
Review Article
Austin J Anal Pharm Chem. 2015; 2(6): 1056.
Ultra Performance Liquid Chromatography (UPLC) - A Review
Taleuzzaman M*, Ali S, Gilani SJ, Imam SS and Hafeez A
Glocal School of Pharmacy,Glocal University, Saharanpur, 247121, U.P, India
*Corresponding author: Mohamad Taleuzzaman, Glocal School of Pharmacy, Glocal University, Saharanpur, India.
Received: December 13, 2015; Accepted: December 29, 2015; Published: December 31, 2015
Abstract
UPLC is a modern technique which gives a new direction for liquid chromatography. UPLC refers to ultra performance liquid chromatography, which enhance mainly in three areas: “speed, resolution and sensitivity. Ultra performance liquid chromatography (UPLC) applicable for particle less than 2μm in diameter to acquire better resolution, speed, and sensitivity compared with high-performance liquid chromatography (HPLC). In twenty first centenary pharmaceutical industries are focusing for new ways to in economy and shorten time for development of drugs. UPLC analysis at the mean time gives the better quality of their products and analytical laboratories are not exception in this trend. The separation and quantification in UPLC is done under very high pressure (up to 100M Pa). As compare to HPLC, under high pressure it is observed that not any negative influence on analytical column and also other components like time and solvent consumption is less in UPLC.
Keywords: Ultra performance liquid chromatography; High separation efficiency; Cost effective; High pressure
Introduction
High performance liquid chromatography (HPLC) has proven to one of the most and predominant technology used in analytical laboratories for the analysis of drugs worldwide during the past 30- plus years [1,2]. One of the basic concerns for the growth of this technique is the packing material which effects the separations. In this separation mechanism the principal apply is Van Deemeter equation, with which any student of chromatography is intimately familiar.
H=A+B/v +Cv
The above equation is an empirical formula that describes the relationship between linear velocity (flow rate) and plate height (HETP, or column efficiency). And, since particle size is one of the variables, a Van Deemter curve can be used to investigate chromatographic performance. Where A, B and C are constants and v is the linear velocity, the carrier gas flow rate.
A= Eddy mixing
B =Axial diffusion
C=Solute’s mass transfer
The A term is independent of velocity and represents “eddy” mixing. It is smallest when the packed column particles are small and uniform. The B term represents axial diffusion or the natural diffusion tendency of molecules. This effect is diminished at high flow rates and so this term is divided by v. The C term is due to kinetic resistance to equilibrium in the separation process. The kinetic resistance is the time lag involved in moving from the gas phase to the packing stationary phase and back again. The greater the flow of gas, the more a molecule on the packing tends to lag behind molecules in the mobile phase. Thus this term is proportional to v [3-5].
Comparison between UPLC and HPLC
Principles are the same but not the performance
The principles of UPLC are same principle as HPLC, the basic difference is in designer of the column material particle size which less than 2-μm. Which make a big deference in performance and to maximize the advantages of these columns, creating a powerful, robust and reliable solution? The familiar design of UPLC H-class’s Quaternary Solvent Manager (QSM) and Sample Manager (SMFTN), with flow-through needle design, gives all the flexibility and usability of your current HPLC while still achieving the highly efficient separations that only UPLC can provide [6-9] (Table 1).
To improve the UPLC efficiency following measures need to be performed
S. No.
Characteristics
HPLC
UPLC
1.
Particle size
3 to 5µm
Less than 2 µm
2.
Maximum back pressure
35-40 MPa
103.5 MPa
3.
Analytical column
Alltima C18
Acquity UPLC BEH C18
4.
Column dimensions
150 X 3.2 mm
150 X 2.1 mm
5.
Column temperature
30°C
65°C
6.
Injection volume
5mL (Std. In100% MeOH)
2mL (Std.In100% MeOH)
Table 1: Comparison between UPLC and HPLC.
1. By employing high temperature which reduce the viscosity of mobile phase and ultimately flow rate if high. Significantly back pressure is reduced.
2. The unique feature of UPLC analysis is interconnected skeletons and interconnected flow paths (through-pores) which are found in monolithic columns make UPLC technique different from HPLC. In UPLC chromatogram it is found that better resolution and separation are found as compared to HPLC along with perform more sensitive analysis, reduce consumption of solvent and has high speed of analysis [8-10].
Small Particle Chemistry
The ideal of the Van Deemter equation cannot be completed without smaller particles than those traditionally used in HPLC. The Van Deemter equation influence by particles size, so the scientist focus on the design and development of sub-2 μm particles is a significant challenge, and researchers have been active in this area for some time to capitalize on their advantages Figure 1 shows Van Deemter plot, illustrating the evolution of particle sizes over the last three decades.
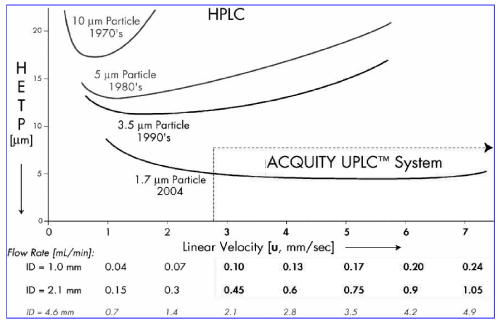
Figure 1: van Demeter plot, illustrating the evolution of particle sizes over the
last three decades.
Researcher scholars are study many decades at “fast LC” to speed up analyses. In drug discovery the “need for speed” has been came from by the selecting so many of samples in different laboratories, and the availability of sophisticated instrument like UPLC with detector as mass spectrometers. The unique features of column that is smaller columns and faster flow rates (amongst other parameters) have been used. During analysis higher temperature, having the dual advantages of lowering viscosity, and increasing mass transfer by increasing the diffusivity of the analytes, has also been investigated. However, using conventional particle sizes and pressures, limit-actions are soon reached and compromises must be made, sacrificing resolution for time.
It found that the analysis by UPLC method and the classic separation method is of HPLC (High Performance Liquid Chromatography). There are many advantages in HPLC like robustness, ease of use, good selectivity and adjustable sensitivity. But its main limitation is the lack of efficiency compared to gas chromatography or the capillary electrophoresis due to low diffusion coefficients in liquid phase, involving slow diffusion of analytes in the stationary phase. In UPLC main advantage is better efficiency with speedy analysis and this achieved by only smaller particle size. The Van Deemter equation shows that efficiency increases with the use of smaller size particles but this leads to a rapid increase in back pressure, while most of the HPLC system can operate only up to 400 bars. That is why short columns filled with particles of about 2μm are used with these systems, to accelerate the analysis without loss of efficiency, while maintaining an acceptable loss of load to improve the efficiency of HPLC separations, the following can be done [10-15].
• Work at higher temperatures- allows high flow rates by reducing the viscosity of mobile phase which significantly reduces back pressure.
• Use of monolithic columns- contains polymerize porous support structure that provides lower flow resistances than conventional particle-packed columns.
By above two parameter UPLC analysis improves in three areas.
1. Produced Chromatogram with resolved peak.
2. Fast analysis
3. Sensitive analysis
It uses fine particles and saves time and reduces solvent consumption. The new method make a very big difference by retains the same analytical separation method as HPLC while other things which drastically changes are speedy analysis, sensitivity and high resolution. Today’s pharmaceutical industries are focusing for new path to reduce cost and less time for development of drugs and in mean time the quality of their products are not suffer analytical laboratories maintain the whole things. Speed allows a greater number of analyses to be performed in a shorter amount of time thereby increasing sample throughput and lab productivity. These are the benefits of faster analysis and hence the ultra performance liquid chromatography. A typical assay was transferred and optimized for UPLC system to achieve both higher sample analysis throughput and better assay sensitivity. Analysis of operation cost and sample throughput found, UPLC cost advantageous over HPLC [16-20].
Chemistry of Small Particles whatever as shown in Figure 1, smaller particles create not only increased efficiency, parallel also the ability to work at higher linear velocity without a loss of efficiency, providing both resolution and speed. Efficiency is the fundamental separation parameter behind UPLC since it relies on the same selectivity and retentivity as HPLC. In the fundamental Resolution (Rs) equation:
Rs = √N/4 (α-1/ α) (k/k+1)
Resolution is proportional to the square root of N. But since N is inversely proportional to particle size (dp):
N α 1/dp
As the particle size is lowered by a factor of three, from, for example, 5 m (HPLC scale) to 1.7 m (UPLC-scale), N is increased by three and resolution by the square root of three or 1.7 N is also inversely proportional to the square of the peak width [20-24].
N α 1/ w²
This illustrates that the narrower the peaks are, the easier they are to separate from each other. Also, peak height is inversely proportional to the peak width:
H α 1/w
So as the particle size decreases to increase N and subsequently Rs, an increase in sensitivity is obtained, since narrower peaks are taller peaks. Narrower peaks also mean more peak capacity per unit time in gradient separations, desirable for many applications, e.g., peptide maps. Still another equation comes into play when migrating toward smaller particles:
F opt α 1/dp
This relationship also is revealed from the Van Deemter plot. As particle size decreases,
The optimum flow ( Fopt). To reach maximum N increases. But since back pressure is proportional to flow rate, smaller particle sizes require much higher operating pressures, and a system properly designed to capitalize on the efficiency gains . A system that can both reliably deliver the requisite pressures and that can maintain the separation efficiency of the small particles with tightly managed volumes. Higher resolution and efficiency can be leveraged even further, however when analysis speed is the primary objective. Efficiency is proportional to column length and inversely proportional to the particle size [17-20].
N α L/dp
Therefore, the column can be shortened by the same factor as the particle size without loss of resolution. Using a flow rate three times higher due to the smaller particles and shortening the column by one third (again due to the smaller particles), the separation is completed in 1/9 the time while maintaining resolution. So if speed, throughput, or sample capacity is a concern, theory can be further leveraged to get much higher throughput. But the design and development of sub-2μm particles is a significant challenge, and researchers have been active in this area for some time, trying to capitalize on their advantages. Although high efficiency nonporous 1.5-μm particles are commercially available, they suffer from poor loading capacity and retention due to low surface area. Silica-based particles have good mechanical strength but can suffer from a number of disadvantages, which include a limited pH range and tailing of basic analytes. Polymeric columns can overcome pH limitations, but they have their own issues including low efficiencies, limited loading capacities, and poor mechanical strength. In 2000, Waters introduced XTerra®, a first generation hybrid chemistry that took advantage of the best of both the silica and polymeric column worlds. XTerra®, columns are mechanically strong, with high efficiency, and operate over an extended pH range. They are produced using a classical sol-gel synthesis that incorporates carbon in the form of methyl groups [21- 22].
Instrumentation
A. Sample Injection
B. UPLC Columns
C. Detectors
A. Sample Injection
In UPLC, sample introduction is critical. Conventional injection valves, either automated or manual, are not designed and hardened to work at extreme pressure. To protect the column from extreme pressure fluctuations, the injection process must be relatively pulsefree and the swept volume of the device also needs to be minimal to reduce potential band spreading. A fast injection cycle time is needed to fully capitalize on the speed afforded by UPLC, which in turn requires a high sample capacity. Low volume injections with minimal carryover are also required to increase sensitivity. There are also direct injection approaches for biological samples.
B. UPLC Columns
Resolution is increased in a 1.7μm particle packed column because efficiency is better. Separation of the components of a sample requires a bonded phase that provides both retention and selectivity. Four bonded phases are available for UPLC separations:
(i) ACQUITY UPLCTM BEH C8 (straight chain alkyl columns),
(ii) ACQUITY UPLCTM BEH C18 (straight chain alkyl columns),
(iii) ACQUITY UPLC BEH Shield RP18 (embedded polar group column) and
(iv) ACQUITY UPLC BEH Phenyl (phenyl group tethered to the silyl functionality with a C6 alkyl),
ACQUITY UPLC BEH Phenyl (phenyl group tethered to the silyl functionality with a C6 alkyl).
Each column chemistry provides a different combination of hydrophobicity, silanol activity, hydrolytic stability and chemical interaction with analytes. ACQUITY UPLC BEH C18 and C8 columns are considered the universal columns of choice for most UPLC separations by providing the widest pH range. They incorporate tri functional ligand bonding chemistries which produce superior low pH stability. This low pH stability is combined with the high pH stability of the 1.7μm BEH particle to deliver the widest usable pH operating range. ACQUITYUPLC BEH Shield RP18 columns are designed to provide selectivity that complements the ACQUITYUPLC BEH C18 and C8 phases. ACQUITY UPLC BEH Phenyl columns utilize a tri functional C6 alkyl tether between the phenyl ring and the silyl functionality. This ligand, combined with the same proprietary end capping processes as the ACQUITY UPLC BEH C18 and C8 columns, provides long column lifetimes and excellent peak shape. This unique combination of ligand and end capping on the 1.7μm BEH particle creates a new dimension in selectivity allowing a quick match to the existing HPLC column. An internal dimension (ID) of 2.1 mm column is used. For maximum resolution, choose a 100 mm length and for faster analysis, and higher sample throughput, choose 50 mm column. Half-height peak widths of less than one second are obtained with 1.7μm particles, which gives significant challenges for the detector. In order to integrate an analyte peak accurately and reproducibly, the detector sampling rate must be high enough to capture enough data points across the peak. The detector cell must have minimal dispersion (volume) to preserve separation efficiency. Conceptually, the sensitivity increase for UPLC detection should be 2-3 times higher than HPLC separations, depending on the detection technique. MS detection is significantly enhanced by UPLC; increased peak concentrations with reduced chromatographic dispersion at lower flow rates promote increased source ionization efficiencies. The ACQUITY UPLC System consists of a binary solvent manager, sample manager including the column heater, detector, and optional sample organizer.
The binary solvent manager uses two individual serial flow pumps to deliver a parallel binary gradient. There are built-in solvent select valves to choose from up to four solvents. There is a 15,000-psi pressure limit (about 1000 bar) to take full advantage of the sub-2μm particles. The sample manager also incorporates several technology advancements. Using pressure assisted sample introduction, low dispersion is maintained through the injection process, and a series of pressures transducers facilitate self-monitoring and diagnostics. It uses needle-in-needle sampling for improved ruggedness and needle calibration sensor increases accuracy. Injection cycle time is 25 seconds without a wash and 60 sec with a dual wash used to further decrease carry over. A variety of micro titer plate formats (deep well, mid height, or vials) can also be accommodated in a thermostatically controlled environment. Using the optional sample organizer, the sample manager can inject from up to 22 micro titer plates. The sample manager also controls the column heater. Column temperatures up to 65°C can be attained. To minimize sample dispersion, a “pivot out” design allows the column outlet to be placed in closer proximity to the source inlet of an MS detector.
C. Detectors
The detectors are use in UPLC analysis is UV/Visible detector. Detection of analytes is conventionally based on absorbance that is concentration sensitivity detectors. In UPLC the flow cell volume would have to be reduced to maintain concentration and signal. Based on Beer’s Law, smaller volume conventional flow cells would also reduce the path length upon which the signal strength depends. A reduction in cross-section means the light path is reduced, and transmission drops with increasing noise. Therefore, if a conventional HPLC flow cell were used, UPLC sensitivity would be compromised. The ACQUITY Tunable UV/Visible detector cell consists of a light guided flow cell equivalent to an optical fiber. Light is efficiently transferred down the flow cell in an internal reflectance mode that still maintains a 10mm flow cell path length with a volume of only 500mL. Tubing and connections in the system are efficiently routed to maintain low dispersion and to take advantage of leak detectors that interact with the software to alert the user to potential problems [23-24] (Figure 2).
Figure 2: Detectors Flow Cell.
Advantages of UPLC
Various advantages of UPLC are as follows:
- Require less run time and enhance sensitivity.
- Provides the selectivity, sensitivity, and dynamic range of LC analysis.
- In chromatogram resolved peaks are obtained.
- Multi residue methods are applied.
- Speedy analysis, quantify accurately analytes and related products.
- Uses of fine particle (2μm) for packing of stationary phase make analysis fast.
- Time and cost both are reduced.
- Consumption of solvents is less.
- More products are analyzed with existing resources.
- Increases sample throughput and enables manufacturers to produce more material that consistently meet or exceeds the product specifications, potentially eliminating variability, failed batches, or the need to re-work material.
- Delivers real-time analysis in step with manufacturing processes.
- Assures end-product quality, including final release testing.
Disadvantages of UPLC
In UPLC analysis the main disadvantage occurs are life of columns, during analysis high pressure developed because the particle size. Increase pressure reduces the life of the columns. Due to increased pressure requires more maintenance and reduces the life of the columns of these types. Using stationary phase of particle size 2μm perform better analysis without the adverse effects of high pressure.
Application
In pharmaceutical industry the demand of UPLC analysis is very high, because of the unique features of UPLC like high resolution in chromatogram, short time analysis which make more analytical work in less time with valuable, reliable and authentic data. Scientist can generate more accurate data by UPLC in faster way. UPLC technique is used for the analysis of herbal product. In analytical laboratory the demand of UPLC is very high because the method developed are accurate and précised and also this expand the research information of the analyte in nano level. By this method the standard of analysis in every respect like qualitative, quantitative and complexity of sample can be differentiate in very high standard. The UPLC/MS system is used to generate a data which solved the complexity of the compound. By using MS as a detector with UPLC the interpretation of analysis is go to depth. Such analysis is very use full in bio-analytical field. The unique features of UPLC that high resolution and speedy analysis also very helpful in pharmacokinetic studies like – adsorption, distribution, metabolism and excretion (ADME). ADME studies measure physical and chemical properties of compound. UPLC/MS/ MS method saves time. For the drug development and formulation process, profiling, detecting and quantifying drug substances and their impurities can be performed very accurately [25-26].
UPLC Analysis can be done like:
1. Amino acid analysis.
2. Analysis of natural medicine and herbal medicine.
3. Analysis of drugs in human plasma (e.g. Levofloxacin and metabolites).
4. Study of metabonomics.
References
- van Deemter JJ, Zuiderweg EJ, Klinkenberg A. Longitudinal diffusion & resistance to mass transfer or cause of non ideality in chromatography. Chem. Eng. Sci. 1965; 5: 271-289.
- Srivastava B, Sharma BK, Baghel US, Yashwant, Sethi N. Ultra Performance Liquid Chromatography (UPLC): A Chromatography Technique. International Journal of Pharmaceutical Quality Assurance. 2010; 2: 19-25.
- Zhang YH, Gong XY, Zhang HM, Larock RC, Yeung ES. Combinatorial screening of homogeneous catalysis and reaction optimization based onmultiplexed capillary electrophoresis. Journal of Combinatorial Chemistry. 2000; 2: 450-452.
- Zhou C, Jin Y, Kenseth JR, Stella M, Wehmeyer KR, Heineman WR. Rapid pKa estimation using vacuum-assisted multiplexed capillary electrophoresis (VAMCE) with ultraviolet detection. J Pharm Sci. 2005; 94: 576-589.
- Zhu J, Goodall DM, Wren SAC. Ultra-High Performance Liquid Chromatography and Its Applications. LCGC. 2005; 23: 54-72.
- Greibrokk T, Andersen T. High-temperature liquid chromatography. J Chromatogr A. 2003; 1000: 743-755.
- Gerber F, Krummen M, Potgeter H, Roth A, Siffrin C, Spoendlin C. Practical aspects of fast reversed-phase high-performance liquid chromatography using 3 microm particle packed columns and monolithic columns in pharmaceutical development and production working under current good manufacturing practice. J Chromatogr A. 2004; 1036: 127-133.
- Tanaka N, Kobayashi H, Nakanishi K, Minakuchi H and Ishizuka N. Monolithic columns-a new type of chromatographic support for liquid chromatography. Anal. Chem. 2001; 73: 420-429.
- Wu N, Dempsey J, Yehl PM, Dovletoglu A, Ellison A. Wyvratt. Practical aspects of fast HPLC separations for pharmaceutical process development using monolithic columns. Journal of Analytical Chemistry. 2004; 523: 149-156.
- Jerkovich AD, Mellors JS, Jorgenson JW. LCGC 2003; 21: 606-611.
- Nguyen DT, Guillarme D, Rudaz S, Veuthey JL. Fast analysis in liquid chromatography using small particle size and high pressure. J Sep Sci. 2006; 29: 1836-1848.
- Unger KK, Kumar D, Grun M, Buchel G, Ludtke S, Adam TH. Synthesis of spherical porous silicas in the micron and submicron size range: challenges and opportunities for miniaturized high-resolution chromatographic and electrokinetic separations. Journal of Chromatography A. 2000; 892: 47-55.
- Jerkovich AD, Mellors JS, and Jorgenson JW. LCGC. 2003; 21: 660–661.
- Wu N, Lippert JA, Lee ML. Practical aspects of ultrahigh pressure capillary liquid chromatography. J Chromatogr A. 2001; 911: 1-12.
- Unger KK, Kumar D, Grün M, Büchel G, Lüdtke S, Adam T, et al. Synthesis of spherical porous silicas in the micron and submicron size range: challenges and opportunities for miniaturized high-resolution chromatographic and electrokinetic separations. J Chromatogr A. 2000; 892: 47-55.
- Swartz ME. and Murphy B. Lab Plus Int. 2004; 18.
- Swartz ME and Murphy B. Pharm. Formulation Quality. 2004; 6: 40.
- Van Deemter JJ, Zuiderweg EJ, Klinkenberg A. Longitudinal diffusion and resistance to mass transfer as causes of non ideality in chromatography. Chem. Eng. Sci. 1956; 5: 271-289.
- Zhang Y, Gong X, Zhang H, Larock RC, Yeung ES. Combinatorial screening of homogeneous catalysis and reaction optimization based on multiplexed capillary electrophoresis J Comb Chem. 2000; 2: 450-452.
- Zhou C, Jin Y, Kenseth JR, Stella M, Wehmeyer KR, Heineman WR. Rapid pKa estimation using vacuum-assisted multiplexed capillary electrophoresis (VAMCE) with ultraviolet detection. J Pharm Sci. 2005; 94: 576-589.
- Zhu J, Goodall DM, and Wren SAC. LCGC. 2005; 23: 54–72.
- Greibrokk T, Andersen T. High-temperature liquid chromatography. J Chromatogr A. 2003; 1000: 743-755.
- Gerber F, Krummen M, Potgeter H, Roth A, Siffrin C, Spoendlin C. Practical aspects of fast reversed-phase high-performance liquid chromatography using 3 microm particle packed columns and monolithic columns in pharmaceutical development and production working under current good manufacturing practice. J Chromatogr A. 2004; 1036: 127-133.
- Tanaka N, Kobayashi H, Nakanishi K, Minakuchi H, Ishizuka N. Monolithic LC columns. Anal Chem. 2001; 73: 420A-429A.
- Wu N, Dempsey J, Yehl PM, Dovletoglu A, Ellison A, Wyvratt J. Practical aspects of fast HPLC separations for pharmaceutical process development using monolithic columns. Anal. Chim. Acta. 2004; 523: 149–156.
- Swartz ME. Ultra Performance Liquid Chromatography (UPLC): An Introduction, Separation Science Re-Defined, LCGC Supplement; 8.