
Research Article
Austin J Anal Pharm Chem. 2015;2(4): 1045.
Development of HPTLC Method for the Estimation of Aceclofenac and Tolperisone Hydrochloride in Combination
Patel DR*, Mehta FA, Shah DA and Chhalotiya UK
Department of Pharmaceutical Chemistry and analysis, Indukaka Ipcowala College Of Pharmacy, India
*Corresponding author: Divya R. Patel, Indukaka Ipcowala College of Pharmacy, Beyond GIDC, P.B. No. 53, Vitthal Udyognagar- 388 121, Gujarat, India.
Received: April 13, 2015; Accepted: June 05, 2015; Published: June 09, 2015
Abstract
A simple, sensitive, and precise high-performance thin-layer chromatographic method has been developed for estimation of Aceclofenac (ACF) and Tolperisone HCl (TOL) in combine dosage form. The method employs thin-layer chromatography (TLC) aluminum plates precoated with silica gel 60 F254 as the stationary phase, while the solvent system was n-butanol: 1, 4-dioxan(6:4v/v). The Rf values were observed to be 0.30 ± 0.02 and 0.60 ± 0.02 for ACF and TOL respectively. The separated band was densitometrically analyzed in absorbance mode at 268 nm. The method was linear in the range of 400-900 ng/band for ACF and 600-1350 ng/band for TOL. The limits of detection for ACF and TOL were found to be 14.41and81.54ng/band respectively. The limits of quantification for ACF and TOL were found to be 43.67 and 247.11 ng/band respectively. The proposed method was validated with respect to linearity, accuracy, precision and robustness.
Keywords: HPTLC; Aceclofenac; Tolperisone Hydrochloride; Validation
Introduction
Aceclofenac (ACF) is 2-[(2,6-dichlorophenyl)amino] phenylacetoxyacetic acid [1]. It is a white or almost white and crystalline powder. Its Molecular formula is C16H13Cl2NO4 and Molecular weight is 354.18 [2]. It is a new non-steroidal antiinflammatory, antirheumatic and analgesic drug of the phenyl acetic acid group. It is used for the treatment of inflammatory disorders and acute pain with better gastric tolerance [3]. ACF inhibits synthesis of the inflammatory cytokines interleukin [IL-1] and tumor necrosis factor and prostaglandin E2 [PGE2] production [4]. Detailed survey of literature for ACF reveled several methods based on titrimetric analysis [5]. ACF is a medicine which is used in rheumatoid arthritis, osteoarthritis and ankylosing spondylitis. ACF helps to reduce inflammation and to reduce pain. ACF can take a few weeks to help improve inflammation but can start to relieve pain after the first few doses [6].
Tolperisone Hydrochloride (TOL), chemically 2-methyl-1-(4- methylphenyl)-3-(1-piperidyl) propan-1-one monohydrochloride, is a piperidine derivative. TOL is a white to off-white crystalline powder. Its molecular formula and molecular weight are C16H13Cl2NO. HCl and 281.83 respectively [7]. TOL is centrally acting muscle relaxant which acts by inhibiting voltage gated sodium and calcium channels. TOL has the unique property of mediating muscle relaxation without concomitant sedation and it does not cause in co-ordination, weakness and mental confusion or withdrawal phenomena, in contrast to other muscle relaxants [8]. TOL is extremely water soluble; it is more stable in acidic medium because it is disposed to decomposition in aqueous solution, which is faster at higher pH. Japanese pharmacopoeia suggests a potentiometric titration for the determination of TOL in bulk [9]. A number of methods such as HPTLC [10], HPLC [1, 11] have been reported for the quantitative estimation of TOL. Potentiometric titration is an accurate but highly tedious method and no other official method is reported. Spectroflourometric [12], densitometric [13,14], HPLC [14,15], spectrophotometric [12,14,16] for the determination of ACF in pharmaceutical formulation. Tolperisone, a centrally acting muscle relaxant agent, which has been in therapeutic use for more than three decades, has been widely used as spasmolytic of choice. It is recently launched drug in India for acute and chronic back pain and spasticity of neurological origin. Tolperisone is an aryl alkyl β-aminoketone having an asymmetric carbon atom α to the carbonyl group. It has higher muscle relaxant activity. It exhibits membrane stabilizing potency, which is characteristic of antiarrhythmic and local anesthetic agents [17].
With the reference of article “Effectiveness of Tolperisone Hydrochloride [TOL] with Aceclofenac [ACF] as combined therapy in acute low back pain”.
It was found that the combination of ACF and TOL have better effect than ACF alone in acute low back pain with no chances of adverse effect by combining both the drugs [9].
One UV spectrophotometric method is reported for estimation of ACF and TOL [17].
There is no HPTLC method reported for the estimation of ACF and TOL, hence an attempt was made to develop HPTLC. .
Experimental Work
Instrumentation
HPTLC instrument consists of CAMAG (Muttenz, Switzerland) Linomat V sample applicator with 100 μL applicator syringe (Hamilton, Bonadauz, Switzerland). Chromatography was performed on 10 cm × 10 cm aluminum TLC plates precoated with silica gel 60- F254 (E. Merck, Darmstadt, Germany). CAMAG TLC scanner 4 was used for the densitometric scanning of the developed chromatogram.
All drugs and chemicals were weighed on METTLER TOLEDO electronic balance (ME204, METTLER TOLEDO GROUP, Mumbai, India).
Chemicals and reagents
Methanol of analytical reagent obtained from S. D. Fine Chemicals, Mumbai, India. n-butanol of analytical reagent obtained from S.D. Fine chem., Mumbai, India. And 1,4-dioxan was obtained from Suvidhinath Laboratories, Baroda, India. The drugs ACF and TOL were obtained as gift sample.
Chromatographic system
Sample application: Standard and synthetic mixture sample of ACF and TOL were applied on the plates in the form of narrow bands of 6 mm length. The bands were applied 10 mm above from the bottom and 15 mm away from left edge of the plate. Samples were applied under a continuous drying stream of nitrogen gas.
Mobile phase and development: Plates were developed using a mobile phase consisting of n-butanol: 1,4-dioxane (6:4 v/v). Linear ascending development was carried out in a twin-trough glass chamber equilibrated with the mobile phase vapors for 30 min. Ten milliliters of the mobile phase (10 ml) were used for each development and were allowed to migrate at a distance of 80 mm. After development, the plates were dried completely.
Densitometric analysis: Densitometric scanning was performed in the absorbance-reflectance mode under control by win CATS planar chromatography software (CAMAG, Muttenz, Switzerland). The source of radiation was the deuterium lamp, and bands were scanned at 268 nm. The slit dimensions were 5 mm length and 0.45 mm width, with a scanning rate of 20 mm/s. Concentrations of the compound were determined from the intensity of diffusely reflected light and evaluated as peak areas against concentrations using a linear regression equation.
Preparation of standard stock solution
10 mg of ACF and 15 mg of TOL were accurately weighed and transferred to two separate 10 ml volumetric flasks and dissolved in a few milliliters of methanol. The volumes were made up to the mark with methanol to yield a solution containing 1000 μg/ml of ACF and 1500 μg/ml TOL. Aliquots from the stock solution of ACF and TOL were appropriately diluted with methanol to obtain working standards of 100 μg/ml and 150 μg/ml respectively. Aliquots from the stock solutions of ACF and TOL were appropriately diluted with methanol to obtain working standards of 100 μg/ml of ACF and 150 μg/ml of TOL.
Validation: Validation of the developed HPTLC method was carried out according to the International Conference on Harmonization (ICH) guidelines Q2 (R1) [18].
Linearity of calibration curves: Linearity of the method was evaluated by constructing calibration curves at six concentration levels over a range of 400–900 ng/band for ACF and 600-1350 ng/ band for TOL. The calibration curves were developed by plotting peak area versus concentration (n = 6).
Accuracy: The accuracy of the method was determined by calculating recoveries of ACF and TOL by method of standard additions. Known amounts of this combination (0, 720, 800, 880 ng/band) ACF and (0, 1080, 1200, 1320 ng/band) TOL were taken from the working standard solution (100μg/band of ACF and 150 μg/ band TOL) and the amounts of this combination was estimated by measuring the areas and by fitting these values to the straight-line equations of the calibration curves.
Precision: Precision was evaluated in terms of intra-day and inter-day precisions. For precision study, working standard solution of 100μg/ml of ACF and150 μg/ml TOL were used for applying bands on the HPTLC plate. Intra-day precision was determined by analyzing sample solutions of this combination (400, 600, 800 ng/ band) of ACF and (600,900,1200 ng/band) of TOL at three levels covering low, medium, and high concentrations of the calibration curve three times on the same day (n = 3). Inter-day precision was determined by analyzing sample solution of ACF (400,600,800 ng/ band) and TOL (600, 900, 1200 ng/ band) and at three levels covering low, medium, and high concentrations over a period of 3 days (n = 3). The peak areas obtained were used to calculate mean and relative standard deviation (% RSD) values.
Repeatability of measurement of peak area was determined by analyzing ACF (600 ng/band) and TOL (900 ng/band) six times without changing the position of plate on the same plate to check the repeatability of injection.
Sensitivity: The limit of detection (LOD) is defined as the lowest concentration of an analyte that can reliably be differentiated from background levels. The limit of quantification (LOQ) of an individual analytical procedure is the lowest amount of analyte that can be quantitatively determined with suitable precision and accuracy.
LOD and LOQ were calculated using the following equation as per ICH guidelines:
LOD = 3.3 × σ/S
LOQ = 10 × σ/S
Where S is the standard deviation of y-intercepts of regression lines and S is the slope of the calibration curve.
Robustness: Small changes in the chamber saturation time and solvent migration distance were introduced, and the effects on the results were examined. Robustness of the method was determined in triplicate at concentration levels of 600 ng/bandand900 ng/spot of this combination. The mean and % RSD values of the peak areas were calculated.
Solution stability: The solutions at analytical concentration (600 ng/band for ACF and 900 ng/spot TOL) were prepared from working standard solutions (100 μg/ml of ACF and 150μg/ml of TOL) and stored at room temperature for 24 h and analyzed at intervals of 0, 6, 12, and 24 h for the presence of any band other than that of this combination. The results were simultaneously compared with the freshly prepared ACF and TOL standard solution of the same concentrations in the form of change in % RSD of the response obtained.
Analysis of formulation: An in-house formulation was used for the assay. The dose of the drugs was 100mg and 150 mg respectively.
Powder equivalent to 100 mg of ACF and 150 mg of TOL was taken in 100 mL volumetric flask. Methanol was added to the above flask, and the flask was sonicated for 15 min. The solution was filtered using Whatman filter paper, and the volume was made up to the mark with methanol.
Appropriate volume of the aliquot was transferred to a 10 mL volumetric flask, and the volume was made up to the mark with the methanol to obtained 100 μg/ml ACF and 150 μg/ml TOL respectively. This solution was used for the estimation of ACF (500 ng/band) and TOL (750 ng/band) respectively.
Results and Discussion
Optimization of the mobile phase
To develop the HPTLC method for the estimation of ACF (Figure 1) and TOL (Figure 2). In dosage form, selection of the mobile phase was carried out on the basis of polarity. A mobile phase that would give a dense and compact band with an appropriate Rf value for this combination was desired. Various mobile phases were tried at initial stage of method development. Mixture of n-butanol: methanol (7:3, v/v), n-butanol: Et Ac: TEA (5:4:1 v/v/v), n-butanol: ethyl Acetate : Acetic Acid (7:3 v/v), n-butanol: acetone: TEA (7:2:1 v/v), n-butanol: ethyl acetate: ammonia (7:2:1 v/v/v ) were tried as mobile phases, but satisfactory resolution of drugs were not achieved. The mobile phase n-butanol: 1, 4-Dioxan (6:4 v/v) was found to be satisfactory and gave good separation for ACF and TOL (Figure 3). It was also observed that chamber saturation time and solvent migration distance were crucial in the chromatographic separation. A chamber saturation time of less than 15 min and solvent migration distances greater than 80 mm resulted in diffusion of the analyte band. So, n-butanol: 1, 4- Dioxan (6:4 v/v) mobile phase with a chamber saturation time of 30 min at ambient condition and solvent migration distance of 80 mm was selected as optimum conditions. These chromatographic conditions produced a well-defined, compact band of ACF and TOL with Rf 0.30 ±0.02 and 0.60 ± 0.02 respectively (Figure 3).
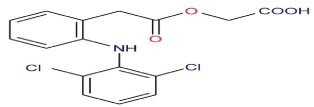
Figure 1: Chemical Structure of Aceclofenac.
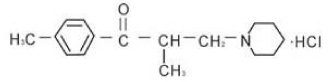
Figure 2: Chemical Structure of Tolperisone hydrochloride.
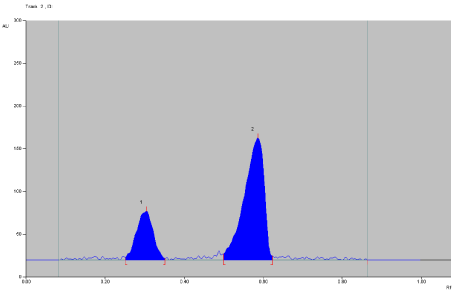
Figure 3: Densitograms of Aceclofenac and Tolperison hydrochloride.
Selection of detection wavelength
The sensitivity of HPTLC method that uses ultraviolet (UV) detection depends upon proper selection of detection wavelength. An ideal wavelength is the one that gives good response for the drugs that are to be detected. In the present study, solution was applied in the form of a band in concentration ranges of 400–900 ng/band of ACF and 600-1350 ng/band TOL were prepared in methanol. A solution was filled in the syringe, and under nitrogen stream, it was applied in form of band on a single plate. The plate was developed using n-butanol: 1, 4- Dioxan (6:4 v/v) at ambient condition and dried in air. The developed plate was subjected to densitometric measurements in scanning mode in the UV region of 200–400 nm, and the overlain spectrum was recorded using CAMAG TLC Scanner 4. The overlay spectra showed that all drugs absorb appreciably at 268 nm. So, it was selected as detection wavelength (Figure 4).
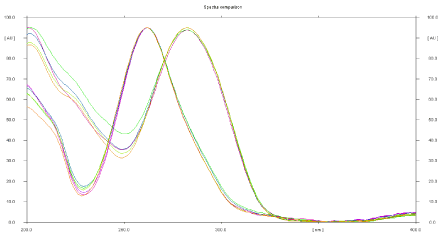
Figure 4: Overlain spectra of Aceclofenac and Tolperisone hydrochloride.
Validation
Linearity and Calibration Curves: Linearity of an analytical method is its ability, within a given range, to obtain test results that are directly, or through a mathematical transformation, proportional to the concentration of the analyte. The method was found to be linear in concentration ranges of 400–900 ng/band of ACF and 600-1350 ng/band TOL. The regression data showed a good linear relationship over the concentration range studied, demonstrating the suitability of the method for analysis (Table 1). Figure 5 displays a three-dimensional overlay of HPTLC densitograms of ACF and TOL, the calibration bands at 268 nm.
Parameters
Aceclofenac
Tolperison hydrochloride
Linearity (ng/band)
400-900
600-1350
Correlation coefficient( r2)
0.995
0.997
Slope of regression equation
2.82
2.001
Standard deviation of slope
0.064
0.098
Intercept of regression
255.04
1140.1
Standard deviation of intercept
12.315
49.591
Table 1: Regression analysis of calibration curve.
Figure 5: Three-dimensional overlay of HPTLC densitograms of calibration
bands.
Accuracy: Accuracy of an analytical method is the closeness of test results to the true value. It was determined by the application of analytical procedure to recovery studies, where a known amount of standard is spiked into pre-analyzed sample solutions. Percent Recoveries was found to be 99.36-100.31% for ACF and99.53-100.81 for TOL (Table 2).
Parameters
Aceclofenac
Tolperison hydrochloride
Linearity ( ng/band)
400-900
600-1350
Retention factor
0.30±0.02
0.60±0.02
Detection limit ( ng/band
14.81
81.54
Quantitation limit ( ng/band)
43.67
247.11
Accuracy (%)
99.36 - 100.31
99.53 - 100.81
Precision (%RSD)
Intra-day (n = 3)
1.05 - 1.31
1.09 - 1.12
Inter-day (n = 3)
1.12 - 1.58
1.07 - 1.47
Instrument precision (%RSD)
Scanner (n=7)
0.51
0.59
Injection (n=7)
0.831
0.77
Table 2: Summary of validation parameters of HPTLC.
Precision: Intra-day precision refers to the use of an analytical procedure within a laboratory over a short period of time by the same operator with the same equipment, whereas inter-day precision involves estimation of variations in analysis when a method is used within a laboratory on different days. The RSD values of the response were less than 2% for intra-day and inter-day precision, respectively. Repeatability of the scanning device and injection was studied by applying and analyzing ACF and TOL. (600 and 900 ng/ band) six times. The RSD values obtained were less than 2% (Table 2), which was under the acceptance criteria of ICH method validation guideline (<2%). The results indicated that the method is repeatable and reproducible (Table 2).
Limit of detection and limit of quantification: Under the experimental conditions used, the lowest amounts of drug that could be detected (LOD) for ACF and TOL were found to be14.41ng/band and 81.54ng/band, respectively. The limits of quantification (LOQ) for ACF and TOL were found to be 43.67ng/band and 247.11ng/ band (Table 2). This indicates that nano gram quantity of all drugs can be estimated accurately and precisely, this shows that the method is sensitive.
Robustness: The % RSD values less than 2% were obtained after introducing small, deliberate changes in parameters of the developed HPTLC method, confirming its robustness (Table 3).
Method Parameters/ Condition
Normal Condition
Deliberate Changes
Mean ± S.D (n=3)
ACF
TOL
Mobile Phase ratio
(n-butanol: 1-4-Dioxane)
6 : 4
5.5:4.5
1.90±0.005
1.63±0.01
6.5:3.5
1.80±0.006
1.38±0.01
Chamber saturation time
(min.)
30
33
1.56±0.003
0.79±0.005
27
1.59±0.005
1.03±0.008
Chamber Size
10*10
20*20
1.79±0.005
0.91±0.005
Table 3: Results of robustness studies.
Solution Stability: The stability of sample solutions was studied at ambient condition for 24 h. All drugs were found to be stable with a recovery of more than 97% (Table 4).
Drug
Time (hr)
Mean ± S.D. (n=3)
% Drug found
ACF
0
2003±1
100
4
1980 ± 10
98.85
8
1933 ± 1
99.5
12
2000 ±1
99.85
TOL
0
2017.66±0.08
100
4
2971 ± 0.03
99.77
8
2014.66 ± 0.05
99.87
12
2916±0.03
99.94
Table 4: Solution stability.
Analysis of formulation: The Formulation was analyzed using the proposed method which gave percentage recovery of more than 99.0% for ACF and TOL. A single band at Rf 0.30 ± 0.02 and 0.60 ± 0.02 was observed in the chromatogram for ACF and TOL, and no interference from the excipients present in the formulation was observed (Table 5).
Formulation
Amount taken (mg)
% Drug recovery
Aceclofenac
Tolperisone hydrochloride
Aceclofenac
Tolperisone hydrochloride
10
15
99.80 ± 1.42
99.53 ± 1.06
Table 5: Analysis of formulation.
Conclusion
Proposed study describes HPTLC method for the estimation of ACF and TOL in formulation. The method was validated and found to be simple, sensitive, accurate and precise. Statistical analysis proved that method was repeatable and selective for the analysis of ACF and TOL in formulation without any interference from the excipients.
References
- Government of India Ministry of Health and Family Welfare, published by The Indian Pharmacopoeia commission, Ghaziabad, 2014; 1466-1467.
- An Encyclopedia of chemicals, Drugs and Biological In Merck Index,14th Edn., published by Merck research Laboratories division of Merck & Co., Inc. White house station, NJ,USA, 2006; pp. -1636.
- Sharma M C, Sharma S. “Application to dissolution studies and HPLC assay method for determination of diacerein and aceclofenac in tablet dosage form.” American-Eurasian Journal of Scientific Research. 2011; 3: 149-154.
- British Pharmacopoeia Published the Stationary Office on behalf of the Medicines and Healthcare products Regulatory Agency (MHRA). London, 2011; 47-48.
- Maheshwari R K, Chturvedi S C, Jain N K. “Analysis of aceclofenac in tablets using hydrotropic solublisation technique”. Indian drugs. 2006; 6: 516-518.
- Koladiya BB, Vaghela VM. “UV spectrophotometric method: A quantitative estimation of tolperisone hydrochloride in bulk and pharmaceutical dosage form.” Int J. of pharm Tech Res. 2012; 4: 1317-1322.
- An Encyclopedia of chemicals, Drugs and Biologicals In Merck Index, 14th Edn., published by Merck research Laboratories division of Merck & Co., Inc. White house station, NJ, USA. 2006; 1637.
- Bhattacharya B, Naser S M. “Effectiveness of tolperiosne hydrochloride with Aceclofenac as combined therapy in acute low back pain.” I J P M R. 2012; 2: 74-78.
- Japanese Pharmacopoeia. The ministry of health, labour and welfare, prefectural office in Japan. 15th Ed. 2006; 1190.
- Liawruangrath S, Liawruangrath B. High performance thin layer chromatographic determination of tolperisone hydrochloride. J Pharm Biomed Anal. 1999; 20: 401-404.
- Raju TV, Seshadri RK, Arutla S, Mohan TS, Rao IM, Nittala SR. Development and Validation of a Precise, Single HPLC Method for the Determination of Tolperisone Impurities in API and Pharmaceutical Dosage Forms. Sci Pharm. 2013; 81: 123-138.
- El Kousy NM. Spectrophotometric and spectrofluorimetric determination of etodolac and aceclofenac. J Pharm Biomed Anal. 1999; 20: 185-194.
- el-Saharty YS, Refaat M, el-Khateeb SZ. Stability-indicating spectrophotometric and densitometric methods for determination of aceclofenac. Drug Dev Ind Pharm. 2002; 28: 571-582.
- Zawilla NH, Mohammad MA, El Kousy NM, El-Moghazy Aly SM. Determination of aceclofenac in bulk and pharmaceutical formulations. J Pharm Biomed Anal. 2002; 27: 243-251.
- Sheriker O D, Puranik M P, Yeole P G. “A validated reversed phase high performance liquid chromatographic method for simultaneous estimation of aceclofenac drug substance and its related traces impurities in solid dosage form.” Int.J. ChemTech Res. 2011; 2: 547-554.
- Gandhi S, Deshpande P, Rajmane V. “Method development and validation for simultaneous estimation of drotaverine hydrochloride and aceclofenac in tablet dosage form by RP-HPLC.” I J P S R R. 2012; 3: 49-52.
- Ahir KB, Naik HS, Patel A, Gandhi C, Solanki D, “Development and Validation of Spectrophotometric Method for Simultaneous Estimation of Tolperisone Hydrochloride and Aceclofenac by Absorbance Ratio Method in Synthetic Mixture.” Inventi Rapid. 2014; 1-5.
- ICH Guidelines. Validation of Analytical Procedures: Methodology; ICH Harmonized Tripartite Guidelines, Q2 (R1). 2005; 5-17.