a section in the ventricular wall of cardiac
muscle of stressed rat treated with DC (Group IV) showing nearly organized
cardiac muscle fibers however, vacuolated sarcoplasm (arrows) was still
present. H&E, × 400.
The mean area % of iNOS immunopositive cells showed a significant difference between the control groups (I &II) and stressed group (Group III). However, dark chocolate treatment (Group IV) resulted in a significant decrease in the mean area % of iNOS immunoreactivity as compared to stressed group (Group III) (Figure 7).
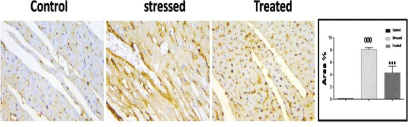
Figure 7: Expression of iNOS immunoreactivity of experimental groups.
Stressed group (Group III) showed a significant increase in expression of
iNOS. ***p < 0.001, compared to the control group. stressed rat treated with
DC (Group IV) significantly down-regulated the (restraint induced stress)
increased in expression of iNOS, 000p < 0.05 compared to the stressed
group (Group III). Immunoperoxidase technique, × 400.
Positive immune histochemical staining for caspase-3 was demonstrated as brown cytoplasmic staining (index for apoptosis). Stressed group (Group III) showed a significant increase in the mean area % of Caspase-3 immunoreactivity (P<0.05) as compared to control groups (Group I & II). This increase was significantly decreased in treated rats (Group IV) as compared to stressed ones (Group III) (Figure 8).

Figure 8: Expression of Caspase 3 immunoreactivity of experimental groups.
Stressed group (Group III) showed a significant increase in the mean area %
of Caspase-3immunoreactive cells (P<0.05) as compared to control group.
This increase was significantly decreased in treated rats (Group IV) as
compared to stress ones (Group III). Immuno peroxidase technique, × 400.
Figure 8 has an expression of Caspase 3 immunoreactivity of experimental groups. Stressed group (Group III) showed a significant increase in the mean area% of Caspase-3 immunoreactive cells (P<0.05) as compared to control group. This increase was significantly decreased in treated rats (Group IV) as compared to stress ones (Group III). Immunoperoxidase technique × 400.
Brain
H & E-stained sections of the external granular layer in control rats (Group I & II) revealed that the pyramidal cells, granular cells, and perineural neuroglia represented the main cellular components of the cortical area. These cells were scattered in an eosinophilic background (neuropil) formed of neuronal and glial cell processes. Pyramidal cells showed vesicular nuclei, basophilic cytoplasm, and long apical dendrites. Granular cells appeared rounded in shape and showed large rounded open face vesicular nuclei with prominent nucleoli. Glial cells appeared smaller in size with small deeply stained nuclei (Figure 9).
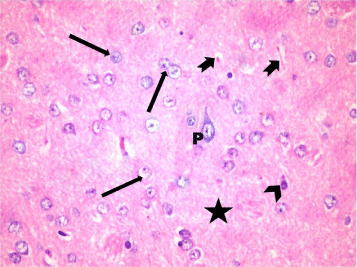
Figure 9: A photomicrograph of a section in layer III of the frontal cortex of a
control rat (Group I)showing Pyramidal cells (P) with large rounded vesicular
nuclei, basophilic cytoplasm, and processes. Note the neuroglia cells with
small dense nuclei (arrow head) and granular cells with open face vesicular
nuclei and prominent nucleoli (?) in the eosinophilic neuropil that forms
the background for the cells (*). Blood capillaries (notched arrow) are seen
among neurons. H&E, × 400.
A photomicrograph of a section in layer III of the frontal cortex of a control rat (Group I) showing Pyramidal cells (P) with large rounded vesicular nuclei, basophilic cytoplasm, and processes. Note the neuroglia cells with small dense nuclei (arrow head) and granular cells with open face vesicular nuclei and prominent nucleoli (?) in the eosinophilic neuropil that forms the background for the cells (*). Blood capillaries (notched arrow) are seen among neurons. H&E, × 400.
Examination of H&E stained sections of stressed rats (Group III). Showed that, most neurons were distorted in shape and shrunken with deeply stained nuclei and surrounded by vacuolated pale areas most probably apoptotic cells. Neuropil showed vascular dilatation. Few cells appeared normal (Figure 10).
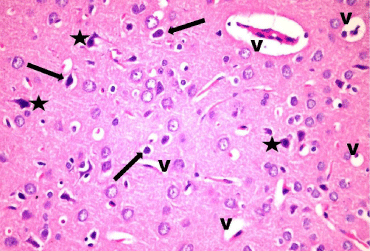
Figure 10: A photomicrograph of a section in layer III of the frontal cortex of
stressed group (Group III). showing that most cells are distorted with deeply
stained nuclei (*). Some cells are surrounded by clear halos (arrow). Notice
the vascular dilatation (V) in the neuropil. H & E, × 400.
In Treated rats (Group IV), multiple neurons were of normal appearance however, few distorted cells and dilated vessels were still present (Figure 11).
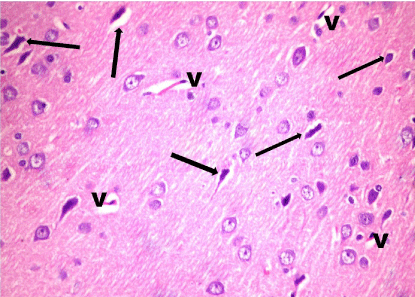
Figure 11: A photomicrograph of a section in layer III of the frontal cortex of
treated rats (Group IV) showing that multiple cells appear normal. Few cells
appear distorted and shrunken with deeply stained nuclei (arrow). Note the
dilated blood Vessels (V). H & E, × 400.
A photomicrograph of a section in layer III of the frontal cortex of treated rats (Group IV) showing that multiple cells appear normal. Few cells appear distorted and shrunken with deeply stained nuclei (arrow). Note the dilated blood vessels (V). H & E, × 400.
A significant up-regulation of iNOS expression, a marker of oxidative distress was detected in stressed group (Group III) compared with control groups (Group I & II). In the treated group (Group IV), iNOS expression was significantly down-regulated compared with stressed rat (Group III) (Figure 12).
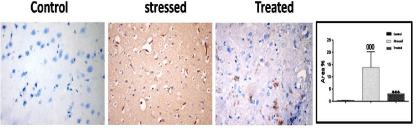
Figure 12: A photomicrograph of immunostaining sections in the frontal
cerebral cortex of different experimental groups showing that, expression of
iNOS immunoreactive cells were significantly increased in stressed group
(Group III). These increases were significantly reduced in the treated group
(Group IV). *** p < 0.001, compared to the control group; and 000p < 0.05
compared to the stressed group (Group III). Immunoperoxidase technique
× 400.
A photomicrograph of immunostaining sections in the frontal cerebral cortex of different experimental groups showing that, expression of iNOS immunoreactive cells were significantly increased in stressed group (Group III). These increases were significantly reduced in the treated group (Group IV). *** p < 0.001, compared to the control group; and 000p < 0.05 compared to the stressed group (Group III). Immunoperoxidase technique, × 400.
The control groups (Group I & II) showed minimal cytoplasmic reaction for caspase-3. Stressed group (Group III) showed more caspase-3 reaction compared with the control groups. The mean area% of caspase-3 reaction in all groups is presented in (Figure 13). There was a significant increase (P=0.05) in caspase-3 reaction in stressed group compared to control groups, whereas this increase was significantly decreased in treated group (Group IV).
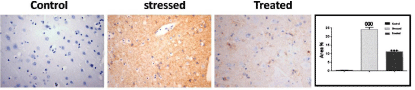
Figure 11: Expression of Caspase 3 immunoreactive cells in the frontal
cortex of experimental groups. Treated group (Group IV) showed a significant
decrease in the mean area % of Caspase-3 immunoreactivity as compared to
stressed group (Group III) which showed a significant increase in the mean
area % of Caspase-3 immunoreactivity (P=0.05) as compared to control
group. Immunoperoxidase technique, × 400.
Expression of Caspase 3 immunoreactive cells in the frontal cortex of experimental groups. Treated group (Group IV) showed a significant decrease in the mean area % of Caspase-3 immunoreactivity as compared to stressed group (Group III) which showed a significant increase in the mean area % of Caspase-3 immunoreactivity (P=0.05) as compared to control group. Immunoperoxidase technique, × 400.
Discussion
Chronic Restraint Stress (CRS) is the easiest and most available method to cause physical stress [18]. Production of free radicals and the imbalance between their production and the antioxidant defense system termed oxidative stress plays an important role in the stress process as well as in the pathogenesis of several disease states. The important consequences of oxidative stress are the peroxidation of membrane lipids [19] which is the main biochemical changes induced by oxidative stress since it produces enzymatically damaging consequences, inducing cell injury including numerous diseases [20- 23]. Thus, lipid peroxidation is a major consequence of free radical toxicity leading to marked changes in the membrane structure and function that may cause even cell death.
Brain is a very susceptible organ to stress because of its high sensitivity to stress-induced degenerative conditions, since it includes high content of polyunsaturated fatty acids which are particularly vulnerable to free radical that can attack proteins, nucleic acids and lipid membranes producing marked damage to the structure and function of cell membranes in these tissues [24]. Data suggest a relationship between stress and the risk of cardiovascular disease [25]. Also [26,27] found that the cardiovascular system is the major target of stress injury.
The purpose of this communication in this study is to survey the aspects of myocardial lesions associated with stress and with other types of alterations in the nervous system.
In the present study, stressed rats had greater levels of enzymatic activities of the SOD, CAT and LPO compared to the control rats indicating occurrence of lipid peroxidation that lead to oxidative stress in heart and brain tissues. These agreed with Matsuura, [25] who reported that CRS augmented cardiac oxidative stress as well as inflammation by significant increase in the superoxide production in myocardial tissue sections, compared with the cont. group and with Nayanatara, [18] Who postulated that repeated immobilization stress significantly increased the oxidative damage biomarker of lipid peroxidation in liver, kidneys, heart, and different brain tissues in rats. The increased level of lipid peroxidation is the evidence most frequently cited in support of the involvement of oxidative stress in tissues. Méndez-Cuesta, [29] suggested that acute restraint stress brings an accurate model for early pro-oxidant responses that can be targeted by broad-spectrum antioxidants like l-CAR.
Numerous pathways can be stimulated in response to stress exposure leading to increased production of free radicals that contribute to the occurrence of pathological conditions [30,31]. Restraint stress may affect the antioxidant defence system, leading to oxidative damage, by changing the balance between oxidant and antioxidant factors [32]. Which may be increased production of oxidizing species or a significant decrease in the effectiveness of antioxidant defenses can occur? The cellular antioxidants as the enzymes Lipid Peroxidase (LPO), Superoxide Dismutase (SOD) & Catalase (CAT) is the best studied indicator of lipid peroxidation (a biomarker of protective oxidative injury) [33].
In the current study, the key histopathological heart findings were that myocardium of stressed rats showing separation, degeneration, cellular infiltration & extravasation of blood. Also the frontal cortex showed damage, where most neurons were distorted in shape and shrunken with deeply stained nuclei and surrounded by vacuolated pale areas most probably apoptotic cells. Neuropil showed vascular dilatation. Few cells appeared normal. These results were in harmony with Nabila, [34] who showed that daily 2 hr immobilized-stress of rats for 5,10 and 30 days provoked structural changes in the cardiac muscle such as degeneration and disorganization of the muscle fibres, vacuolation of the sarcoplasm, pyknosis of the nuclei and congestion of the blood vessels. Lennon, [35] found that apoptosis can occur in response to moderate oxidation, while necrosis can follow more intense stresses.
In this present study, induction of stress was associated with a significant increase in the mean area % of iNOS immunopositive cells. A previous work by Lee, [36] suggested that chronic stress activates inflammatory mechanisms and found increased microglial activation, which exert cytotoxic effects by releasing inflammatory mediators, including iNOS.
Expression of Caspase 3 in this study showed a significant increase (P=0.05) in stressed group compared to control groups confirming the occurrence of apoptosis in heart & brain tissues subjected to RS. These results are in agreement with Ustunel, [37] who found increased levels of apelin inhibited stress-induced apoptosis in heart andLennon, [35] who reported the occurance of apoptosis in response to moderate oxidation. Fan, [38] stated the occurrence of cardiomyocyte hypertrophy and apoptosisin response to stress. However, the results are in contrast withBartekova, [39] who demonstrated elevation of anti-apoptotic proteins (Bcl-2) and downregulation of pro-apoptotic proteins (cleaved caspase-3) following repeated restraint stress.
Use of natural antioxidants is a new plan for alleviating oxidative damage. Many of the negative effects of oxidative stress are decreased after supplementation with certain dietary antioxidants as flavonoids [40]. Flavonoids are phenolic compounds have been reported that most of them are effective antioxidants [41].
For the first time, the present study demonstrates that Dark Chocolate (DC), a high constituent of cocoa, which has a quite large percentage of antioxidant molecules, mainly flavonoids, Interfere with and reduce oxidative stress and apoptosis triggered by counteracting the effects induced by ROS, that cause lipid peroxidation and apoptosis, biochemically, histologically and immune histochemically.
Treatment with DC in stressed rats causes a significant reduction in the SOD and CAT, as well as the levels of LPO as compared to stressed rats thus protects the depletion of non-enzymatic antioxidants via its antioxidant property and may minimize the usage of these antioxidants, thus restoring their normal levels confirming its antioxidant capability to block the stress-induced oxidative stress in the heart and brain tissues of rats as findings reported by Mattera, [42] who demonstrated the ability of polyphenols to counteract oxidative stress-induced cardiomyocytes damage and death. Polyphenols exert long-lasting effects compared to other direct anti-oxidant agents, because they induce a transcription-mediated signaling, thus activating endogenous anti-oxidant enzymatic defense systems. Pari, [43] explained that an antioxidant is a molecule capable of slowing or preventing the oxidation of other molecules. Oxidation reaction transfers electrons from a substance to an oxidizing agent and produce free radicals, which start chain reactions that damage cells. Antioxidants terminate these chain reactions by removing free radical intermediates, and inhibit other oxidation reactions by being oxidized themselves. Also the enzymatic antioxidants SOD and CAT and LPO play a vital role during the process of scavenging reactive oxygen species or preventing their formation. They constitute the major enzymatic antioxidant defenses which convert active oxygen molecules in to non-toxic compounds. Superoxide dismutase is a ubiquitous enzyme with an essential function in protecting aerobic cells against oxidative stress. It is primarily mitochondrial enzyme usually found in the plasma membrane. Catalase is a tetrameric heme protein that undergoes alternative divalent oxidation and reduction at its active site in the presence of hydrogen peroxide.
In the current study, stressed rats treated with DC showing histological improvement where the cardiac muscle fibers are nearly organized while vacuolated sarcoplasm was still present. Also the frontal cortex showing that multiple cells appear normal. Few cells appear distorted and shrunken with deeply stained nuclei. DC treatment resulted in a significant decrease in the mean area% of iNOS immunoreactivity and significant decrease in the cytoplasmic reaction for caspase-3 as compared to stressed group. In agreement with Nehlig, [9] who stated that, flavonoids found abundantly in cocoa powder and chocolate display several beneficial actions on the brain, the vascular system and cerebral blood flow in the form of inhibition of neuronal death by apoptosis induced by neurotoxicants such as oxygen radicals and promote neuronal survival and synaptic plasticity by interaction with signalization cascades involving protein and lipid kinases. Also Kerimi and Williamson, [44] reported lowering of lipid peroxidation and improving cardiovascular risk following chocolate consumption, especially DC.
In summary, we demonstrated that CRS promotes the progression of oxidative stress and apoptosis responsible for the biochemical, histological and immune histochemical alterations induced in the cardiac muscle and frontal cortex of male albino rats and DC could alleviate these alterations due to its antioxidant and antiapoptotic potential by scavenging the harmful effects of free radicals biochemically, histologically and immunohistochemically. So DC could be used as a potentially effective anti-stress therapeutic intervention in clinical conditions associated with free radical damage following exposure to stress. Further studies are needed to explain the mechanism of DC in interfering with apoptosis by reducing ROS.
References
- WHO. The World Health Report: Mental Health: New Understanding New Hope, World Health Organization, Geneva, Switzerland, 2001.
- Munhoz CD, Garcia-Bueno B, Madrigal JL, Lepsch LB, Scavone C, Leza JC. Stress-induced neuro inflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res. 2008; 41: 1037–1046.
- Brzozowski T, Konturek PC, Konturek SJ, Drozdowicz D, Pajdo R, Pawlik M, et al. Expression of Cyclooxygenase (COX)-1 and COX-2 in adaptive cytoprotection induced by mild stress. J Physiol Pharmacol. 2000; 94: 83–91.
- Bugajski AJ, Chlap Z, Gadek-Michalska A, Borycz J, Bugajski J. Degranulation and decrease in histamine levels of thalamic mast cells coincides with corticosterone secretion induced by compound 48/80. Inflamm Res. 1995; 44: 50–51.
- Szymañska M, Suska A, Budziszewska B, Jaworska-Feil L, Basta-Kaim A, Leoekiewicz M, et al. Prenatal stress decreases glycogen synthase kinase-3 phosphorylation in the rat frontal cortex. Pharmacol Rep. 2009; 61: 612–620.
- Zafir A, Banu N. Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Indian J Biochem Biophys. 2009; 46: 53-58.
- McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species. Exp Neurol. 1996; 141: 201-206.
- Ishikawa Y, Kitamura M. Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int. 2000; 58: 1078- 1087.
- Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br J Clin Pharmacol. 2013; 75: 716-727.
- Ding EL, Hutfless SM, Ding X, Girotra S. Chocolate and prevention of cardiovascular disease: a systematic review. Nutr Metab (Lond). 2006; 3: 2.
- Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011; 15: 2779-2811.
- Sentürk T, Günay S. The mysterious light of dark chocolate. Turk Kardiyol Dern Ars. 2015; 43: 199-207.
- Marcilhac A, Dakine N, Bourhim N, Guillaume V, Grino M, Drieu K. Effect of chronic administration of Ginkgo biloba extract or Ginkgolide on the hypothalamic-pituitary-adrenal axis in the rat. Life Sci. 1998; 62: 2329-40.
- Purcell TL, Buhimschi IA, Given R, Chwalisz K, Garfield RE. Inducible nitric oxide synthase is present in the rat placenta at the fetal-maternal interface and decreases prior to labour. Mol Hum Reprod. 1997; 3: 485-491.
- Giorno R. A comparison of two immunoperoxidase staining methods based on the avidin-biotin interaction. Diagn Immunol. 1984; 2: 161-166.
- Krebs JF, Armstrong RC, Srinivasan A, Aja T, Wong AM, Aboy A, et al. New Insights into Retinal Degenerative Diseases. J Cell Biol. 1999; 144: 915–926.
- Jackson P, Blythe D, Bancroft JD, Gample M. Immunohistochemical techniques in theory and practice of histological technique (6th ed.), Elsevier, China. 2008; 423.
- Nayanatara AK, Tripathi Y, Nagaraja HS, Jeganathan PS, Ramaswamy C, Ganaraja B, et al. Effect of Chronic Immobilization Stress on some selected Physiological, Biochemical and Lipid Parameters in Wistar Albino Rats. Research Journal of Pharmaceutical, Biological and Chemical Sciences. January. 2012; 3: 34.
- Matsumoto K, Yobimoto K, Huong NT, Abdel-Fattah M, Hien TV, Watanabe H. Psychological stress-induced enhancement of lipid peroxidation via nitric oxide systems and its modulation by anxiolytic and anxiogenic drugs in mice. Brain Res. 1999; 839: 74-84.
- Shaheen A, Hamdy MA, Kheir-Eldin AA, Lindstrom P, El-Fattah AA. Effect of pretreatment with vitamin E or diazepam on brain metabolism of stressed rats. Biochem Pharmacol. 1993; 46: 194-197.
- Liu J, Wang X, Mori A. Immobilization stress-induced antioxidant defense changes in rat plasma: effect of treatment with reduced glutathione. Int J Biochem. 1994; 26: 511-517.
- Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Fernandez AP, Rodrigo J, et al. Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J Neurochem. 2000; 74: 785-791.
- Zaidi SM, Banu N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta. 2004; 340: 229-233.
- Jain A, Martensson J, Stole E, Auld PA, Meister A. Glutathione deficiency leads to mitochondrial damage in the brain. Proc Natl Acad Sci USA. 1991; 88: 1913-1917.
- Zhao Y, Wang W, Qian L. Hsp70 may protect cardiomyocytes from stressinduced injury by inhibiting Fas-mediated apoptosis. Cell Stress Chaperones. 2007; 12: 83-95.
- Wang S, Song P, Zou MH. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci (Lond). 2012; 122: 555-573.
- Ippoliti F, Canitano N, Businaro R. Stress and obesity as risk factors in cardiovascular diseases: a neuroimmune perspective. J Neuroimmune Pharmacol. 2013; 8: 212-226.
- Matsuura N, Nagasawa K, Minagawa Y, Ito S, Sano Y, Yamada Y, et al. Restraint stress exacerbates cardiac and adipose tissue pathology via β adrenergic signaling in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol. 2015; 15: 308: 1275-1286.
- Méndez-Cuesta LA, Márquez-Valadez B, Pérez-De la Cruz V, Maldonado PD, Santana RA, Escobar-Briones C, et al. Early changes in oxidative stress markers in a rat model of acute stress: effect of l-carnitine on the striatum. Basic Clin Pharmacol Toxicol. 2011; 109: 123-139.
- Bagchi D, Carryl OR, Tran TX, Bagchi M, Garg A, Milnes MM, et al. Acute and chronic stress-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. Mol Cell Biochem. 1999; 196: 109-116.
- Rai J, Pandey SN, Srivastava RK. Effect of immobilization stress on spermatogenesis of albino rats. J Anat Soc India. 2003; 52: 55-57.
- Ganesan B, Anandan R, Lakshmanan PT. Studies on the protective effects of betaine against oxidative damage during experimentally-induced restraint stress in Wistar albino rats. Cell Stress Chaperon. 2011; 16: 641-652.
- Schafer FQ, Buettner GR. “Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple”. Free Radic Biol Med. 2001; 30: 1191–1212.
- Nabila I. El-Desouki, Amal I. El-Refaiy Dalia FA. Histochemical, and Immunohistochemical Studies of the Cardiac Muscle of the Albino Rat under Immobilization Stress and the Curative Role of Diazepam. Egypt J Exp Biol. 2012; 8: 273-285.
- Lennon SV, Martin SJ, Cotter TG. “Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli”. Cell Prolif. 1991; 24: 203–214.
- Lee S, Kang BM, Shin MK, Min J, Heo C, Lee Y, et al. Chronic Stress Decreases Cerebrovascular Responses During Rat Hindlimb Electrical Stimulation. Front Neurosci. 2015; 23: 462.
- Ustunel I, Acar N, Gemici B, Ozbey O, Edizer I, Soylu H, et al. The effects of water immersion and restraint stress on the expressions of apelin, apelin receptor (APJR) and apoptosis rate in the rat heart. Acta Histochem. 2014; 116: 675-681.
- Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012; 5: 15.
- Bartekova M, Barancik M, Pokusa M, Prokopova B, Radosinska J, Rusnak A, et al. Molecular changes induced by repeated restraint stress in the heart: the effect of oxytocinreceptor antagonist atosiban. Can J Physiol Pharmacol. 2015; 93: 827-834.
- Halli well. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovascular Research. 2007; 73: 341–347.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996; 20: 933-956.
- Mattera R, Benvenuto M, Giganti MG, Tresoldi I, Pluchinotta FR5, Bergante S, et al. Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes. Nutrients. 2017; 20: 9.
- Pari L, Karthikeyan A, Karthika P, Rathinam A. Protective effects of hesperidinon oxidative stress, dyslipidaemia and histological changes in iron induced hepatic and renal toxicity in rats. Toxicology Reports. 2015; 2: 46-55.
- Kerimi A, Williamson G. The cardiovascular benefits of dark chocolate. Vascul Pharmacol. 2015; 71: 11–15.