
Special Article - Green Chemistry
Austin J Anal Pharm Chem. 2015;2(1): 1034.
Influence of Various Parameters on Cellulase and Xylanase Production by Different Strains of Trichoderma Species
Tanveer Bilal1*, Bisma Malik1, Reiaz ul Rehman1 and Manoj Kumar2
1Department of Bioresources, University of Kashmir, India
2School of Biotechnology and Bio-sciences, Lovely Professional University, India
*Corresponding author: Tanveer Bilal, Department of Bioresources, University of Kashmir, Srinagar -190006, Jammu & Kashmir, India.
Received: February 16, 2015; Accepted: March 02, 2015; Published: March 03, 2015;
Abstract
The main purpose of this study is to reduce the production cost of cellulase by using alternative carbon source such as wheat bran residue and optimized fermentation medium for high yields. In the present investigation, to isolate the novel cellulase producing fungi, Trichoderma viride and T. reesei from the soil samples and to optimize the physicochemical and nutritional parameters for cellulase and xylanase production. Wheat bran residue was found to be the best substrate source for the production of cellulase and xylanase by Trichoderma spp. Optimal concentration of wheat bran residue for the production of cellulase and xylanase was 1.0% (w/v). Optimum temperature, pH and incubation time of the medium for the cellulase and xylanase production by T. viride and T.reesei was 28° C, 5.5 and 120 h respectively. Cellulase production from T. reesei can be an advantage as the enzyme production rate is normally higher as compared to other fungi.
Keywords: Trichoderma spp. Cellulase; Xylanase; Wheat bran; Cellulose powder; Bio fuel
Introduction
Cellulases are multi-enzyme complex proteins secreted by diverse microorganisms that catalyze the conversion of most abundant organic polymer i.e. cellulose to smaller sugar units. These enzymes are considered as third largest industrial enzymes because of their tremendous application in cotton processing, paper recycling as well as feed additives. Currently due to rising oil prices and global warming, cellulase enzymes gained much importance as it plays a pivotal role in producing sustainable biofuel by the degradation of cellulosic biomass. Trichoderma species is widely studied fungi for cellulose degradation [1]. The cellulase produced by Trichoderma is resistant to chemical inhibitors and stable in stirred tank reactors at pH 4.8, 50° C for 48h or longer [2]. Majority of the cellulolytic microorganisms (Trichoderma spp.) produce complete cellulase enzymes that acts synergistically on native cellulose [3] besides producing other protein byproducts that helps in cellulose degradation [4]. T.reesei ß-glucosidase (BGL) is subject to product inhibition and though it is sufficient to support growth on cellulosic material; it is often supplemented with Aspergillus BGLs for complete biomass saccharification at industrial level [5]. Nevertheless, it is the best cellulase available today.
The major bottle neck against comprehensive application of cellulases in biofuel industry is the high cost of the enzyme production and source of substrate. Therefore, extensive research is needed to enhance the enzyme activity so that less enzyme is needed for the complete hydrolysis of biomass. In this investigation, cellulase producing strains of Trichoderma spp. isolated from soil samples were subjected to optimization of media and cultivation parameters for cellulase and xylanase production.
Materials and Methods
Chemical reagents
All chemicals and reagents used were of analytical grade. Different carbon sources such as birch wood xylan, Salicin, carboxymethyl cellulose (CMC) and glucose were obtained from Hi-media, Mumbai India; whereas wheat bran was purchased from local market of Srinagar, Kashmir, India.
Isolation of fungal culture
To isolate cellulase producing microorganisms, soil sample from decomposing sites of Botanical garden of Kashmir University was taken. The decomposing residue brought in the laboratory was kept at 4°C ± 1. The cellulose-hydrolytic fungus was isolated by direct agar plate method of Warcup [6] using Czapek Dox Agar medium.
Purification of fungal isolate
The contaminated cultures of fungal isolates were purified and derived through single spores, using dilution plating [7]. To ensure purity, cultures were derived from single spore in each case. The bacterial contamination was prevented by using antibiotic streptomycin (50mg/100 ml) in the medium during isolation and purification of fungi. After ensuring purity, the cultures were maintained in potato dextrose agar (PDA) slants at 4°C.
Screening for cellulolytic activity
Screening for cellulolytic activity was made using PDA media supplemented with 5% carboxymethyl cellulose (CMC). After certain incubation, the plates were stained by Congo red followed discolouration by 1M NaCl. The cellulase activity of each culture was determined by measuring the zone of clearance on agar plate [8].
Preparation of enzyme
Isolated fungal cultures were grown in 500 ml flask containing Mandel and Reese medium [9] supplemented with different carbon sources (1% of wheat bran, cellulose powder) at 28° C for 7 days. 10 ml samples were withdrawn on 7th day of inoculation and mycelia was separated by centrifugation at 10000 RPM for 20 min. Supernatant obtained was used as source of crude enzyme [10].
Quantitative enzyme assays
Assay of endoglucanase activity (CMCase assay): Endoglucanase activity was measured as per method described by Mandels et al. [11] with slight modification. Reaction mixture consisting of 0.5 ml of 1% CMC in citrate buffer (pH 4.8), 0.5 ml of enzyme sample and the reaction mixture is incubated at 50°C for 30 minutes. The reaction was stopped by adding 3 ml of DNS in the boiling water. After cooling down, the absorbance was taken at 540 nm [12].
Assay of exo-glucanase (FPase): Fpase activity was assayed by measurement of reducing sugars in the reaction mixture containing Whatman No. 1 filter paper (1.0 cm × 6.0 cm ~ 50.0 mg) as substrate in 1ml sodium citrate buffer (pH 4.8) and 0.5 ml enzyme sample and the reaction mixture is incubated at 50°C for 60 minutes and finally reaction is stopped by adding 3 ml of DNS reagent. The suspension is mixed well and the tubes are transferred to a boiling water bath for exactly 5 min. Tubes are immediately cooled in the cold water and absorbance is then measured at 540nm [11].
Assay of β–glucosidase (BGL) activity: BGL activity in measured as per the method described by Mandels [13]. Reaction mixture consisting of 0.5 ml of 1% salicin prepared in citrate buffer (pH 4.8), 0.5 ml of enzyme sample and then incubated at 50° C for 30 minutes. After appropriate period of incubation, the reaction is stopped by adding 3 ml of DNS reagent and the reaction mixture is boiled for exactly 5 minutes in vigorously boiling water bath. The tubes are cooled immediately in cold water and the absorbance is then measured at 540 nm. In accord with the International Union of Biochemistry, one enzyme unit (U) equals to 1μmol of glucose released per minute.
Xylanase activity: Xylanase activity was determined according to method described by Bailey et al. [14]. Reaction mixture consisting of 1.0 ml of crude extracellular enzyme sample, 1 ml of 1% birch wood xylan (prepared in 0.05 M Na-citrate buffer, pH 5.3) and 1 ml of 0.05 M citrate buffer. The mixture was incubated at 55°C for 10 min. The reaction was stopped by the addition of 3.0 ml of 3, 5- dinitrosalicylic acid (DNS) and the contents were boiled for 15 min [15]. After cooling, the color developed was read at 540 nm.
Optimization of culture conditions for enzyme production
Effect of incubation period in enzyme production: Fermentation period is important parameter for enzyme production by Trichoderma spp. In this study, experiments fermentation was carried out up to 6days and production rate measured at 24 h intervals.
Effect of pH and temperature on enzyme production: The most suitable pH of the fermentation medium was determined by adjusting the pH of the culture medium at different levels in the range of pH 4 to 5.5 using different buffers. In order to determine the effective temperature for cellulase production by the Trichoderma spp., fermentation was carried out at 28 to 32 ± 2°C.
Effect of various carbon sources on enzyme production: Effect of various carbon compounds viz., cellulose powder, wheat bran were used for studying. The broth was distributed into different flasks and 1% of each carbon sources were then added before inoculation of the strain and after culture inoculation, the flasks were incubated for 7 days at 28 ± 2°C.
Statistical analysis
Data presented on the average of three replicates (±SE) obtained from there independent experiments.
Results and Discussion
Isolation and identification of fungal strain
A total of two isolates of fungi were isolated and identified. Identification of fungal strain was made on the basis of morphological and microscopic characteristics by using standard reference manuals [16, 17] (Figure1, 2).

Figure 1a: Trichoderma viride.

Figure 1b: Trichoderma reesei.
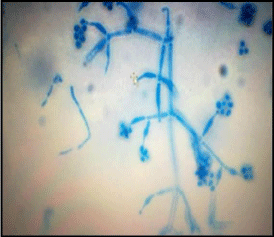
Figure 2: Conidia and Conidiophore branching in T.reesei using lacto phenol
cotton blue (100X).
Screening of fungal isolate for cellulolytic activity
Screening of fungus was conducted by using Congo red test as a preliminary study for identifying cellulase producer. The fungal isolates which showed a maximum clear zone on the cellulose and xylose containing medium after staining with Congo red were selected. The diameter of hydrolytic zones was measured which was found to be 10mm and 19mm. (Figure 3a, b)
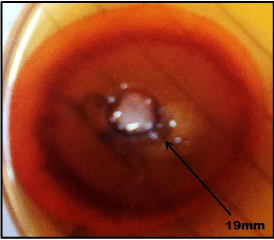
Figure 3a: Xylanase activity is revealed by hydrolyzed zone (19cm) around
the well.
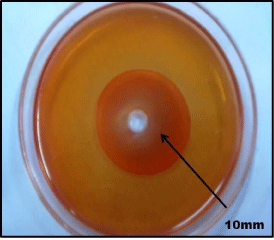
Figure 3b: EG activity is revealed by pale reddish zone around the well.
Cellulase assay
CMCase, FPase and BGL assays were carried out respectively for measurement of endoglucanase, exoglucanase and β-glucosidase activity (Table 1). The result shows that culture of T.reesei shows higher cellulase activity (Endoglucanase (EG)-5.89 IU/ml, Exo-glucanase (EXG)-4.32 IU/ml and β-glucosidase (BGL)-6.08 IU/ml; pH 5.5) than the T. viride with wheat bran as sole carbon source. The results reveal that T. reesei shows highest endoglucanase and exoglucanase activity than T. viride. Earlier it has been reported that endoglucanase was induced by CMC but repressed by glucose [18]. It was also found that Wheat bran, carboxymethyl cellulose and cellulose powder was preferred substrate for EG and EXG production [19].
S.No.
Species
Substrate
pH
EG activity (IU/ml)
EXG activity (IU/ml)
BGL activity (IU/ml)
Xylanase activity (IU/ml)
1.
T. reesei
Cellulose powder
4.0
3.11±0.03
0.46±0.02
3.2±0.02
12.04±0.01
5.0
3.816±0.05
1.315±0.01
4.07±0.04
1.146±0.05
5.5
4.692±0.04
2.759±0.04
6.01±0.06
14.73±0.07
Wheat bran
4.0
2.173±0.02
0.622±0.06
1.84±0.05
10.73±0.04
5.0
5.382±0.01
3.274±0.04
4.89±0.07
1.766±0.03
5.5
5.89±0.08
4.32±0.08
6.08±0.08
15.96±0.08
2.
T. viride
Cellulose powder
4.0
2.12±0.07
0.39±0.05
2.18±0.03
9.89±0.05
5.0
2.89±0.04
1.03±0.04
3.11±0.05
1.104±0.07
5.5
3.26±0.02
1.52±0.08
4.05±0.07
10.02±0.09
Wheat bran
4.0
2.10±0.05
0.46±0.03
1.82±0.04
8.46±0.06
5.0
4.330.04
2.11±0.06
4.22±0.06
1.56±0.05
5.5
3.67±0.05
3.16±0.02
5.06±0.09
12.06±0.08
Table 1: Comparative analysis between T. reesei and T. viride for Cellulases (EG, EXG and BGL) and Xylanase production on different carbon sources at various pH.
Xylanase assay
Among the two cultures T.reesei exhibits highest xylanase activity (15.96 IU/ml) as compared to other isolate with wheat bran as a substrate. Earlier it has been reported that it is one of the most popular components of complex media for xylanase production [20]. Several substances have been indicated in the literature as suitable carbon sources for xylanase-producing micro-organisms, birch wood xylan [21], oat spelt xylan [22], wheat bran arabinoxylan [23], wheat bran and rice bran [24] are few recommended sources.
Optimization of culture conditions for enzyme production
Effect of pH on enzyme production: Cellulase yield by different strains of T. species were found to be pH dependent. Results illustrated by Table 1 clearly show that cellulase and xylanase production expressed as enzyme activity gradually increased as the pH value increased from 4-5.5 and reached its optimum level of pH 5.5 as also reported by Lee et al. [25]. Further increase in pH declines the enzyme activity.
Effect of Incubation temperature on enzyme production: The effect of temperature on the activity of the enzymes is shown in Figure 4. The enzymes were activated at 28° to 32°C after which the activity began to drop. The optimum temperature for the two strains was found to be 28°C. At higher temperature than the optimum, enzyme activity decreases because of denaturation as reported earlier [26].
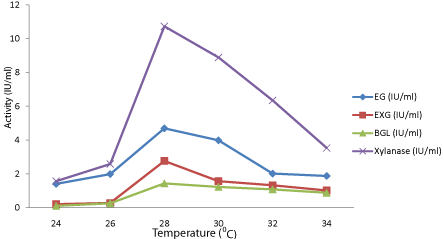
Figure 4: Effect of temperature on cellulase and xylanase production by
parent culture of T.reesei.
Effect of incubation period on enzyme production: Time course for cellulases production of T.reesei was also investigated. Optimum EXG, EG and BGL production was achieved at 120 h of incubation at pH 5 and 280C (Figure 5). Further increase in incubation time resulted in decrease in EXG, EG and BGL activities. It may be due to the depletion of nutrients in the medium which stressed the fungal physiology resulting in the inactivation of secretary machinery of the enzymes [27]. Time course of 120 h for optimum cellulases production by T. reesei is in accordance with earlier reports. Cellulolytic enzymes were produced by Aspergillus phoenix at 120 h incubation [28].
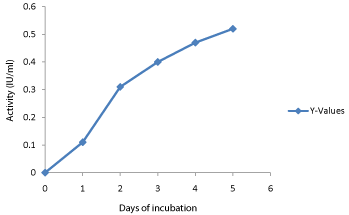
Figure 5: Influence of Time on Cellulase Enzyme Synthesis in Reese and
Mandels Mineral Salts medium using Trichoderma reesei. –�•–represents the
Enzyme activity.
Effect of carbon sources on enzyme production: Data presented in table 1 shows that cellulase and xylanase production by different strains of T. species was significantly influenced by the type of carbon source in the basal salt medium. In this study different carbon sources viz., wheat bran and cellulose powder were investigated. The results revealed that culture of T.reesei shows highest cellulase and xylanase production than other species. This indicates that 1% wheat bran is the most effective sole carbon source for cellulase and xylanase production followed by cellulose powder. Earlier Mandel and Reese [9] reported that maximum yields of cellulase were obtained on 1% cellulose powder carbon substrate using T.viride culture. Reduction in the cost of cellulase production can be achieved by the use of cheap and easily available substrates. Niranjane et al. [29] observed highest yields of cellulases on CMC. In this study, we found that wheat bran proved to be the best and cheap substrate for cellulase and xylanase production.
References
- Pirzadah T, Garg S, Singh J, Vyas A, Kumar M, Gaur N, et al. Characterization of Actinomycetes and Trichoderma spp. for cellulase production utilizing crude substrates by response surface methodology. BMC Springer Plus. 2014. 3: 622.
- Mandels M, Reese ET (1965). Ann. Rev. Phytopathol. 1965. 3: 85.
- Wilson DB. Three microbial strategies for plant cell wall degradation. Ann N Y Acad Sci. 2008; 1125: 289-297.
- Wang Y, Tang R, Tao J, Gao G, Wang X, Mu Y, et al. Quantitative investigation of non-hydrolytic disruptive activity on crystalline cellulose and application to recombinant swollenin. Appl Microbiol Biotechnol. 2011; 91: 1353-1363.
- Reczey K, Brumbauer A, Bollok M, Szengyel Z, Zacchi G. Use of hemi cellulose hydrolysate for ß-glucosidase fermentation. App. Biochem. Biotechnol. 1998. vol. 70-72, 225-235.
- Warcup. On the origin of colonies of fungi developing on soil dilution plates. Trans. Br. Mycol. Soc. 1955. 38: 298-301.
- Waksman SA. Principle of soil microbiology, Wilkim & Wilkims Co. Baltimore, 1st edition. 1927.
- Limtong PS, Vangnai V, Sunanthapongsuk S, Piriyaprin. Isolation and selection of thermophilic cellulolytic microorganisms for compost production in Thailand. Kasetsart J. Nat Sci. 1990. 24:108-115.
- MANDELS M, REESE ET. Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol. 1957; 73: 269-278.
- Vyas A, Vyas D, Vyas KM. Production and optimization of pretreated groundnut shell by Aspergillus terreus AV49. J. Sci. Ind. Res. 2005. 64: 281-286.
- Mandels M, Andreotti R, Roche R. Bioteh. Bioeng. Symp.1976. 6:17-37.
- Ghose TK, (1987). Measurement cellulase of activities. Pure & App. Chem, 59: 257-268.
- Mandels, M. Production and application of cellulase. Laboratory Procedures. U.S. Army Natick Laboratories, 1974. USA. 22 p.
- Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992. 23: 257-270.
- Miller. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959. 31: 426-428.
- Ellis MB. More Dematiaceous Hyphomycetes. CAB International Mycological Institute, Kew, UK. 1976. 507 pp.
- Raper KB, Fennell DI. The Genus Aspergillus. Baltimore MD: Williams & Wilkins 1965.
- Ahmed S, Aslam N, Latif F, Rajoka MI, Jamil A. Molecular cloning of cellulase genes from Trichoderma harzianum. (Eds.): Attaur-Rehman/ Choudhary/ Khan, Frontiers in Natural Product Chemistry. Bentham Science Publishers, The Netherlands. 2005.1: 73-75.
- Lucas R, Robles A, Garc´ia MT, Alvarez de Cienfuegos G, Gálvez A. Production, puri?cation and properties of an endoglucanase produced by the hyphomycete Chalara (syn. Thielaviopsis) paradoxa CH32. J Agric Food Chem. 2001. 49: 79–85.
- Sá-Pereira P, Costa-Ferreira M, Aires-Barros MR. Enzymatic properties of a neutral endo-1,3(4)-beta-xylanase Xyl II from Bacillus subtilis. J Biotechnol. 2002; 94: 265-275.
- Tseng MT, Yap MN, Ratanakhanokchai K, Kyu KL, Chen ST. Purification and characterization of two cellulase free xylanases from an alkalophilic Bacillus firmus. Enzyme Microbiol. Technol. 2002. 30: 590-595.
- Chivero ET, Mutukumira AN, Zvauya R. Partial purification and characterization of a xylanase enzyme produced by a microorganism isolated from selected indigenous fruits of Zimbabwe. Food Chem. 2001. 72: 179-185.
- Bataillon M, Nunes Cardinali A, Castillon N, Duchiron F. Purification and characterization of a moderately thermostable xylanase from Bacillus sp. strain SPS-0. Enzyme Microb Technol. 2000; 26: 187-192.
- Dhillon A, Gupta JK, Khanna S. Enhanced production, purification and characterization of a novel cellulase poor thermostable, alkali-tolerant xylanase from Bacillus circulans AB 16. Process Biochem. 2000. 35: 849-856.
- Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002; 66: 506-577, table of contents.
- Kang SW, Park YS, Lee JS, Hong SI, Kim SW. Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour Technol. 2004; 91: 153-156.
- Nochure SV, Roberts MF, Demain AI (1993). True cellulases production by Clostridium thermocellum grown on different carbon sources. Biotech. Lett. 1993. 15: 641-646.
- Dedavid e Silva LA, Lopes FC, Silveira ST, Brandelli A. Production of cellulolytic enzymes by Aspergillus phoenicis in grape waste using response surface methodology. Appl Biochem Biotechnol. 2009; 152: 295-305.
- Niranjane AP, Madhou P, Stevenson TW. The effect of carbohydrate carbon sources on the production of cellulase by Phlebia gigantean. Enzy Microbial Technol. 2007. 40: 1464-1468.