
Research Article
Austin J Anat. 2023; 10(1): 1111.
Copper Content in Lumbar Vertebrae across Age Groups
Mavrych V¹*; Bolgova O¹; Shypilova I²; Eryomin A³
¹Department of Anatomy and Genetics, COM Alfaisal University, Saudi Arabia
²Biochemistry Department, SOM St. Matthews University, Cayman Islands
³Anatomy Department, Luhansk State Medical University, Ukraine
*Corresponding author: Mavrych V Department of Anatomy and Genetics, COM Alfaisal University, P.O. Box 50927, Riyadh 11533, Saudi Arabia Riyadh, Saudi Arabia. Tel: +966 11 215 7629 Email: prof.mavrych@gmail.com
Received: June 14, 2023 Accepted: July 14, 2023 Published: July 21, 2023
Abstract
Copper, an essential trace element in bone tissue, plays a crucial role in the active centers of vital enzymes. It is involved in the synthesis of key connective tissue proteins like collagen and elastin, which form the structural matrix of bones and cartilage. This research aims to investigate the copper content in human lumbar vertebrae and explore how it changes with age, with a particular focus on gender differences. The study involved the analysis of the third lumbar vertebrae from 211 individuals spanning ages 0 to 90 years. Copper content was determined using an atomic absorption spectrophotometer C-115M1 (PA “Electron,” Ukraine), while the organic component of bone tissue was calculated using the weighing method. The obtained data were statistically analyzed using ANOVA and independent samples t-tests. The results revealed that copper levels in the spongy bone of human lumbar vertebrae ranged from 2.45 to 6.12 mg/kg dw, and the organic component of bone tissue varied from 6.5% to 49.6%. The statistical analysis demonstrated a positive correlation between the organic component and copper content in the spongy bone tissue for both males (r=0.5195, p<0.01, n=106) and females (r=0.6101, p<0.01, n=105). Our study demonstrated that bone copper levels decrease with age, irrespective of gender. A strong positive correlation was observed between bone copper and organic content in the spongy bone of human lumbar vertebrae.
Keywords: Lumbar vertebra; Copper; Bone composition; Trace elements
Introduction
Bone is a calcified tissue that responds to changes in dietary intake and nutritional status. It consists of approximately 60% inorganic components (hydroxyapatite), 10% water, and 30% organic components (proteins). The primary functions of bone are to provide mechanical support for movement, protect vital internal organs, and regulate mineral balance within the body. In addition to well-known macro minerals, trace elements such as iron, zinc, copper, and selenium also play a role in bone metabolism [1].
The bone tissue undergoes a constant turnover process involving resorption by osteoclasts and formation by osteoblasts. The overall balance of bone depends on the relative contribution of these processes. Trace elements can influence skeletal metabolism and tissue properties indirectly by regulating macromineral metabolism or directly by affecting the proliferation or activity of osteoblasts and osteoclasts, which are incorporated into the bone mineral matrix [1].
Deficiency in trace elements can hinder the increase of bone mass during childhood and adolescence and accelerate bone loss after menopause or in old age. Deterioration in bone quality increases the risk of fractures. To identify and treat individuals at risk of non-traumatic fractures, it is important to monitor the homeostasis of trace elements, measure bone density, and assess biochemical markers of bone metabolism [2].
Within the human body, copper content ranges from 50 to 120 mg, with about two-thirds of the total copper found in the muscles and skeleton [3,4]. Copper plays a crucial role in the formation of enzymes that facilitate electron transfer and the reduction of molecular oxygen, making it essential for cellular energy metabolism [5,6]. One of these enzymes, lysyl oxidase, utilizes lysine and hydroxylysine as substrates to produce cross-links necessary for the development of connective tissues, including those in bones [6-8].
Numerous in vitro studies have confirmed the beneficial impact of copper on the cells involved in bone metabolism. Some studies demonstrated that copper ions could inhibit osteoclastic resorption [9], while other researchers have shown that the effects of copper are dependent on its dosage. Specifically, low concentrations of copper have been found to enhance the viability and growth of osteoblastic cells, whereas higher concentrations can be cytotoxic [10].
Under normal physiological conditions, copper ions can create a hypoxic microenvironment similar to hypoxia-mimicking ions and increase bone mineral density by promoting the expression of bone-related genes such as ALP, OPN, and OPN. This inhibition of active bone resorption and promotion of angiogenesis is achieved through the upregulation of vascular endothelial growth factor [11-13]. Copper has been found to downregulate the expression of Runx2 and other genes related to osteogenic differentiation, as well as inhibit collagen formation while stimulating angiogenesis in vivo studies [14]. Additionally, copper has been observed to hinder cytoskeletal changes in Bone Mesenchymal Stem Cells (BMSCs) during osteogenic differentiation, suggesting its interference with mesenchymal stem cells involved in osteogenesis by affecting cytoskeleton organization [14]. Other studies indicate that copper can also promote both osteogenesis and adipogenic differentiation of BMSCs, with a preference for the osteogenic lineage [15]. Higher concentrations of copper have been found to induce apoptosis in BMSCs [16].
In vivo, experiments on bone tissue formation have demonstrated that copper inhibits the accumulation of collagen type I and the formation of connective tissue while stimulating microvascular formation [14]. These findings suggest a balance between inhibiting collagen accumulation and promoting microvascular formation with copper supplementation, which can aid in the development of copper-containing implants to promote angiogenesis [17-19].
The insufficient amount of copper ions has detrimental effects on bone tissue, leading to increased bone resorption and disruption of lysyl oxidase [20]. Copper deficiency can result in decreased cancellous bone, weakened bone formation and mineralization, impaired cartilage integrity, and ultimately reduced bone density. Animal experiments have shown that copper deficiency leads to bone deformities, hypoplasia, fragile bones, and frequent fractures [21].
The impact of copper supplementation on bone health has been examined in several studies, primarily focusing on menopausal women. One study investigated the effects of a 3 mg copper daily intake in the diet of healthy women over a span of 2 years. The results showed decreased vertebral bone density loss compared to the control group without supplementation [22]. Another study conducted by Baker et al. explored the influence of a 3-week diet with varying levels of copper (1.6 mg/d vs. 0.7 mg/d vs. 6 mg/d) on healthy men. The transition from a copper-poor to a copper-rich diet led to a significant reduction in markers of bone resorption [23]. Thus, it can be inferred that nontoxic doses of copper are unlikely to induce detrimental changes in bone tissue.
Furthermore, another study indirectly supports the role of copper in maintaining bone integrity [24]. Cu and Zn SOD-deficient mice, which exhibited increased cytoplasmic superoxide, displayed compromised bone integrity. These mice demonstrated greater weakness in bone stiffness and decreased bone mineral density. In the lumbar vertebrae of SOD-deficient mice, there was a decrease in the presence of osteoblasts and osteoclasts on the surface area. Primary osteoblasts exhibited enhanced cell death and reduced proliferation, likely due to decreased antioxidant activity. These findings may be attributed not only to decreased collagen crosslinking but also to increased free radical production [25].
Results of studies aimed at determining the effects of excessive copper levels indicated that copper exhibits direct toxicity to cartilage and bone tissue in chicken embryos, primarily due to the inhibition of collagen synthesis [26,27], and elevated serum copper level is associated with decreased bone size and density in C57 mice [28]. Another study demonstrated that copper-induced inhibition of mineral and matrix formation remained unaffected by zinc intake [29].
In patients with Wilson's disease, copper overload can interfere with bone metabolism, resulting in a generalized loss of bone density, rickets, and abnormal osteophytes [12]. Epidemiological studies in individuals with Wilson's disease have revealed normal bone mineral density in cortical and cancellous bone but an increased risk of fractures [30]. Moreover, excessive copper levels and decreased bone strength have been associated with other conditions, such as rickets and abnormal osteophytes [31].
Excessive copper also leads to the generation of high levels of free radicals, triggering lipid peroxidation and interfering with bone metabolism, resulting in decreased bone cortex and strength. Oxidation itself can promote aging and reduce bone strength [32].
Furthermore, high concentrations of copper ions have the potential to impact collagen synthesis negatively. Ascorbic acid is well-known for its essential role in collagen production. However, at elevated levels of copper, free copper ions can reduce the half-life of ascorbic acid, leading to reduced collagen accumulation [7].
Impaired bone growth is frequently observed in various conditions linked to disrupted copper metabolism, implying a physiological significance of copper in bone tissue growth and mineralization [33]. However, recent studies examining the relationship between copper and bone are rare, both in humans and animals. One of the main reasons for this could be the relatively minor prevalence of copper deficiency among most population groups in the United States [34].
Therefore, the aim of this study was to investigate the physiological changes in copper content within the spongy bone of human lumbar vertebrae across different age groups.
Materials and Methods
This study utilized a collection of 211 regular lumbar spine specimens gathered from diseased residents of Luhansk City in Ukraine. The study was approved by the Bioethics Committee of Luhansk State Medical University (3/10112005) and the MPH of Ukraine (2000/20.23.23). The samples of the third lumbar vertebrae (L3) consisted of 106 male and 105 female individuals spanning an age range from 0 to 88 years, with an average age of 40-year-old. All specimens were sourced from individuals who experienced trauma, poisoning, asphyxia, or sudden death due to vascular disorders without spinal damage recorded (Table 1).
Age Group
Year-old
Males
(n)Females
(n)1
0
11
12
2
0-1
7
5
3
1-3
5
4
4
3-7
3
4
5
8-12
4
3
6
13-16
5
3
7
17-20
7
6
8
21-35
18
15
9
36-60
21
17
10
61-74
14
22
11
75-90
11
15
Total
106
105
Table 1: Material of the research: number of lumbar spine specimens - age distribution.
The bone samples obtained from the L3 vertebral body underwent a series of procedures to analyze the chemical composition of the spongy bone tissue. First, the samples underwent a dry-heating process at 105°C until a constant weight was achieved. Then they were incinerated at a temperature of 450°C for 48 hours to convert to ashes. A percentile of organic substances in the bone tissue was calculated by weighing the samples before and after the incineration.
The quantitative determination of copper (324.7 nm) was performed using an atomic absorption spectrophotometer C-115M1 (PA "Electron," Ukraine). Results were expressed as micrograms of element per kilogram of dry bone weight.
Descriptive statistics were calculated for all measurements, and the relationships between Cu content, age, and organic bone component were assessed using analysis of variance (ANOVA) in TIBCO Statistica® software. An independent samples t-test was employed to compare the data between male and female groups. Statistical significance was determined at a threshold of P<0.05.
Results
Figure 1 illustrates age-related changes in Cu content in the vertebral body of L3. Our data demonstrated that the average Cu content in spongy bone tissue of L3 was 3.82±0.70 mg/kg dw, with a range of 2.45–5.63 mg/kg dw for men and 3.87±0.73 mg/kg dw, with a range of 2.72–6.12 mg/kg dw for women. The independent group t-test did not show significant differences in copper content between male and female groups (t-value 0.547, p=0.589).
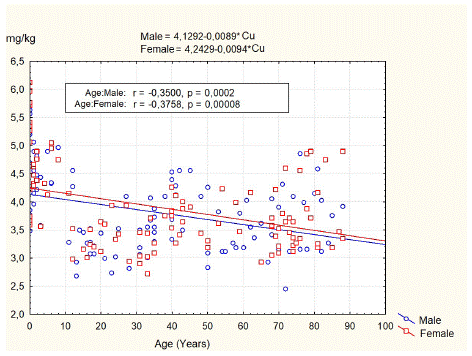
Figure 1: Age-related changes of the copper content in spongy bone of L3.
The least squares fit equation for males was Copper (mg/kg dw) = 4.1292–0.0089* Age (years), n=106, p<0.05, correlation coefficient = -0.35. For females, the equation was Copper (mg/kg dw) = 4.2429–0.0094* Age (years), n=105, p<0.05, correlation coefficient = -0.37.
The data of Cu and organic content in the L3 vertebral body for persons of different age groups and gender are summarized in Table 2. According to the results of our study, the concentration of Cu in the spongy bone of the L3 body of newborns ranges from 3.48 mg/kg dw to 6.12 mg/kg dw, averaging 4.84±0.89 mg/kg dw. In boys (4.68±0.84 mg/kg dw), the average concentration of this element is lower than in girls (4.99±0.94 mg/kg dw), but these differences are not statistically significant (p=0.34). In children of 1–12 years old, Cu content in spongy bone of L3 was in the range of 3.28 mg/kg dw to 5.06 mg/kg dw with an average of 4.4±0.46 mg/kg dw for boys and in the range of 2.98 mg/kg dw to 5.04 mg/kg dw with an average of 4.34±0.55 mg/kg dw for girls. In teenagers, there is a decrease in the content of copper in the spongy bone of the L3 by 26.3%, and the average concentration of this element is 3.19±0.31 mg/kg dw (range of recorded values from 2.68 mg/kg dw to 3.52 mg/kg dw). In other age periods, the content of copper in the bone tissue was relatively stable. It averaged 3.25±0.21 mg/kg dw for adolescent subjects, 3.36±0.39 mg/kg dw for adults, and 3.74±0.47 mg/kg dw, in the elderly -3.55±0.45 mg/kg dw and in the senile -3.87±0.65 mg/kg dw.
Age group
Sex
Cu
(mg/kg dw)Organic
(%)Mean ± SD
Mean ± SD
Range
Range
(1) 0 yr
M
4.68±0.84
43,4±2,6
39,3-46,5
3.48-5.63
F
4.99±0.94
44,3±2,5
41,0-49,6
3.59-6.12
M+F
4.84±0.89
43,9±2,5
39,3-49,6
3.48-6.12
(2) 0-1 yr
M
4.49±0.42
34,1±2,0
31,6-36,2
3.96-5.06
F
4.48±0.17
33,6±2,7
30,2-36,4
4.29-4.67
M+F
4.49±0.32
33,9±2,2
30,2-36,4
3.96-5.06
(3) 1-3 yr
M
4.31±0.46
29,2±1,5
27,1-31,1
3.58-4.82
F
4.40±0.59
29,1±2,0
26,7-31,5
3.57-4.89
M+F
4.35±0.49
29,1±1,6
26,7-31,5
3.57-4.89
(4) 3-7 yr
M
4.52±0.32
22,9±1,2
21,6-23,8
4.32-4.89
F
4.61±0.46
23,6±0,9
22,3-24,3
4.12-5.04
M+F
4.57±0.38
23,3±1,0
21,6-24,3
4.12-5.04
(5) 8-12 yr
M
4.27±0.72
17,5±1,5
16,3-19,4
3.28-4.97
F
4.45±0.42
17,0±0,6
16,5-17,4
4.15-4.75
M+F
4.33±0.6
17,3±1,2
16,3-19,4
3.28-4.97
(6) 13-16 yr
M
3.16±0.35
17,1±1,2
15,9-18,9
2.68-3.50
F
3.22±0.27
17,5±0,7
16,9-18,3
2.98-3.52
M+F
3.19±0.31
17,3±1,0
15,9-18,9
2.68-3.52
(7) 17-20 yr
M
3.23±0.20
15,6±2,6
12,0-18,8
2.99-3.52
F
3.29±0.25
15,8±1,5
13,0-17,2
3.00-3.65
M+F
3.25±0.21
15,7±2,1
12,0-18,8
2.99-3.65
(8) 21-35 yr
M
3.40±0.41
13,9±2,9
6,5-17,0
2.73-4.09
F
3.32±0.37
14,5±2,4
10,7-18,5
2.72-3.93
M+F
3.36±0.39
14,1±2,7
6,5-18,5
2.72-4.09
(9) 36-60 yr
M
3.75±0.57
14,3±2,1
10,9-17,2
2.83-4.56
F
3.71±0.33
14,6±2,5
11,3-19,0
3.19-4.26
M+F
3.74±0.47
14,5±2,3
10,9-19,0
2.83-4.56
(10) 61-74 yr
M
3.53±0.52
14,9±1,4
12,3-17,5
2.45-4.31
F
3.56±0.41
15,1±2,7
9,8-21,2
2.93-4.60
M+F
3.55±0.45
15,0±2,2
9,8-21,2
2.45-4.60
(11) 75-90 yr
M
3.81±0.60
13,0±2,0
9,2-15,8
3.11-4.86
F
3.92±0.70
13,7±2,2
10,2-16,1
3.18-4.89
M+F
3.87±0.65
13,4±2,1
9,2-16,1
3.11-4.89
Table 2: The copper and organic bone content in spongy bone of the L3 for 212 persons of different age groups.
The results of our study demonstrated that the average organic component of L3 spongy bone tissue was 19.79±10.13%, with a range of 6.5–46.5% for men, and 19.95±10.34%, with a range of 9.8–49.6% for women, generally decreasing with age.
The independent group t-test did not show statistical differences in the organic component of L3 between male and female groups (t-value 0.114, p=0.909).
Discussion
Copper is vital in various physiological processes, including forming and maintaining connective tissues, bone health, and enzymatic reactions. While copper is present in different tissues and organs throughout the body, its concentration may vary depending on different factors such as age, sex, and overall health [12].
Imbalances in trace metal elements, whether excessively high or low, can have detrimental effects on bone mineral formation and the synthesis of the bone matrix, there by increasing the risk of osteoporosis and other bone disorders [35]. The association between copper deficiency and radiographic signs of metabolic bone diseases, including osteoporosis, metaphyseal changes, and disruptions of the physical structure, has been observed in premature infants [33]. Copper deficiency significantly impacts bone metabolism, particularly in newborns with Menkes disease, a genetic disorder caused by mutations in the ATP7A gene. This condition affects copper absorption and leads to various systemic manifestations, including bone abnormalities such as delayed growth, generalized osteoporosis, and abnormal development of long bones [36]. The impact of copper deficiency on bone changes primarily arises from functional impairments in osteoblasts, while osteoclast activity remains unaffected [37]. This imbalance, with reduced osteoblast function and unaltered osteoclast activity, disrupts the normal remodeling of bone tissue, ultimately resulting in osteopenia [38].
On the contrary, excessive levels of copper can have adverse effects. The excess copper can generate Reactive Oxygen Species (ROS), leading to the peroxidation of lipids and interference with bone metabolism. As a result, there is a general reduction in bone density, the occurrence of rickets, and the formation of abnormal osteophytes in individuals with Wilson's disease, a genetic disorder characterized by impaired copper metabolism [32]. Thus, maintaining balanced copper homeostasis is vital for supporting skeletal growth during childhood and promoting optimal bone health in adulthood [2]. To ensure adequate bone quality, it is recommended that adults maintain a daily intake of 0.9 mg of copper [39].
According to the results of our study, copper content in the spongy bone of human lumbar vertebrae is in the range of 2.45–6.12 mg/kg dw. A value of 5–6 mg/kg dw was considered the physiological level of Cu in bone tissue, with the range varying according to people’s age and/or bone type [40,41]. In a systematic review, Ciosek et al. [42] analyzed the average copper concentrations in the various bone types available in scientific literature. Copper levels range from 0.16 to 6.30 mg/kg dw, with the highest value recorded in ribs. Comparing bone types, copper concentrations can be arranged in the following descending series: tibia > femur > ribs.
When comparing the content of copper in the different tissues of patients living in rural areas and cities, some studies indicated an elevated level [43]. In contrast, others did not show statistically significant differences [44]. Also, some studies indicated that copper levels are higher in spongy bone than in cortical bone [45,46]. Since Luhansk is a city in a very industrial region and we studied spongy bone specimens, this can explain slightly higher copper levels. We have not found specific data for copper levels in the human vertebrae.
Based on the conducted studies, it can be observed that the average copper level in the lumbar vertebrae of newborns is 4.84±0.89 mg/kg dw versus 3.87±0.65 mg/kg dw in elderly people. copper level in spongy bone of L3 generally declines with age despite the significant variation. This data supports other studies on different human bones that have shown the same trend [40,47,48]. In general, copper concentrations in the body tend to decrease with age due to reduced absorption and increased excretion. However, it's important to note that bone composition and mineral content are influenced by factors beyond copper alone, such as calcium, phosphorus, and other trace minerals [12].
Our data did not show statistically significant gender differences for copper levels in lumbar vertebrae, as well as it was not found in other studies for the ribs [40,41,48], femur [47,49], and tibia bones [44].
mineral portion of the bone is comprised of hydroxyapatite, which contributes up to 65% of the weight of the bone. The remaining 20–30% of the bone weight contains organic components, primarily type I collagen (~90%), and the remaining ~10% noncollagenous proteins. The last 10% of the bone weight includes water molecules in the collagen and mineral portions [50].
Our data indicated that the organic component of bone tissue in lumbar vertebral bodies lies in the range of 6.5–49.6% of the bone weight for the different age groups, with no statistical differences between male and female groups.
Statistical analysis of our data shows a positive correlation between organic components and copper content in spongy bone tissue of L3. The least squares fit equation for males was Organic (%) = -8.8395+7.4965* Cu (mg/kg dw), n=106, p<0.01, correlation coefficient = 0.5195 (Figure 2). For females, the equation was Organic (%) = -2.9706+8.5007* Cu (mg/kg dw), n=105, p<0.01, correlation coefficient = 0.6101 (Figure 3).
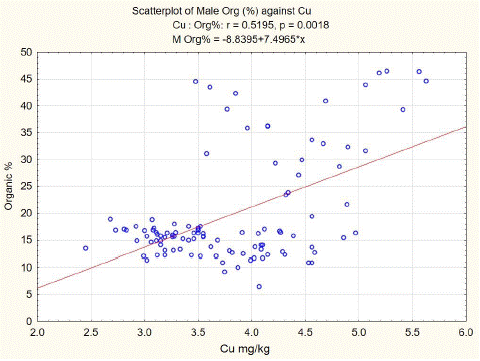
Figure 2: L3 spongy bone organic component versus Cu content in males.
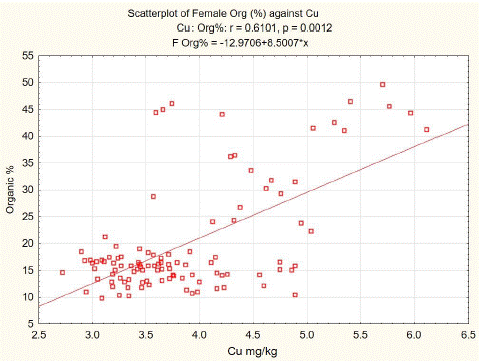
Figure 3: L3 spongy bone organic component versus Cu content in females.
Our findings align with previous research, providing further evidence that copper plays a vital role in the synthesis and stabilization of collagen, a crucial organic component of bones [1]. Additionally, copper actively participates in enzymatic reactions that regulate bone remodeling and mineralization processes. Animal studies have demonstrated the influence of copper on collagen accumulation and angiogenesis during in vivo bone formation [14]. Numerous studies have established that copper deficiency impairs the mechanical strength of bones by reducing the crosslinking of elastin and collagen [51,52]. Furthermore, the dosage of copper has been emphasized by other authors, as low concentrations of copper (0.1% w/w) have been shown to improve the viability and growth of osteoblastic cells, while higher concentrations (2.5% and 1% w/w) have proven to be cytotoxic [10,53].
The biochemical processes within the skeletal system are complex, with a delicate balance between cells, organic compounds, and inorganic elements [54]. Disruptions in this equilibrium, whether through excessive or insufficient amounts, can significantly impact bone metabolism. Despite numerous publications addressing this subject, there is still much to uncover to comprehensively understand the influence of minerals on bone metabolism.
Conclusion
In conclusion, our study demonstrated that bone copper level declines with age without gender differences. There was a strong positive correlation between bone copper and organic content in the spongy bone of human lumbar vertebrae. This correlation suggests that copper plays an essential role in bone metabolism and collagen interactions.
Author Statements
Ethical Approval
The study was approved by the Bioethics Committee of Luhansk State Medical University (3/10112005) and the MPH of Ukraine (2000/20.23.23).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Gaffney-Stomberg E. The impact of trace minerals on bone metabolism. Biol Trace Elem Res. 2019; 188: 26-34.
- Zofkova I, Davis M, Blahos J. Trace elements have beneficial, as well as detrimental effects on bone homeostasis. Physiol Res. 2017; 66: 391-402.
- Rondanelli M, Faliva MA, Infantino V, Gasparri C, Iannello G, et al. Copper as dietary supplement for bone metabolism: a review. Nutrients. 2021; 13: 22-46.
- Olivares M, Uauy R. Limits of metabolic tolerance to copper and biological basis for present recommendations and regulations. Am J Clin Nutr. 1996; 63: 846S-52S.
- Kubiak K, Klimczak A, Dziki L, Modranka R, Malinowska K. Wplyw kompleksu miedzi (LL) na aktywnosc wybranych enzymów antyoksydacyjnych [Influence of copper (II) complex on the activity of selected oxidative enzymes]. Pol Merkur Lekarski. 2010; 28: 22-5.
- Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996; 63: 797S-811S.
- Dahl SL, Rucker RB, Niklason LE. Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant. 2005; 14: 367-74.
- Rucker RB, Kosonen T, Clegg MS, Mitchell AE, Rucker BR, et al. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am J Clin Nutr. 1998; 67: 996S-1002S.
- Li BB, Yu SF. In vitro study of the effects of copper ion on osteoclastic resorption in various dental mineralized tissues. Zhonghua Kou Qiang Yi Xue Za Zhi. 2007; 42: 110-3.
- Milkovic L, Hoppe A, Detsch R, Boccaccini AR, Zarkovic N. Effects of Cu-doped 45S5 bioactive glass on the lipid peroxidation-associated growth of human osteoblast-like cells in vitro. J Biomed Mater Res A. 2014; 102: 3556-61.
- Bari A, Bloise N, Fiorilli S, Novajra G, Vallet-Regí M, et al. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017; 55: 493-504.
- Qu X, He Z, Qiao H, Zhai Z, Mao Z, et al. Serum copper levels are associated with bone mineral density and total fracture. J Orthop Translat. 2018; 14: 34-44.
- Wilson T, Katz JM, Gray DH. Inhibition of active bone resorption by copper. Calcif Tissue Int. 1981; 33: 35-9.
- Li S, Wang M, Chen X, Li SF, Li-Ling J, et al. Inhibition of osteogenic differentiation of mesenchymal stem cells by copper supplementation. Cell Prolif. 2014; 47: 81-90.
- Rodríguez JP, Ríos S, González M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J Cell Biochem. 2002; 85: 92-100.
- Burghardt I, Lüthen F, Prinz C, Kreikemeyer B, Zietz C, et al. A dual function of copper in designing regenerative implants. Biomaterials. 2015; 44: 36-44.
- Glenske K, Donkiewicz P, Köwitsch A, Milosevic-Oljaca N, Rider P, et al. Applications of metals for bone regeneration. Int J Mol Sci. 2018; 19: 826.
- Tsiklin IL, Shabunin AV, Kolsanov AV, Volova LT. In vivo bone tissue engineering strategies: advances and prospects. Polymers (Basel). 2022; 14: 3222.
- Shen Q, Qi Y, Kong Y, Bao H, Wang Y, et al. Advances in copper-based biomaterials with antibacterial and osteogenic properties for bone tissue engineering. Front Bioeng Biotechnol. 2021; 9: 795425.
- Sarazin M, Alexandre C, Thomas T. Influence on bone metabolism of dietary trace elements, protein, fat, carbohydrates, and vitamins. Joint Bone Spine. 2000; 67: 408-18.
- Rico H, Roca-Botran C, Hernández ER, Seco C, Paez E, et al. The effect of supplemental copper on osteopenia induced by ovariectomy in rats. Menopause. 2000; 7: 413-6.
- Strause L, Saltman P, Smith KT, Bracker M, Andon MB. Spinal bone loss in postmenopausal women supplemented with calcium and trace minerals. J Nutr. 1994; 124: 1060-4.
- Baker A, Harvey L, Majask-Newman G, Fairweather-Tait S, Flynn A, et al. Effect of dietary copper intakes on biochemical markers of bone metabolism in healthy adult males. Eur J Clin Nutr. 1999; 53: 408-12.
- Nojiri H, Saita Y, Morikawa D, Kobayashi K, Tsuda C, et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res. 2011; 26: 2682-94.
- Medeiros DM. Copper, iron, and selenium dietary deficiencies negatively impact skeletal integrity: a review. Exp Biol Med (Maywood). 2016; 241: 1316-22.
- Rest JR. The histological effects of copper and zinc on chick embryo skeletal tissues in organ culture. Br J Nutr. 1976; 36: 243-54.
- Kaji T, Kawatani R, Takata M, Hoshino T, Miyahara T, et al. The effects of cadmium, copper or zinc on formation of embryonic chick bone in tissue culture. Toxicology. 1988; 50: 303-16.
- Massie HR, Aiello VR, Shumway ME, Armstrong T. Calcium, iron, copper, boron, collagen, and density changes in bone with aging in C57BL/6J male mice. Exp Gerontol. 1990; 25: 469-81.
- Kaji T, Takata M, Miyahara T, Kozuka H, Koizumi F. Interaction of zinc with cadmium and copper on ossification of embryonic chick bone in tissue culture. Arch Environ Contam Toxicol. 1990; 19: 653-6.
- Quemeneur AS, Trocello JM, Ea HK, Ostertag A, Leyendecker A, et al. Bone status and fractures in 85 adults with Wilson’s disease. Osteoporos Int. 2014; 25: 2573-80.
- Beattie JH, Avenell A. Trace element nutrition and bone metabolism. Nutr Res Rev. 1992; 5: 167-88.
- Dermience M, Lognay G, Mathieu F, Goyens P. Effects of thirty elements on bone metabolism. J Trace Elem Med Biol. 2015; 32: 86-106.
- Marquardt ML, Done SL, Sandrock M, Berdon WE, Feldman KW. Copper deficiency presenting as metabolic bone disease in extremely low birth weight, short-gut infants. Pediatrics. 2012; 130: e695-8.
- Klevay LM, Medeiros DM. Deliberations and evaluations of the approaches, endpoints and paradigms for dietary recommendations about copper. J Nutr. 1996; 126: 2419S-26S.
- Lin S, Chen C, Cai X, Yang F, Fan Y. The concentrations of bone calcium, phosphorus and trace metal elements in elderly patients with intertrochanteric hip fractures. Front Endocrinol. 2022; 13: 1005637.
- Kodama H, Murata Y, Kobayashi M. Clinical manifestations and treatment of Menkes disease and its variants. Pediatr Int. 1999; 41: 423-9.
- Gehrke M. Copper and manganese in the pathogenesis of diseases of the skeletal system of animals. Med. Wet. 1997; 53: 644-6.
- de Romaña DL, Olivares M, Uauy R, Araya M. Risks and benefits of copper in light of new insights of copper homeostasis. J Trace Elem Med Biol. 2011; 25: 3-13.
- Pepa GD, Brandi ML. Microelements for bone boost: the last but not the least. Clin Cases Miner Bone Metab. 2016; 13: 181-5.
- Zaichick S, Zaichick V. The effect of age and gender on 38 chemical element contents in human femoral neck investigated by instrumental neutron activation analysis. Biol Trace Elem Res. 2010; 137: 1-12.
- Zaichick V, Zaichick S. Instrumental neutron activation analysis of trace element contents in the rib bone of healthy men. J Radioanal Nucl Chem. 2009; 281: 47-52.
- Ciosek Z, Kot K, Rotter I. Iron, Zinc, copper, cadmium, mercury, and bone tissue. Int J Environ Res Public Health. 2023; 20: 2197.
- Brodziak-Dopierala B, Kwapulinski J, Sobczyk K, Kowol J. The occurrence of nickel and other elements in tissues of the hip joint. Ecotoxicol Environ Saf. 2011; 74: 630-5.
- Roczniak W, Brodziak-Dopierala B, Cipora E, Jakóbik-Kolon A, Kluczka J, et al. Factors that affect the content of cadmium, nickel, copper and zinc in tissues of the knee joint. Biol Trace Elem Res. 2017; 178: 201-9.
- Samudralwar DL, Robertson JD. Determination of major and trace elements in bones by simultaneous PIXE/PIGE analysis. J Radioanal Nucl Chem Artic. 1993; 169: 259-67.
- Lin S, Chen C, Cai X, Yang F, Fan Y. The concentrations of bone calcium, phosphorus and trace metal elements in elderly patients with intertrochanteric hip fractures. Front Endocrinol (Lausanne). 2022; 13: 1005637.
- Brodziak-Dopierala B, Kwapulinski J, Paukszto A, Kowol J, Bogunia M, et al. Interactions of copper and iron with other elements in the osseous tissue of the femur head. Fresenius Environ Bull. 2009; 18: 1963-6.
- Kuo HW, Kuo SM, Chou CH, Lee TC. Determination of 14 elements in Taiwanese bones. Sci Total Environ. 2000; 255: 45-54.
- Messer JG, Kilbarger AK, Erikson KM, Kipp DE. Iron overload alters iron-regulatory genes and proteins, down-regulates osteoblastic phenotype, and is associated with apoptosis in fetal rat calvaria cultures. Bone. 2009; 45: 972-9.
- Brodziak-Dopierala B, Kwapulinski J, Sobczyk K, Wiechula D. Analysis of the content of cadmium and zinc in parts of the human hip joint. Biol Trace Elem Res. 2015; 163: 73-80.
- Feng X. Chemical and biochemical basis of cell-bone matrix interaction in health and disease. Curr Chem Biol. 2009; 3: 189-96.
- Jonas J, Burns J, Abel EW, Cresswell MJ, Strain JJ, et al. Impaired mechanical strength of bone in experimental copper deficiency. Ann Nutr Metab. 1993; 37: 245-52.
- Rondanelli M, Faliva MA, Infantino V, Gasparri C, Iannello G, et al. Copper as dietary supplement for bone metabolism: a review. Nutrients. 2021; 13: 2246.
- Skrajnowska D, Jagielska A, Ruszczynska A, Idkowiak J, Bobrowska-Korczak B. Effect of copper and selenium supplementation on the level of elements in rats’ femurs under neoplastic conditions. Nutrients. 2022; 14: 1285.
- Luo Y, Amromanoh O. Bone organic-inorganic phase ratio is a fundamental determinant of bone material quality. Appl Bionics Biomech. 2021; 2021: 4928396.