
Special Article - Green Chemistry
Austin J Anal Pharm Chem. 2015;2(3): 1043.
Green Analytical Chemistry in Teaching Laboratory:Spectrophotometric Determination of Fe Ions with Using Green Tea to Demonstrate the Principles of Sequential Injection Analysis
Martinovic Bevanda A¹*, Talić S¹ and Ivankovic A²
1Department of Chemistry, Faculty of Science and Education, University of Mostar, Bosnia and Herzegovina
2Faculty of Agronomy and Food Technology, University of Mostar, Bosnia and Herzegovina
*Corresponding author: MMartinovic Bevanda Anita, Faculty of Science and Education, University of Mostar, Bosnia and Herzegovina.
Received: April 28, 2015; Accepted: May 28, 2015; Published: May 29, 2015
Abstract
An interesting, simple and an inexpensive experiment that can be successfully applied in student laboratory for the course of green analytical chemistry was proposed. The students have goal to design SIA method through experiments optimization and finally take advantage of this method in the analysis of real samples. Optimization procedures of SIA system are suggested and the achieved results presented. The proposed method, based on reaction between green tea polyphenols and Fe ions, can allowed the determination of Fe ions in the concentration range of 4×10-5 to 4×10-4 mol/L with sample throughput 122 h-1. The formed stable complex has the absorption maximum at λ = 570 nm. This optimized method was successfully applied to the determination of Fe ions in laboratory samples and pharmaceuticals.
Keywords: Green analytical chemistry; Sequential injection analysis; Teaching experiment; Green tea extracts; Natural reagents; Spectrophotometry
Introduction
The achievements of green chemistry in the years since it emerged as a cohesive field in the early 1990s has been remarkable. It is important to recognize the scientific breakthroughs that have been made through excellent research as well as through the other elements of education, industrial engagement, and outreach that are required to provide the broader structure needed to drive the field [1].
The timeframe of green chemistry implies that it is a „green” area; however the principles of green chemistry, suggested by Anastas and Warner [2], are well known to the scientific community in the field of chemistry. One editorial of this journal [3] is dealing with principles of green analytical chemistry. Different aspects of green chemistry idea have their foundation in sustainable development and in some present and future goals of all aspects of human activity.
The ideas of a green chemistry should become part of chemists and chemical engineers training from the very start. Students of chemistry programs at university should be guided to develop a deep consciousness of the importance of sustainability strategies in chemistry research and industry, and also to develop knowledge and skills to operate them [4].
In a special way the application of sequential injection analysis (SIA) can enhance green chemistry experiment or any other experiment and it can make it „greener“.
The main components of manifold SIA were injection pump and selection valve with ports for support of all solutions involved in the analysis. The main characteristic of SIA is the sequential aspiration of the determined small volumes of solutions to a holding coil and then flow reversal for the propulsion of reaction zone to the detector. The detector should be equipped to accept a solution in flow (eg. flow cell for spectrophotometry). Let us mention only the basic advantages provided by SIA: less waste due to a decrease in the consumption of the solution of reagents and samples, complete automation and control using software, a simple adaptation to different methods without significant changes of manifold.
In addition, green analytical methodology implies a combination of flow techniques and reagents from nature, as a ”green” reagents.
A symbiotic relationship exists between research and teaching aspects of any academic discipline. The next generation of researchers “learn their trade” in the undergraduate classroom and modern teaching practices are shaped by current research trends [5].
There are numerous possibilities to use natural plant [6-8] extracts as a tool in chemical analysis or in education chemical analysis. Several advantages arises from these approach: many plants to choose, low cost or no cost at all, renewable source, simple extraction, less ethical conflict, extended teaching/learning topics can be covered [6].
Interestingly, Cucurbira pepo L extracts can be used at optimized experimental condition for the synthesis of gold nanoparticles [9]. Also, the group of scientists has proposed educational experiment for preparation of gold nanoparticles using the extract of black tea leaves [10].
It is obviously from the available literature that very few research groups explore the potential of application of plant extracts in analytical chemistry and in educational chemistry.
Recently Pinyou and co-workers [11] have developed a new analytical method for determination of iron ions using flow injection analysis and natural reagent extracted from green tea. It seemed interesting to apply and adjust this experiment for teaching analytical chemistry in our undergraduate laboratory. The SIA system with spectrophotometric detector in combination with the extract of green tea as natural chromogenic reagents was used for determination of Fe (III) and Fe (II). This green analytical method is based on formation of Fe-polyphenol complex that was monitored at 570 nm. The suggested experiment can be divided in two experimental parts. The first one is the optimization of the SIA system. The second one is the application of the optimized method in the analysis of the real samples. For the successful work in laboratory it is important to get familiar with all the principles earlier, that the experiment is based on and the cooperation of students with their instructor.
Experimental
Reagents and chemicals
Standard solutions, 100 mL each of 0.1 M Fe(II) and Fe(III) were prepared by dissolving 3.9214 g Fe(NH4)2(SO4)2×6H2O and 4.8219 g Fe(NH4)(SO4)2×12H2O, respectively, in deionized (DI) water containing 1 % (v/v) concentrated H2SO4. Green tea is purchased from local stores. Extract (GTE) suspension was prepared with 4.0 g green tea from tea bags, in 150 mL acetate buffer (0.2 M, pH = 4.8) [11]. The suspension was shaken for 20 min. Then, the suspension was filtered and diluted to a volume of 500 mL with acetate buffer (pH= 4.8). The extract was prepared daily.
Sample solutions preparation. Twenty tablets were accurately weighed and ground. A portion of powder was weighted and mixed with DI water, concentrated HCl, then was boiled and it was allowed to cool down to room temperature and diluted to 1000 mL with water. The mixture was filtered. The aliquot of the filtrate was transfered to a 25 mL volumetric flask. Hydrogen peroxide (0.3 mL) was added, to ensure the complete oxidation of Fe(II) to Fe(III).
Aparatus
The system for sequential injection analysis is illustrated in Figure 1. It was constructed from syringe pump M6-Pump VICI (Valco Instruments, Houston, Texas, USA), a 10-port electrically actuated selection valve C25- 3180EMH Cheminert (Valco Instruments, Houston, Texas, USA), a Shimadzu UV mini-1240 (Shimadzu, Kyoto, Japan) UV-Vis spectrophotometer equipped with a flow cell (Hellma, Müllheim, Germany) of 80 μL internal volume and 10 mm optical path. Spectrophotometric data acquisition and control of measurement were achieved by coupling detector with personal computer and using UVmini-1240 data manager software and plug-in memory card with kinetics program both from Shimadzu (Shimadzu, Kyoto, Japan). M6-LHS -M6 Liquid Handling Software (Valco Instruments, Houston, Texas, USA) has been used for writing sequences of analysis and for the pump and the valve control. All used tubing were PTFE tubing of 1/16” OD and 0.75 mm ID supplied by VICI (Valco Instruments, Houston, Texas, USA)
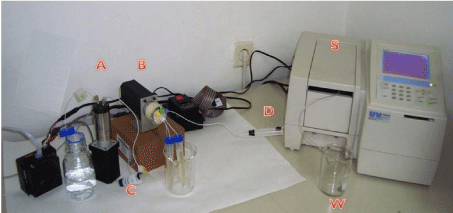
Figure 1: Sequential injection system. A-Siringe pump, B-Selection valve,
C-holding coil, D-reaction coil; S-UV-Vis Spectrophotometer with flow cell (V
= 80 μL), W- waste.
Recommended procedure for optimization SIA system
Before beginning of the analysis, all reagents and samples/ standards must be aspirated in holding coli (HC), and washed from HC dispensing carrier with flow reversal. This is necessary for filling all flow lines.
The optimization experiments were performed following sequences that had been written using the software (mentioned above) M6-LHS.
Briefly described steps to be taken during the optimization procedure are:
Valve position 1: At a selected flow the injection pump aspirates some volume of the green tea extract into the holding coil.
Valve positions 2-9: At a selected flow the injection pump aspirates the solution of the standard Fe ions into the holding coil.
Valve position 10 (detector line): With flow reversal the pump dispense 5 mL of the carrier solution (acetate buffer, pH = 4.8) and during this process it drives reagent and standard zones from the holding to the reaction coil and subsequently to detector.
After optimization experiments, obtained results can be used for determination of Fe ions in real samples. In Table 1 are presented the SIA protocol sequence (steps of analyses, injection volume and flow rate) that can be applied for analysis of real samples.
Operation
Valve port
Volume, µL
Flow rate,µL/min
Stacking of zones:
GTE aspiration
Sample/standard aspiration
Measurement of absorbance:
Dispense to detector
1
300
3500
2-9
350
3500
10
4000
3500
Table 1: Sequential injection system. A-Siringe pump, B-Selection valve, C-holding coil, D-reaction coil; S-UV-Vis Spectrophotometer with flow cell (V = 80 μL), W- waste.
Results and Discussion
The well-known principles of established green method were used as a starting material for the optimization of SIA system. This teaching experiment has so popular story about green tea polyphenols that can attract the interest of students for analytical chemistry experiment for development and optimization of SIA system. The obtained results will certainly be inspiring for the discussion upon completing the experiment.
Optimization of the SIA system
Before laboratory experiments and during discussions with their instructors students are made familiar with the well-known principles described previously (preparation of the green tea extract, influence of possible interferences, reagent stability) [5]. Moreover, students have to become familiar with different application possibilities and principles, which the system for the sequential injection analysis is based on.
System for sequential injection analysis (SIA) shown in Figure 1 was used for the optimization experiment and for the determination of iron ions with the green tea extracts (GTE). This spectrophotometric method was based on the reaction of the formation of stable complex between Fe ion and polyphenols from green tea extract. The advantage of SIA was injection of certain volume of analyte (Fe ions) and reagent (GTE) in the holding coil at a selected flow. Zone of iron solution was injected next to the zone of the solution of GTE. Zone dispersions begin from the moment of injection solution of reagents and analyte in the holding coil (Figure 2). Flow reverse, oriented towards the detector, promote mixing (zone dispersion) of sample and reagent zone, increasing amounts of formed complex which absorbs at the wavelength of λ = 570 nm. The optimization of variables that significantly affect the SIA system included: the injection volumes, carrier flow-rate, holding coil and reaction coil lengths.
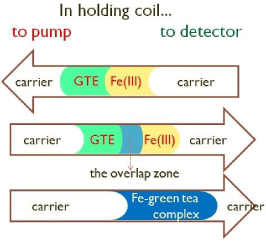
Figure 2: In holding coil. GTE-green tea extract.
The SIA method sensitivity is determined by dispersion degree of the reagent and sample zones under the flow conditions. The influence of the injected volumes on method sensitivity can be tested in determining Fe ions in solutions at the concentration, c {Fe (III)} = 1 × 10-4 mol / L. During the measurement different volumes of reagent solutions (GTE) and analyte were injected. Under the flow conditions these zones disperse, get mixed and amount of the formed complex increase. Furthermore, by increasing the volume of reagents and analyte, the amount of species mutually reacting is also increased, which results in greater amount of the formed complex and greater recorded absorbance. However, at a specific combination of volumes a considerable dilution can be expected as a consequence of dispersion and the concentration of the absorbing species is decreased in the overlapping zone and proportionally the measured absorbance is decreased as well. The volumes of Fe ions solutions in range of 80-400 μL and volumes of GTE solution in range of 200-400 μL can be tested.
The SIA method sensitivity is determined by dispersion degree of the reagent and sample zones under the flow conditions. The influence of the injected volumes on method sensitivity can be tested in determining Fe ions in solutions at the concentration, c {Fe (III)} = 1 × 10-4 mol / L. During the measurement different volumes of reagent solutions (GTE) and analyte were injected. Under the flow conditions these zones disperse, get mixed and amount of the formed complex increase. Furthermore, by increasing the volume of reagents and analyte, the amount of species mutually reacting is also increased, which results in greater amount of the formed complex and greater recorded absorbance. However, at a specific combination of volumes a considerable dilution can be expected as a consequence of dispersion and the concentration of the absorbing species is decreased in the overlapping zone and proportionally the measured absorbance is decreased as well. The volumes of Fe ions solutions in range of 80-400 μL and volumes of GTE solution in range of 200-400 μL can be tested. expected to get the concentration gradients (peaks) with narrower bases by increasing the flow-rate. Likewise, the height of peak is also increased by increasing the flow-rate. The optimal one is the flow-rate at which the maximal absorbance is recorded. Also, it can observe the reduction of the recorded absorbance in flows-rate greater than the optimal one. The recomended tested flow-rates range for this optimization experiments is 2000-5000 μL/min
The characteristic of the SIA system is the holding-coil in which the sample and reagent zones disperse. The flow direction is changed in the holding-coil, the mixing of zones is more successful, but dispersion is also increased. The holding coil with a optimal length gave the optimal result, implying: accommodation of the stacks of the sample and the reagent zone, initial dispersion, stable base line due to an uncontaminated carrier. The formation of complex is practically instantaneous, and higher peaks can be obtained with a shorter coil length due to less dispersion. At optimization of the holding-coil length the compromise between the reaction kinetics and dispersion has to be entirely achieved, which results in the maximal recorded absorbance and increases the method sensitivity. Generally, at optimal length of the holding coil the concentration gradients with narrower bases were recorded. A longer reaction coil can increased the residence time of the complex, while increasing the dispersion and decreasing the peak heights. The holding and reaction coils length can be tested over the range of 50.0 – 200.0 cm.
In the optimization process of the SIA system it is important to consider the influence of the internal diameter of the tubes in which the flow is in progress, the shape of the holding coil, and the whole length of the system. The internal diameter of the tubules, which the used SIA system is composed of, is adjusted to the dimensions of the valves and injection pump and it is Φ = 0.75 mm. In order to reduce dispersion even more, the holding coil can be spirally coiled around a solid base of a specific diameter, Φ = 10.0 mm. The total length of the tube system determines, as well, the total volume of the carrier solution, needed for enabling the flow of the overlapping zone by the means of the system through the detector. The properly chosen volume of the carrier solution, flowing through the flow system between the two sequences enables the quality of the system rinsing, differentiation of signals of the two measurements, the return to the baseline, and determining the next sample without the contribution of analyte remained from the previous measurement.
In table 2 is presented an overview of the parameters optimized by the teaching experiment and of values that are chosen as optimal. After the optimization of the SIA system it is important to get the good method sensitivity, to decrease dispersion and to improve the measurement dynamics.
Parameter /Optimization range
Suitable condition
Volume of GTE, 200-400 µL a,b,c,e
300 µL
Volume of standard Fe , 80-400 µL a,b,d,e
350 µL
Holding coil, 50-200 cm a,b,c,d,e
Reaction coil, 50-200 cm a,b,c,d,e
200 cm
50 cm
Flow rate, 2000-5000 µL/min a,c,d,e,
3500 µL/min
Experimental condition: a. c{Fe(III)} = 1×10-4 M; b. Flow rate: 2000 µL/min;
c. V(Fe(III)) = 100 µL; d. V (GTE) = 300 µL; e. Volume of flow cell: 80 µL.
Table 2: The parameters optimized by the teaching experiment and values that are chosen as optimal.
Analytical application of the optimized SIA system
The high of the concentration gradient (signal) is changed by changing the concentration of Fe ions, that is, the recorded absorbance is increased by increasing the concentration. The obtained piks have to be sharp and repeatable. Under the optimal conditions the linear dynamic range of concentrations is obtained, in which Fe (III) ions can be determined by the application of the SIA system: 4 × 10-5 mol/L to 4 × 10-4 mol/L. The optimized SIA system allows sample throughput of 122 h-1.
The equation of the calibration line, R² and the limits of detection and determination are presented in the table 3.
Linear regression equation
R2
LDR, mol/L
Detection limit, mol/L
Determination limit, mol/L
A=652.09c(Fe(II)) + 0.0183
0.9992
4 × 10-5 - 4 × 10-4
5.29×10-6
1.76×10-5
Table 3: Linear regression equation and figures of merit for Fe (II).
The method is tested in the pharmaceutics analysis. Regarding the fact that pharmaceuticals contain Fe (II) ions, the amount of 0.3 mL H2O2 was added into the working solution of the sample, in order for Fe (II) to oxidize into Fe (III).
The results of the analysis of the two commercially available pharmaceuticals are given in the table 4.
Sample
Labeled amount, mg
Determinated ± SD, mg
Recovery, %
Heferol
115
112.70 ± 0.005
98.00
Retafer
100
96.08 ± 0.003
96.08
Table 4: Results obtained for determination of Fe(II) in pharmaceuticals n = 3.
Conclusion
The process of the SIA system optimization, aiming at determining Fe ions by the application of the natural reagent, is suggested in this paper. The development of flow based technique such as SIA provides a new opportunity for the development of green analytical chemistry methods. This method offers good analytical characteristics due to their simplicity, high analytical frequency and capacity to reduce reagent consumption when compared with FIA or batch procedure. This method is interesting, inexpensive and suitable for the courses of analytical chemistry in under graduate studies and also in quality control laboratory for determination of Fe ions in pharmaceuticals. The applied method meets basic principles of green chemistry.
References
- Anastas Paul T, Beach Evan S. Green chemistry: the emergence of a transformative framework, Green Chem Lett Rev. 2007; 1: 9-24.
- P T Anastas, J C Warner. Green Chemistry: Theory and Practice, Oxford University Press, New York (1998).
- Samanidou Victoria F. Pharmaceutical Analysis from a Green Perspective. Austin J Anal Pharm Chem. 2014; 1: 1016.
- Eilks I, Rauch F. Sustainable development and green chemistry in chemistry education. Chem Educ Res Pract. 2012; 13: 57–58.
- Andraos J and Dicks Andrew P. Green chemistry teaching in higher education: a review of effective practices. Chem Educ Res Pract. 2012; 13: 69-79.
- Kradtap Hartwell S. Exploring the potential for using inexpensive natural reagents extracted from plants to teach chemical analysis. Chem Educ Res Pract. 2012; 13: 135–146.
- Grudpan K, Kradtap Hartwell S, Lapanantnoppakhun S and McKelvie I. The case for the use of unrefined natural reagents in analytical chemistry—A green chemical perspective. Anal Methods. 2010; 2: 1651–1661.
- Grudpan K, Kradtap Hartwell S, Wongwilai W, Grudpan S and Lapanantnoppakhun S. Exploiting green analytical procedures for acidity and iron assays employing flowanalysis with simple natural reagent extracts. Talanta. 2011; 84: 1396–1400.
- Insain P, Khonyoung S, Sooksamiti P, Lapanantnoppakhun S, Jakmunee J, Grudpan K, et al. Green Analytical Methodology Using Indian Almond (Terminalia Catappa L.) Leaf Extract for Determination of Aluminum Ion in Waste Water from Ceramic Factories. Anal. Sci. 2013; 29: 655-659.
- Sharma R K, Gulati S, and Mehta S. Preparation of Gold Nanoparticles Using Tea: A Green Chemistry Experiment. J. Chem. Educ. 2012; 89: 1316–1318.
- Pinyou P, Kradtap Hartwell S, Jakmunee J, Lapanantnoppakhun S, Grudpan K. Flow injection determination of iron ions with green tea extracts as a natural chromogenic reagent. Anal Sci. 2010; 26: 619-623.