
Research Article
Austin J Anat. 2015;2(1): 1030.
Modulating Role of Panax Ginseng in Experimentally Induced Benign Prostatic Hyperplasia in Adult Male Albino Rats
*Abeer El-Said El-Mehi and Neveen Mohamed El-Sherif
Department of Anatomy and Embryology, Menoufiya University, Egypt
*Corresponding author: Abeer El-Said El-Mehi, Department of Anatomy and Embryology, Menoufiya University, Egypt.
Received: February 04, 2015; Accepted: March 10, 2015 Published: March 13, 2015
Abstract
Benign Prostatic Hyperplasia (BPH) is a common non-malignant disease in elderly men. It has a significant impact on the quality of life. Ginseng is one of the most commonly used herbal medicines with a wide range of beneficial effects. The aim of this study was to investigate the beneficial effects of ginseng on experimentally induced benign prostatic hyperplasia in adult male albino rat. Twenty four (3-month old) male albino rats, with average weight 180-200 gm, were assigned into three groups: Group I (control group), Group II (ginseng treated) and Group III (BPH). The latter group was then subdivided into 2 groups; BPH+basal diet and BPH+ ginseng. BPH was induced by testosterone propionate 7.5 mg/kg body weight/day intramuscularly for 10 days. Panax ginseng was administered at a dose of 10 mg/kg body weight /day orally by gastric tube for 4 weeks. Induction of BPH resulted in significantly increased prostatic weight and increased Dihydrotestosterone (DHT) level in prostate. The histopathological features showed evidence of hyperplasia and inflammation. Quantitative immunohistochemical assessment showed significant upregulation of a-Smooth muscle actin (a-SMA) and Tumor necrosis factor-a (TNF-a). On the other hand, there was significant down regulation of transforming growth factor-β receptor1 (TGFΒRI) and Caspase-3. Panax ginseng treatment significantly ameliorated the changes associated. Panax ginseng treatment inhibits prostatic hyperplasia by reducing epithelial cell proliferation and smooth muscle hyperplasia that may be attributed to potential anti-inflammatory, antiproliferative, anti-oxidant and apoptotic effects. This provides a new insight into the management of BPH using natural agents.
Keywords: Panax ginseng; Benign prostatic hyperplasia; Immunohistochemistry
Introduction
The prostate is a key gland in the sexual physiology of male mammals. Its location in the reproductive tract influences several vital functions such as those related to micturition, seminal emission, and ejaculation [1]. The general pattern of the prostate gland is common to all rodents and human [2]. The ventral lobe as well as the lateral lobe of rat prostate is the most homologous to human prostate, although the ventral one is an essential target for prostatic hyperplasia [3].
Benign Prostatic Hyperplasia (BPH) is a urological disorder characterized by the noncancerous enlargement of the prostate. Problems associated with BPH shows a prevalence of 30% to 60% in menolder than 60 years and 80% in men by age 80 years [4]. As the prostate enlarges, it puts pressure on the urethra and causes the muscles around the urethra to contract leading to a weak urinary stream, incomplete bladder emptying, nocturia, dysuria, bladder outlet obstruction and recurrent infections [5].
BPH is characterized by proliferation of the cellular elements of the prostate. Glandular enlargement may result from an imbalance between prostate cell growth and apoptosis. This imbalance is complex and influenced by factors that stimulate proliferation and minimize cell apoptosis such as growth factors, cytokines and steroid hormones [6,7].
Testosterone produced through hypothalamic-pituitarygonadal axis activity is believed to regulate prostate growth [8]. The enzyme 5a-reductase, found in prostatic cells, catalyzes testosterone conversion into the potent androgen Dihydrotestosterone (DHT). DHT can stimulate a variety of growth factors that accelerate hyperplasia of the stromal and epithelial cells of the prostate resulting in prostatic enlargement. Inflammation can play an important role in BPH. The more the inflammation, the larger the prostate will be [9].
At present, pharmacotherapy becomes the modality of choice for BPH treatment with a shift from surgical treatment. The main drugs used for the management of BHP symptoms include a-blockers and 5a-reductase inhibitors. However, these drugs are limited because of their side effects, including decreased libido, ejaculatory or erectile dysfunction [10].
Due to these risks, natural products appear to have important role in the treatment of BPH. Ginseng (the root of Panax ginseng) is a widely used herbal medicine [11]. Ginseng roots contain multiple active constituents, ginsenosides being the major biologically active compounds [12]. The potential therapeutic effects of ginseng have been attributed to its immunostimulatory, antioxidant, vasorelaxant, anti-neoplastic and anti-inflammatory activities [13]. With respect to the mechanism responsible for the anticancer effects of ginseng, most investigations have focused on cell growth arrest or apoptosis [14]. As BPH is attributable to the uncontrolled growth, we investigate whether ginseng prevents BPH by controlling prostate cell proliferation and apoptosis.
Materials and Methods
Animals
Twenty four male Wistar albino rats (3 months old, weighing 180–200 g) were obtained and housed in the animal house of Faculty of Medicine, Menoufia University, Egypt. Animals of each group were housed in separate hygienic cages at the room temperature. The animals received a standard diet for rodents and allowed free access to water. All experimental procedures were conducted with approval of the Research Ethics Committee, Faculty of Medicine, Menoufia University.
Drugs / Material
- Testosterone propionate: ampoules (25 mg/1ml) for intramuscular injection with the trade name ‘Testone-E’ (Misr Co. for Pharmaceutical industry, Cairo, Egypt).
- Panax ginseng, a product of Pharco Pharmaceuticals, Alexandria, Egypt, was available in the form of capsules with the trade name ‘Ginseng 100’. Each capsule contained 100 mg of the dried roots of Panax ginseng. The contents of the capsule was withdrawn by a syringe and dissolved in distilled water.
- a-Smooth muscle actin (a-SMA) antibody: a mouse monoclonal antibody clone 1A4 Lab Vision Corporation, (Neomarkers laboratories, Westinghouse, Fremont, CA, USA.).
- Tumor necrosis factor-a (TNF-a) antibody: a rabbit polyclonal antibody Lab Vision Corporation (Neomarkers laboratories, Westinghouse, Fremont, CA, USA.).
- Transforming growth factor Β receptor I (TGFΒRI) antibody: a rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA).
- Caspase-3 antibody: a mouse monoclonal antibody (Sigma Company of pharmaceutical industries).
Experimental design
Animals were divided into three groups:
Group I (control group): consisted of six rats.
Group II (ginseng treated group) (six rats) received basal diet for 10 days followed by panax ginseng, at a dose of 10 mg/kg body weight (dissolved in distilled water), orally by a gastric tube once daily for 4 weeks [15].
Group III (12 rats) received testosterone propionate 7.5 mg/kg body weight/day intramuscularly for 10 days to induce prostatic hyperplasia [16]. This group was then subdivided into two equal subgroups:
Subgroup IIIa: received ordinary diet for 4 weeks.
Subgroup IIIb (testosterone+ginseng treated group): Received panax ginseng, at the same dose mentioned before, for 4 weeks.
At the end of each experimental period, animals were fasted overnight. Rats were killed by cervical dislocation under ether anesthesia. The prostatic gland was excised, weighed and divided into two halves. One half was fixed in Bouin’s solution for 48 hours and processed to prepare paraffin sections. The other half was stored at -80C in liquid nitrogen for hormonal assay.
Tissue biochemical study
Prostatic tissue levels of DHT were determined using an Enzyme-Linked Immunosorbent Assay (ELISA) kit according to the manufacturer’s instructions (ALPCO Diagnostics, Salem, NH, USA). The absorbance was measured at 450 nm using a microplate ELISA reader (Bio-Rad Laboratories, Inc.). Values were expressed per mg protein for the prostate.
Histological and immunohistochemical studies
Paraffin sections were prepared with a thickness of 5 microns, mounted on glass slides and stained with H&E and Mallory’s trichrome technique [17]. In each slide, the ventral lobe of the prostate was chosen, according to its histological criteria, for examination and description.
For immunohistochemical staining, Sections were rinsed with PBS, blocked for 30 min in 0.1% H2O2 as inhibitor for endogenous peroxidase activity. After rinsing in PBS, sections were incubated for 60 min in blocking solution (10% normal goat serum) at Room Temperature (RT) (22oC). The sections were then incubated with the primary antibody. Sections were rinsed with PBS, followed by 20 min of incubation at RT with secondary biotinylated antibody. After rinsing the sections in PBS, enzyme conjugate “Streptavidin- Horseradish peroxidase” solution was applied to the sections for 10 min. Secondary antibody binding was visualized using 3, 3-Diaminobenzoic Acid (DAB) dissolved in PBS with the addition of H2O2 to a concentration of 0.03% immediately before use. Finally, sections were PBS rinsed and counterstaining of slides was done using two drops of hemotoxylin. Slides were washed in distilled water until the sections turned blue. Finally, slides were dehydrated in ascending grades of ethanol (70%, 95%, and 100%) for 5 min each and were cleared in xylene and finally coverslipped using histomount mounting solution [18]. Sections were examined with an Olympus light microscope (BX51TF; Olympus, Tokyo, Japan) and photographed.
Quantitative assessment and statistical analysis
Data were obtained using “Leica Qwin-C500” image analyzer computer system (England). The following parameters were measured:
- The mean area % of collagen in Mallory’s trichrome stained sections.
- The mean area % of positive a-SMA, TNF-a and TGFΒRI immunoreactivity.
- The percent of Caspase-3 positive cells in immunosained sections. The number of positive cells was counted and the data were presented as percentage of the total number of cells.
For each parameter, 10 non overlapping fields for every specimen, at magnification X 400, were examined.
Statistical analysis
The data obtained were presented as mean ± SD. Data was statistically analyzed using one-way ANOVA followed by Tukey’s post-test. All statistical analyses were performed using Prism version 4.03 for Windows (GraphPad software Inc., San Diego, California, USA). P Value =0.05 was considered statistically significant [19].
Results
There was no significant difference between Group I (control) and Group II (ginseng treated) rats in all the outcomes at each time point used in the study; therefore, these two groups were pooled in one group (control).
Gross observations
There were no deaths in any of the groups. Testosterone treated group revealed prostatic enlargement by inspection.
Prostatic weight
The mean prostatic weight of testosterone treated rat was significantly higher than that of the control group. However, ginseng treated group was significantly lower than that of testosterone treated rats. No significant difference was found between ginseng treated and control groups (Graph 1).
Measurement of DHT level in prostate
The DHT level in the prostate of testosterone treated group was significantly higher than in controls. However, prostatic DHT level in the ginseng treated group was significantly lower than in testosterone treated group to become non significant from control group (Graph 2).
Histological results
H&E stained sections: Sections of control rats showed that the prostatic parenchyma was composed of packed acini of different sizes. Acini were lined with simple columnar cells with basal nuclei and a small number of epithelial papillary folds. The acini were separated by minimal fibromuscular stroma. Some acini contained homogenous acidophilic secretion (Figure 1a &1b).
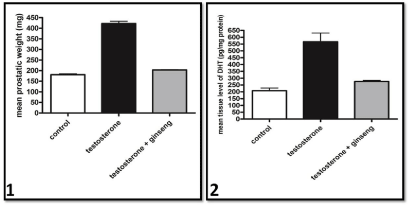
Graphs 1 & 2 Mean Prostatic weight and tissue level of DHT of the different studied groups respectively.
Sections from the testosterone treated group showed larger epithelial cell layer and stromal space compared with controls. Desquamated cells were observed within the lumina of some acini. The acini showed many epithelial folds where the epithelial cells were arranged as multiple unorganized layers. The acini were widely separated with thick fibromuscular stroma that showed areas of hemorrhage and cellular infiltration. Sections from the central region of ventral prostate ofthis group showed enormous dilatation of prostatic acini that were filled with secretion. Thinning and flattening of lining epithelium in some areas of the acini was clearly noted (Figure 1c-1e).
On examination of prostatic sections from the testosterone + ginseng treated group, markedly improved glandular morphology was observed. The acini exhibited a reduction in the lining epithelium, reverting to the simple columnar form. Some epithelial cells exhibited small dark nuclei. Lumina of the acini appeared wide whereas the epithelial folds were diminished. The acini were separated with a reduced amount of stroma compared to the testosterone-treated group (Figure 1f & 1g).
Mallory’s trichrome stained sections: Sections examined from the testosterone group showed significantly increased (P < 0.01) content of collagen fibers in the fibromuscular stroma separating the acini compared to control. This increase was significantly decreased (P < 0.05) in ginseng treated group (Figure 2A-2C and Graph 3).
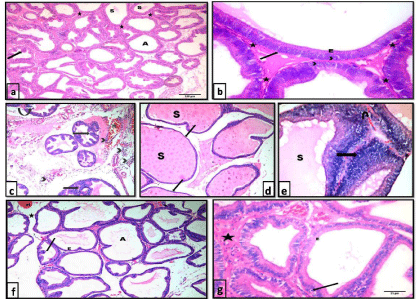
Figure 1: Photomicrographs of HX&E stained sections of the ventral prostate of different groups: a), b) control group showing packed prostatic acini (A) of variable
sizes, infolded mucosa (arrow), fibromuscular stroma (*) and homogenous acidophilic secretion (S) in some acini. The prostatic acini are lined with simple columnar
epithelium (E) with basal nuclei (arrow head) and intact basement membrane (arrow). a x100 , b x400. c,d & e testosterone group showing c) widely separated
prostatic acini with many papillary folds (arrow), desquamated epithelial cells (curved arrow) and focal areas of epithelial proliferation (notched arrow). Notice the
areas of hemorrhage (H) and inflammatory cellular infiltration (arrow head) within the thick fibromuscular stroma. H&E, x100. d) enormous dilatation of prostatic
acini filled with secretion (S) with thinning and flattening of lining epithelium in some areas of the acini (arrow). H&E, x100. e) an area of epithelial proliferation
(arrow) where cells are arranged as multiple unorganized layers. Lumina are distended with secretions (S). Stroma shows areas of hemorrhage (U-turn arrow)
H&E, x400. f) & g) testosterone+ginseng group showing widening of the lumina of the acini (A). Epithelial height is reduced (E) and epithelial folds are diminished
(?). Acini are separated by a reduced amount of fibromuscular stroma (*) that still shows areas of hemorrhage (H). Some epithelial cells show small dark nuclei
(arrow). f x100, gx400.
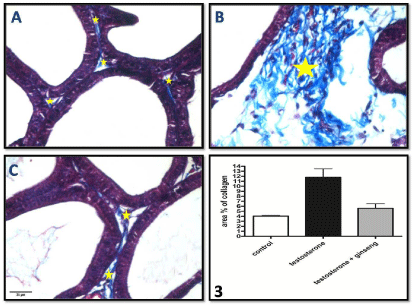
Figure 2: Photomicrographs of Mallory’s trichrome stained sections of the ventral prostate of different groups A) control group, B) testosterone treated group & C)
testosterone+ginseng group showing significant increase of collagen fiber deposition (*), in the connective tissue stroma between acini, in group B which become
markedly decreased in group C. Mallory’s trichrome, x400.
Graph (3) shows the area % of collagen in the different studied groups.
Immunohistochemical results
The testosterone treated group showed a significant increase (P <0.001) in the area % of positive a-SMA and TNF-a immunostaining as compared to controls. On the other hand, immunostained sections of testosterone+ginseng group did not record a significant difference from that of control group but showed a highly significant decrease (P < 0.001) when compared to testosterone group (Figure 3a–3f and Graphs 4,5).
Immunostained prostatic sections with TGFΒRI in the control group showed positive immunoreactivity in some epithelial cells with focal expression in basal and stromal cells. In testosterone treated rats, TGFΒRI showed nearly no expression in epithelial or stromal cells. Ginseng treatment caused a highly significant increase (P < 0.001) in the area % of TGFΒRI positive immunoreactivity as compared to testosterone group (Figure 3g-3i and Graph 6).
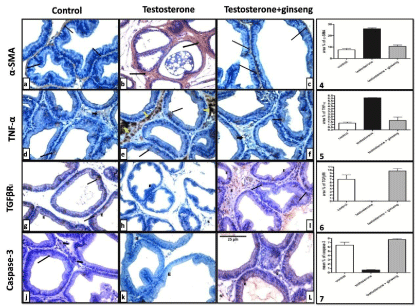
Figure 3: Representative immunostaining of rat ventral prostate of different groups. a-SMA and TNF-a immunoreactivity were dramatically increased in testosterone
group (b&e). These increases were significantly reduced in the testosterone + ginseng group (c&f). On the other hand, testosterone group showed significant
decrease of TGFΒRI and Caspase-3 (h&k). Ginseng treatment caused significant increase of these markers (I&L). x400.
Graph (4), (5), (6) & (7) show mean area % of positive a-SMA, TNF-a and TGFΒRI immunoreactivity and mean percent of Caspase-3 positive cells between the
different studied groups respectively.
In testosterone treated rats, the mean percent of caspase-3 expression was significantly downregulated (P < 0.001). Ginseng treated rats showed a significant enhancement of positivity (P< 0.001) mainly in acinar epithelial cells (Figure 3j-3l and Graph 7).
Discussion
Benign prostatic hyperplasia (BPH) is the most common prostatic disease. Despite currently available medical therapies, a significant number of men still require surgical intervention with a lifetime risk for surgery of around 25–30% [1].
Ginseng was proved to inhibit proliferation of androgendependent and androgen-independent prostate cancer cells [20]. These findings encourage us to suggest the possibility that RG is a potential anti-BPHagent because BPH and prostate cancer have the sameetiology of uncontrolled growth.
Yamashita et al. [21] reported that the hyperplasic changes in the ventral prostate develop with advancing age in albino rats, and that hyperplasic changes in the ventral prostate are observed at 15-20weeks of age. These reports indicate that the prostate, especially the ventral prostate of albino rats can be a good model for human BPH [22].
In our study, animals with BPH showed prostatic enlargement by inspection evidenced by increased absolute prostatic weight. According to previous studies, increased prostate weight is an important marker indicating the development of BPH [23,24]. When sufficiently large, the prostate constricts the urethral canal to cause partial or sometimes complete obstruction. For these reasons, many studies have tested the inhibitory effects of various substances on the development of BPH by measuring prostate weights [25].
In this study, prostatic specimens of BPH group showed a significant increase in the DHT level. The role of DHT in BPH is well known, as it is the androgen responsible for prostate growth [26]. Mizokami et al. [27] stated that, because DHT has a 10 times higher affinity for the androgen receptors than testosterone, DHT easily binds to androgen receptors thus stimulating the transcription of growth factors that are mitogenic for the epithelial and stromal cells of the prostate. Therefore, DHT is ultimately responsible for prostatic epithelial and stromal cell hyperplasia.
Prostatic specimens of animals treated with testosterone in the current study showed acinar epithelial hyperplasia with many epithelial folds. Acini were surrounded by thick fibromuscular stroma rich in smooth muscle fibers as confirmed by significant upregulation of a-Smooth muscle actin. Similar hyperplasic changes have been previously reported in the rat ventral prostate by other authors [28]. They attributed the papillary protrusions to budding and branching of the newly formed acinar structures, focal proliferation of the stroma or by a combination of both. In this study, desquamated cells were observed within the lumina of some acini. A previous report [29] found that the prostatic epithelial cells usually exfoliate into the ejaculate and they may be increased with prostate diseases. It is likely that the increase in desquamation of these cells is associated with the disturbance of their secretory patterns.
In the current work, testosterone treatment resulted in significant increase in density of the collagen fiber content. Moreover, we found
a significant increase in the expression a-SMA in the testosterone treated group. In BPH, stromal mass (collagen fibers and smooth muscle cells) is predominant and very dense such that the stroma: epithelium ratio is increased to 5: 1 compared to a ratio of 2: 1 in the healthy prostate [30,31]. It was postulated that stromal hyperplasia in BPH promotes cellular proliferation through enhanced expression of specific growth factors [32]. It was postulated that secretion accumulation in the acinar lumina may exert a small pressure on the acinar wall. Collagen fiber deposition could be a response of the stretched fibroblasts to the increased intraluminal pressure. In accordance with our study, Tuxhorn et al., [33] stated that in the hyperplastic prostate 40% of the cellular volume is composed of smooth muscle. De WeverandMareel, [34] explained that fibroblasts are considered to transdifferentiate into myofibroblasts by expression of both a-actin as well as vimentin microfilaments. They added that myofibroblasts and their precursor fibrocytes, in this case, could be involved in the synthesis of stromal fibrillar constituents.
Results of the present study showed significant decrease of TGFΒRI and caspase-3 and increase of TNF-a, in BPH specimens, reflecting enhanced proliferation and inflammation and inhibited apoptosis. It was reported that the infiltrating cells are capable of secreting growth factors and pro-inflammatory cytokines such as TFN-a.It is well accepted that regions of prostatic inflammation can generate free radicals, such as Nitric Oxide (NO) and various species of oxygen. In particular, macrophages and neutrophil infiltrations provide a source of free radicals that can induce hyperplastic or precancerous transformations through the oxidative stress to the tissue and DNA [35].
In this study, the hyperplastic changes were accompanied with decreased expression of TGFâRI. In line with this, Shariat et al. 3[36] observed that tissue expression of TGF-â1 receptors is decreased in patients with BPH compared to healthy controls. The authors explained that the loss of functional TGFâRIcontributes to the resistance of TGF-â1 growth inhibition in prostate cancer cells. TGF-â1 is the only known growth factor that can suppress tissue proliferation and induce cell apoptosis [37].
While aberrant regulation of cell growth has traditionally been viewed as the major underlying mechanism for the formation of Benign Prostatic Hyperplasia (BPH), it is becoming increasingly clear that cellular changes that lead to apoptosis inhibition have an essential role in BPH development [38]. This goes in line with the results of this work and with previous studieswhere BPH group showed significantly decreased immunoexpression of caspase-3, the final effector of cell lysis, compared to the control group [4,39].
Results of the present study showed that ginseng administration resulted in significant reduction in DHT level in the prostate compared to the BPH animals. These results were consistent with the changes in prostate weight, immunohistochemical and histomorphological results. These findings indicate that ginseng suppressed the development of BPH in a manner closely associated with reductions in DHT level. This was supported by previous researchers who suggested that development of BPH entails disruption of the DHTsupported homeostasis between cell proliferation and cell death such that proliferative processes predominate and apoptotic processes are inhibited [40].
The potential therapeutic effects of ginseng on prostatic tissue have been attributed to its anti-neoplastic and anti-inflammatory activities [13]. Davy and Allan, [41] reported that ginseng inhibits different inducers-activated signaling protein kinases and transcription factor nuclear factor-kappaB leading to decreases in the production of cytokines and mediators of inflammation including TFN-a.
In this work, ginseng administration was found to enhance expression of TGFΒRI and caspase-3. TGF-Β1 has been shown to activate caspase-3 to induce apoptosis in the NRP-154 prostate epithelial cell line [42]. In addition, in vitro as well as in vivo treatment with a1-adrenergic receptor (a1-ADR) antagonists, used for the treatment of patients with BPH, resulted in significantly enhanced TGF-Β1 expression [43]. Consequently, this led to upregulation of p27kip-1, a downstream intracellular effector of TGF-Β1 apoptotic signaling, and possibly activation of the caspase cascade [44].
Jang et al. [45] noted a significant relaxation effect of ginseng saponin on the bladder and prostatic urethra in both in vitro and in vivo studies. The mechanism by which ginseng saponin induces relaxation appears to involve the nitric oxide/nitric oxide synthase pathway. In addition, Bae et al. [14] proved that red ginseng prevented prostate overgrowth and epithelial thickening induced by testosterone in rats. Moreover, ginseng inhibited testosteroneinduced cell proliferation, arrested cell cycle by inducing p21 and p27, and induced apoptosis in human prostate cells. They found that ginseng suppressed kallikrein-S3, a useful marker for evaluating rat prostate hyperplasia. The authors added that ginseng downregulated androgen receptors, which are major drivers of prostate cell growth and survival, by facilitating the degradation of androgen receptor protein.
BPH is not a life-threatening disease. Accordingly, the safety of therapeutic agents should be primarily considered. Drugs, currently used for the treatment of BPH, have been reported to produce serious side effects, including reduced libido and erectile dysfunction [46]. Therefore, it is worthwhile to search for new safe drugs for BPH therapy. Ginseng is believed to be very safe because it has been widely used in Asia for 1000 years. It has been demonstrated that ginseng is not toxic to the heart, liver, kidney, and the nervous system [47]. In addition to its safety, a double-blind test suggested that ginseng has a beneficial effect in improving male erectile function [48].
Taken together, we propose that ginseng can be viewed as a candidate novel medication for BPH therapy.
References
- Sarma AV, Wei JT. Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N Engl J Med. 2012; 367: 248-257.
- Sluczanowska-Glabowska S, Laszczynska M, Wylot M, Glabowski W, Piasecka M, Gacarzewicz D. Morphological and immunohistochemical comparison of three rat prostate lobes (lateral, dorsal and ventral) in experimental hyperprolactinemia. Folia Histochem Cytobiol. 2010; 48: 447-454.
- Sluczanowska-Glabowska S, Laszczynska M, Glabowski W, Wylot M. Morphology of the epithelial cells and expression of androgen receptor in rat prostate dorsal lobe in experimental hyperprolactinemia. Folia HistochemCytobiol. 2006; 44: 25-30.
- Shariat SF, Ashfaq R, Roehrborn CG, Slawin KM, Lotan Y. Expression of survivin and apoptotic biomarkers in benign prostatic hyperplasia. J Urol. 2005; 174: 2046-2050.
- Bosch JL, Hop WC, Kirkels WJ, Schröder FH. Natural history of benign prostatic hyperplasia: appropriate case definition and estimation of its prevalence in the community. Urology. 1995; 46: 34-40.
- Roehrborn CG1. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin North Am. 2011; 95: 87-100.
- Nilsson PM, Møller L, Solstad K. Adverse effects of psychosocial stress on gonadal function and insulin levels in middle-aged males. J Intern Med. 1995; 237: 479-486.
- Sciarra A, Mariotti G, Salciccia S, Autran Gomez A, Monti S, Toscano V, et al. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008; 108: 254-260.
- Lee C, Kozlowski JM, Grayhack JT. Etiology of benign prostatic hyperplasia. Urol Clin North Am. 1995; 22: 237-246.
- Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5a-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011; 8: 872-884.
- Yeo M, Kim DK, Cho SW, Hong HD. Ginseng, the root of Panax ginseng C.A. Meyer, protects ethanol-induced gastric damages in rat through the induction of cytoprotective heat-shock protein 27. Dig Dis Sci. 2008; 53: 606-613.
- Lee DC, Yang CL, Chik SC, Li JC, Rong JH, Chan GC, et al. Bioactivity-guided identification and cell signaling technology to delineate the immunomodulatory effects of Panax ginseng on human promonocytic U937 cells. J Transl Med. 2009; 7: 34.
- Lü JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009; 7: 293-302.
- Bae JS, Park HS, Park JW, Li SH, Chun YS. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J Nat Med. 2012; 66: 476-485.
- Mahour K, Saxena PN. Modulating role of Panax ginseng in phase-II reaction of hepato-biotransformation in albino rats following mercuric chloride intoxication. Iran J Toxicol. 2009; 2: 273-280.
- Tian HL, Zhao D, Ren LM, Su XH, Kang YH. Effects of (-)doxazosin on histomorphologic and cell apoptotic changes of the hyperplastic prostate in castrated rats. Am J Med Sci. 2009; 338: 196-200.
- Bancroft JD, Layton C. The Hematoxylin and eosin. Suvarna SK, Layton C, Bancroft JD, editors. In: Theory & Practice of histological techniques. 7th edn. Churchill Livingstone of El Sevier. Philadelphia. Chapter 10 and 11. 2013; 172 - 214.
- Jackson P, Blythe D. Immunohistochemical techniques. Suvarna SK, Layton C and Bancroft JD, editors. In: Theory & Practice of histological techniques. 7th edn. Churchill Livingstone of El Sevier. Philadelphia. Chapter 18. 2013; 381 - 434.
- Mould RF. Introductory medical statistics. 2nd edn. Bristol, Philadelphia,USA. Adam Hilger. 1989.
- Liu J, Shimizu K, Yu H, Zhang C, Jin F, Kondo R. Stereospecificity of hydroxyl group at C-20 in antiproliferative action of ginsenoside Rh2 on prostate cancer cells. Fitoterapia. 2010; 81: 902-905.
- Yamashita M, Zhang X, Shiraishi T, Uetsuki H, Kakehi Y. Determination of percent area density of epithelial and stromal components in development of prostatic hyperplasia in spontaneously hypertensive rats. Urology. 2003; 61: 484-489.
- Thielen JL, Volzing KG, Collier LS, Green LE, Largaespada DA, Marker PC. Markers of prostate region-specific epithelial identity define anatomical locations in the mouse prostate that are molecularly similar to human prostate cancers. Differentiation. 2007; 75: 49-61.
- Gat Y, Gornish M, Heiblum M, Joshua S. Reversal of benign prostate hyperplasia by selective occlusion of impaired venous drainage in the male reproductive system: novel mechanism, new treatment. Andrologia. 2008; 40: 273-281.
- Morcos MA, Afifi NM. Effect of doxazocin on experimentally induced prostatichyperplasia in adult male albino rats: a histological and immunohistochemical study. Egypt J Histol. 2011; 34: 870-882.
- Engelstein D, Shmueli J, Bruhis S, Servadio C, Abramovici A. Citral and testosterone interactions in inducing benign and atypical prostatic hyperplasia in rats. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996; 115: 169-177.
- Rittmaster R, Hahn RG, Ray P, Shannon JB, Wurzel R. Effect of dutasteride on intraprostatic androgen levels in men with benign prostatic hyperplasia or prostate cancer. Urology. 2008; 72: 808-812.
- Mizokami A, Koh E, Izumi K, Narimoto K, Takeda M, Honma S, et al. Prostate cancer stromal cells and LNCaP cells coordinately activate the androgen receptor through synthesis of testosterone and dihydrotestosterone from dehydroepiandrosterone. EndocrRelat Cancer. 2009; 16: 1139-1155.
- Golomb E, Rosenzweig N, Eilam R, Abramovici A. Spontaneous hyperplasia of the ventral lobe of the prostate in aging genetically hypertensive rats. J Androl. 2000; 21: 58-64.
- Andrade-Rocha FT1. Assessment of exfoliated prostate cells in semen: relationship with the secretory function of the prostate. Am J Clin Pathol. 2007; 128: 788-793.
- Wennbo H, Kindblom J, Isaksson OG, Törnell J. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology. 1997; 138: 4410-4415.
- Shapiro E, Becich MJ, Hartanto V, Lepor H. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J Urol. 1992; 147: 1293-1297.
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Hittmair A, Zhang J, et al. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996; 28: 392-405.
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002; 8: 2912-2923.
- De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003; 200: 429-447.
- Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005; 26: 1170-1181.
- Shariat S F, Menesses-Diaz A, Kim IY, Muramoto M, Wheeler TM, Slawin KM. Tissue expression of transforming growth factor-beta1 and its receptors: correlation with pathologic features and biochemical progression in patients undergoing radical prostatectomy. Urology. 2004; 63:1191-1197.
- Soulitzis N, Karyotis I, Delakas D, Spandidos DA. Expression analysis of peptide growth factors VEGF, FGF2, TGFB, EGF and IGF1 in prostate cancer and benign prostatic hyperplasia. Int J Oncol. 2006; 29: 305-314.
- Vacherot F, Azzouz M, Gil-Diez-De-Medina S, Colombel M, De La Taille A, Lefrère Belda MA, et al. Induction of apoptosis and inhibition of cell proliferation by the lipido-sterolic extract of Serenoa repens (LSESr, Permixon in benign prostatic hyperplasia. Prostate. 2000; 45: 259-266.
- Kosova F, Temeltas G, Ari Z, Lekili M. Possible relations between oxidative damage and apoptosis in benign prostate hyperplasia and prostate cancer patients. Tumour Biol. 2014; 35: 4295-4299.
- Shin IS, Lee MY, Ha HK, Seo CS, Shin HK. Inhibitory effect of Yukmijihwang-tang, a traditional herbal formula against testosterone-induced benign prostatic hyperplasia in rats. BMC Complementary and Alternative Medicine. 2012; 12: 48.
- Lee DC, Lau AS. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules. 2011; 16: 2802-2816.
- Glassman DT, Chon JK, Borkowski A, Jacobs SC, Kyprianou N. Combined effect of terazosin and finasteride on apoptosis, cell proliferation, and transforming growth factor-beta expression in benign prostatic hyperplasia. Prostate. 2001; 46: 45-51.
- Chipuk JE, Bhat M, Hsing AY, Ma J, Danielpour D. Bcl-xL blocks transforming growth factor-beta 1-induced apoptosis by inhibiting cytochrome c release and not by directly antagonizing Apaf-1-dependent caspase activation in prostate epithelial cells. J Biol Chem. 2001; 276: 26614-26621.
- Papadopoulos G, Vlachodimitropoulos D, Kyroudi A, Kouloukoussa M, Perrea D, Mitropoulos D. Terazosin treatment induces caspase-3 expression in the rat ventral prostate. J Clin Med Res. 2013; 5: 127-131.https://www.ncbi.nlm.nih.gov/pubmed/22507987
- Jang HA, Cho S, Kang SG, Ko YH, Kang SH, Bae JH, et al. The relaxant effect of ginseng saponin on the bladder and prostatic urethra: an in vitro and in vivo study. Urol Int. 2012; 88: 463-469.
- Gravas S, Oelke M. Current status of 5alpha-reductase inhibitors in the management of lower urinary tract symptoms and BPH. World J Urol. 2010; 28: 9-15.
- Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002; 25: 323-344.
- Kim TH, Jeon SH, Hahn EJ, Paek KY, Park JK, Youn NY, et al. Effects of tissue-cultured mountain ginseng (Panax ginseng CA Meyer) extract on male patients with erectile dysfunction. Asian J Androl. 2009; 11: 356-361.