1Key Laboratory of Analytical Chemistry for Biology and Medicine (Ministry of Education), College of Chemistry and molecular Sciences, Wuhan University, China
2Quality Supervision and Measure Station, Tobacco Company of Guizhou Province, China
*Corresponding author: Hong-Wu Tang, Key Laboratory of Analytical Chemistry for Biology and Medicine (Ministry of Education), College of Chemistry and molecular Sciences, Wuhan University, China.
*Corresponding author: Jian Rao, Quality Supervision and Measure Station, Tobacco Company of Guizhou Province,China.
Received: November 26, 2014; Accepted: December 04, 2014; Published: December 05, 2014
Citation: Cai L, Wang W, Xiao-Yong Yang, Zhou P, Hong-Wu Tang, et al. Preparation of Fluorescent-Magnetic Silica Nanoprobes for Recognition and Separation of Human Lung Cancer Cells. Austin J Anal Pharm Chem. 2014;1(6): 1027.
In this work, we have prepared fluorescent-magnetic silica nanoparticles (NPs) based on RuBpy-doped silica NPs and super paramagnetic Fe3O4 NPs using coating method. RuBpy-doped silica NPs (~60 nm) and Fe3O4 NPs (~10 nm) are first prepared using the reverse micro emulsion method (W/O) and the high temperature oil cracking method, respectively. Polyethyleneimine (PEI) is then conjugated to the surface of RuBpy-doped silica NPs. Coordination reaction between amines of PEI modified RuBpy-doped silica NPs and Fe3O4 is further performed in the n-hexanol to realize magnetic materials coating. After silanization and carboxylation of prepared fluorescent-magnetic silica nanoparticles (FMNPs), wheat germ agglutinin (WGA) molecules are conjugated to carboxyl-modified fluorescent-magnetic silica NPs (FMNPs-COOH, 100 nm). To demonstrate the biological application of as-prepared fluorescent-magnetic silica NPs, WGA-conjugated fluorescent-magnetic silica NPs (FMNPs-WGA) are incubated with human lung cancer A549 cells and separated by a magnetic field and then imaged using fluorescence microscopy. The results of fluorescence imaging show that fluorescent-magnetic-bifunctional nanoprobes prepared by this method could be used for highly efficient recognition and separation of cancer cells.
Keywords: Dye-doped silica nanoparticles; Fe3O4 nanoparticles; Fluorescent-magnetic silica nanoparticles; Wheat germ agglutinin; Cancer cells
Rapid and sensitive detection of multiple components in complex biological system is a major problem faced by modern analytical chemistry and biotechnology. Although some fluorescent nonmaterial’s, such as quantum dots, carbon dots and dye-doped silica NPs have displayed great advantages in bioassays [1-6], they can only be functionalized for single signal response from the targeted analyte. In addition, some super paramagnetic particles, such as α-Fe, Fe3O4, and α-Fe2O3 have attracted great attention in biomedicine [7-9], but they have single separation functions. To overcome these difficulties, it is thus highly desirable to fabricate fluorescent-magnetic-bifunctional NPs for bioassays.
Dye-doped silica nanoparticles take advantages of resistance to photo bleaching, facile surface modification and good biocompatibility [10-12]. In addition, super paramagnetic Fe3O4 nanoparticles can aggregate rapidly in the presence of external magnetic field and disperse rapidly in solution in the absence of external magnetic field. So Fe3O4 is a very ideal material in bio separation and especially in the enrichment and separation of cancer cells [13]. Preparation methods of fluorescent-magnetic-bifunctional nanoparticles based on dye-doped silica nanoparticles and super paramagnetic Fe3O4 nanoparticles are mainly focused on embedding method and coating method at present. The theory of embedding method is based on Stöber method that hydrophilic Fe3O4 NPs and fluorescent dyes are together wrapped in the silica networks in the presence of tetraethyl orthosilicate (TEOS) and NH4OH. Although the embedding method is relatively simple and can be carried out in only a few hours, it is limited by the non-uniformity of the obtained products. In addition, the numbers of Fe3O4 NPs wrapped in different silica particles are different [14-17]. The theory of coating method is that polyethyleneimine (PEI) is first conjugated to the surface of some fluorescent nanomaterials, such as dye-doped silica NPs. PEI is an cationic polymer with a larger number of amines, which can preferentially bind to same metal atoms such as iron due to nitrogen-atoms of PEI containing lone-pair electron occupies the unoccupied orbitals of ferri ion (Fe3+) and ferrous ion (Fe2+) resulting in coordination reaction between polyethyleneimine (PEI) modified fluorescent nanomaterials and Fe3O4. The size of fluorescent-magnetic NPs prepared using coating method is uniform and Fe3O4 can be covered uniformly on the surface of fluorescent nanomaterials [18, 19].
Inspired by the extraordinary properties of dye-doped silica nanoparticles and super paramagnetic Fe3O4 nanoparticles, we fabricate fluorescent-magnetic nanoparticles based on RuBpy-doped silica nanoparticles and super paramagnetic Fe3O4 nanoparticles using coating method. Polyethyleneimine (PEI) is first conjugated to the surface of RuBpy-doped silica nanoparticles. Coordination reaction between amines of PEI modified silica nanoparticles and Fe3O4 is further performed in the medium of n-hexanol to realize magnetic nanoparticles coating. To demonstrate the biological application of as-prepared fluorescent-magnetic-bifunctional NPs (FMNPs), wheat germ agglutinin (WGA)-conjugated FMNPs were used for recognition and separation of human lung cancer A549 cells.
Triton X-100, n-hexanol, cyclohexane, tetraethyl orthosilicate (TEOS), ammonium hydroxide (NH4OH, 25-28 wt %), 1-ethyl- 3-(3-dimethylaminopropyl) carbodiimid ehydrochloride (EDC), N-hydroxysulfosuccinimide sodium salt (sulfo-NHS), hexahydrate (RuBpy), iron(III) acetylacetonate (Fe(acac)), hexadecylamine (HDA), Oleic acid (OA), polyethyleneimine (PEI, 25 kD), (3-aminopropyl) triethoxysilane (APTES), wheat germ agglutinin (WGA), and polyvinylpyrrolidone (PVP-k30) were purchased from sigma chemical Co. (St. Louis. MO). Carboxyethlsilanetriol sodium salt (CEOS) was purchased from ABCR Chemical Co. (Germany). Human lung cancer A549 cells lines were obtained from China Center for Type Culture Collection (Wuhan, China).
The size and uniformity of synthesized silica NPs were measured with a transmission electron microscope (JEOL, JEM 100 CXII and Japan). Zeta potential measurement was carried out using a zetasizer (Nano-ZS, Malvern Instruments, Malvern, U.K.). Photoluminescence was measured using a fluorometer (Hitachi, F-4600, Japan) equipped with a 150W xenon lamp. Fluorescence images were recorded with a Nikon ECLIPSE TE2000-U inverted fluorescence microscope equipped with a Nikon INTENSILIGHT C-HGFI lamp and Q-IMAGING RETIGA 2000R CCD. Superparamagnetism was measured using a magnetometer (Lakeshore, F-4600, USA).
Dye-doped silica nanoparticles (SiNPs-COOH) were prepared by the reverse micro emulsion method. Briefly, a water-in-oil micro emulsion was prepared by mixing 1.77 mL of Triton X-100, 7.5 mL of cyclohexane, 1.6 mL of n-hexanol, 80 μL of 0.1 M RuBpy aqueous solution, 400 μL of water, and 100 μL of tetraethyl orthosilicate (TEOS) that was stirred for 30 min at room temperature, and then 60 μL of NH4OH was added to initiate silica polymerization. The reaction was allowed to continue for 24 h at room temperature to form SiNPs with free hydroxyl groups. A post coating of carboxyl-modified silica (SiNPs-COOH) was then prepared by adding 50 μL of TEOS and 40 μL of carboxylethysilanetriol sodium salt. This polymerization was allowed to proceed for 24 h. After the reaction completed, the nanoparticles were isolated by acetone, followed by centrifuging and washing with 95 % ethanol three times and deionized water one time. The NPs were finally re suspended in 0.1 M PBS buffer (pH 7.4) and stored at 4°C until use.
Fe3O4 nanoparticles were synthesized by the high temperature oil cracking method [20-23]. Briefly, 10 mL of diphenyl ether, 2 mL of oleic acid, 0.7 g of iron(III) acetylacetonate (Fe(acac)), and 1.6 g of hexadecylamine were immediately added to a 250mL round-bottomed flask. After heated to 185°C slowly and the reaction was kept for 10min under this temperature, the reaction was then heated to 270°C quickly. After reflux reaction for 2h, the reaction solution was cooled to room temperature and 35mL of 100% ethanol was added. After mixing and centrifugation (16000 rpm×5min), the obtained depositions were re suspended in 10mL of n-hexane and then centrifuged (16000 rpm×5min). Finally, the collected supernatant was stored at 4°C until use.
Fabrication of fluorescent-magnetic-bifunctional nanoparticles: 1mg of EDC, 2.5mg of Sulfo-NHS, and 500μL of polyethyleneimine (PEI) diluted in 0.01M PBS (pH 7.2) at a concentration of 0.2g/mL were added to 2mL of carboxyl-modified RuBpy-doped silica NPs dispersed in 0.1M PBS (pH 7.2). The solution was then incubated for 24h at room temperature with gentle shaking. After the reaction was completed, the nanoparticles were thoroughly washed by three rounds of centrifugation and then washed with 0.5 M NaCl, deionized water and 100% ethanol, respectively. The collected NPs were resuspended in 0.5mL of n-hexanol. The synthesized Fe3O4 nanoparticles dispersed in n-hexane were precipitated by ethanol. After centrifugation and supernatant removal, the Fe3O4 nanoparticles were resuspended in 0.5mL of n-hexanol. Then 0.5mL of PEI-modified silica NPs dispersed in n-hexane and 0.5 mL of Fe3O4 nanoparticles dispersed in n-hexane were mixed together and incubated for 2h at room temperature with gentle shaking. After the reaction was completed, the solution was thoroughly washed by three rounds of centrifugation and then washed with n-hexane, 100% ethanol and deionized water, respectively. The collected NPs were resuspended in 0.1M PBS (pH 7.2). 500μL of 0.2g/mL PEI (25 kD) in 0.01 M PBS (pH 7.2) was added to the solution of NPs and then incubated for 2h at room temperature with gentle shaking. After the reaction was completed, the particles were thoroughly washed by three rounds of centrifugation and then washed with 0.1 M PBS (pH 7.2). The collected NPs were finally resuspended in deionized water until use.
Carboxylation of fluorescent-magnetic-bifunctional nanoparticles: First, 1mL of fluorescent-magnetic-biofunctional nanoparticles in deionized water was centrifuged. After the supernatant removal, the pellet was washed by centrifuging four times with 100% ethanol and then added to 2mL of PVP-k30 diluted in ethanol at a concentration of 20mg/mL. The solution was incubated for 12 h at room temperature with gentle shaking. After the reaction was completed, 60μL of NH4OH and 20μL of tetraethyl orthosilicate (TEOS) were added to the solution and then incubated for 12h at room temperature with gentle shaking. After the reaction was completed, 20μL of tetraethyl orthosilicate (TEOS) was added to the solution. The reaction was allowed to continue for 12h at room temperature, followed by addition of 30μL of APTES and reacting 24h at room temperature. After the reaction was completed, the product was thoroughly washed by three rounds of centrifugation and then washed with deionized water and 100% ethanol, respectively. The collected NPs were resuspended in 5 mL DMF. Then 5mL solution of succinic anhydride in DMF (10%) was added to the NPs solution and incubated for 24 h at room temperature with gentle shaking by the protection of nitrogen [24]. After the reaction was completed, the product was thoroughly washed by three rounds of centrifugation and then washed with 100% ethanol and deionized water, respectively. The collected NPs were finally resuspended in 1 mL of deionized water until use.
Preparation of WGA-conjugated fluorescent-magnetic-bifunctional nanoparticles: 0.5 mL of carboxyl-modified fluorescent-magnetic-bifunctional nanoparticles in deionized water was centrifuged and then resuspended in 0.1 M PBS (pH 7.2). 2 mg EDC, 5 mg Sulfo-NHS and 0.5 mg WGA were immediately added to the NPs solution. The solution was then incubated for 2 h at room temperature with gentle shaking. After the reaction was completed, the product was thoroughly washed by three rounds of centrifugation and then washed with 0.1 M PBS (pH 7.2). WGA-conjugated fluorescent-magnetic-bifunctional NPs were finally resuspended in 0.5 mL of 0.1 M PBS (pH 7.2) until use.
Human lung cancer A549 cells were cultured in DMEM medium supplemented with 10% fetal bovine, 1% penicillin-streptomycin, and insulin and incubated at 37°C in a 5% CO2 environment. A549 cells were cultured until the covering rate on the six-well plate reached 70-90%. After the removal of the medium, the adherent cells were digested by pancreatin. Fresh medium was then added to the digested cells on the six-well plate. The suspended cells were added to 2 mL of centrifuge tube and centrifuged (1000 rpm×5 min). After the removal of the supernatant, the collected cells were resuspended in 1 mL PBS. 100 μL of cell suspension was diluted 10-fold with PBS solution. 50 μL of WGA-conjugated fluorescent-magnetic-bifunctional nanoparticles was then added to the solution. The solution was incubated for 40 min at room temperature with gentle shaking. After the reaction was completed, the cells were separated by a magnetic frame and then observed and analyzed with a fluorescence microscope equipped with a CCD for optical imaging. Carboxyl-modified RuBpy-doped silica NPs were used as the control group.
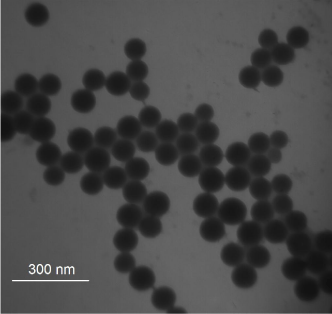
The silica NPs were prepared using the reverse micro emulsion method (W/O), which is a robust and efficient method of NPs preparation. The micro emulsion yields mono disperses particles in the nanometer range. Although the Stöber method is relatively simple and can be carried out in only a few hours (Stöber and Fink, 1968), it is limited by the non-uniformity of the products obtained, filtration and further separation for the products. The size of SiNPs-COOH was measured using a transmission electron microscope (TEM). As shown in Figure 1, the NPs were uniform in shape with average diameter ~60 nm. The functionalized NPs were dispersed very well in aqueous solution and no aggregation was observed due to the electrostatic force between the NPs. The particle size distribution for the synthesized NPs was carried out using dynamic light scattering. The average hydrodynamic size and polydispersity index of NPs were 80.60 ± 0.33 and 0.04 ± 0.01, respectively (data not shown).
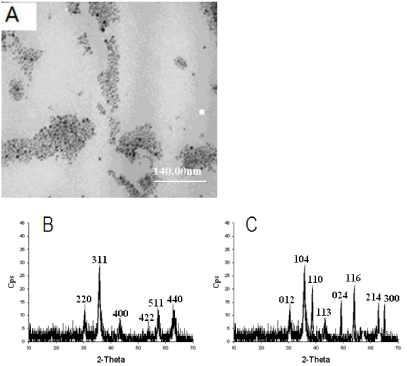
Oil soluble Fe3O4 nanoparticles were synthesized by the high temperature oil cracking method based on iron (III) acetylacetonate (Fe (acac)) as the precursor and hexadecylamine (HDA) and oleic acid (OA) as the ligand. In addition, in the process of preparation, diphenyl ether with high boiling point was used as the reaction medium. If the temperature was below 185°C, Fe (acac) cannot be dissociated and the prepared materials would not display magnetism. While if the temperature was above 185 oC, the size of the obtained materials would not be uniform. So it is critical that temperature should be controlled at 185°C and the reaction should be kept for 10- 20 min under this temperature. The size of Fe3O4 nanoparticles was measured using a transmission electron microscope (TEM). As shown in Figure 2A, the NPs were uniform in shape with average diameter ~10 nm. In order to further verify the prepared materials are Fe3O4, the prepared magnetic materials were characterized by XRD. Compared with the standard diffraction card, we cannot differentiate that the prepared materials are Fe3O4 or γ-Fe2O3 because Fe3O4 and γ-Fe2O3 have the same XRD pattern (Figure 2B). After high temperature calcinations in the air, the prepared magnetic materials were characterized by XRD. The XRD pattern is the same as the pattern of α-Fe2O3 because both Fe3O4 and γ-Fe2O3 can be converted into α-Fe2O3. In order to further confirm the prepared magnetic materials, the prepared magnetic materials were characterized by XRD after calcinated with high temperature in the argon. We found that the XRD pattern is significantly different from the pattern of α-Fe2O3 (Figure 2C). Because Fe3O4 is only converted into α-Fe2O3 in the presence of oxygen and γ-Fe2O3 can be converted into α-Fe2O3 in the absence of oxygen, we conclude that the prepared magnetic materials are Fe3O4.
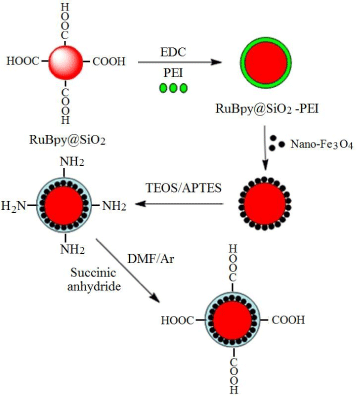
Coating method was used for the fabrication of fluorescent-magnetic-bifunctional nanoparticles (FMNPs) based on carboxyl-modified RuBpy-doped silica NPs and magnetic Fe3O4 NPs. As shown in Figure 3, the magnetic Fe3O4 NPs were conjugated to carboxyl-modified RuBpy-doped silica NPs surface to form a cladding. However, carboxyl-modified RuBpy-doped silica NPs are hydrophilic and magnetic Fe3O4 NPs are oleophilic. To solve this problem, amphiphilic polyethyleneimine (PEI) was used as a mediator and was first conjugated to carboxyl-modified RuBpy-doped silica NPs surface. PEI is a cationic polymer with a larger number of amines [25-27], which can preferentially bind to same metal atoms such as iron because nitrogen-atoms of PEI containing lone-pair electrons and they can occupy the unoccupied orbitals of ferri ion (Fe3+) and ferrous ion (Fe2+), thus resulting in coordination reaction between polyethyleneimine (PEI) modified silica NPs and Fe3O4 NPs. In addition, both PEI-modified RuBpy-doped silica NPs and Fe3O4 NPs have good solubility in n-hexanol, thus providing a favorable condition for the coordination reaction. The surface of RuBpy-doped silica NPs is coated by a layer of Fe3O4 and it is oleophilic. To enhance the water-solubility of FMNPs, the second coordination reaction between PEI molecules and RuBpy-doped silica NPs surface coated by a layer of Fe3O4 was performed in n-hexanol. At this time, the molecular structure of RuBpy-doped silica NPs surface can be vividly called a sandwich structure (PEI-Fe3O4 -PEI-NPs).
The surface modification of FMNPs is important because the preparation of nanoprobes is usually required to conjugate the desirable bio molecules (proteins or aptamers). The prepared FMNPs mentioned above were first incubated with PVP-k30 in ethanol. PVP-k30 can be adsorbed on the surface of NPs and it prevents the aggregation of NPs. NH4OH and TOES were then added to the solution. TEOS was hydrolyzed and polymerized to form silica coating around the surface of FMNPs. After the reaction was completed, APTES was added to the solution of FMNPs coated by silica. Amine-modified FMNPs are further generated by polymerization reaction between APTES and silica. In order to prepare the carboxyl-modified FMNPs, carboxylation of amine-modified FMNPs can be realized by adding amine-modified FMNPs to the solution of succinic anhydride in DMF.
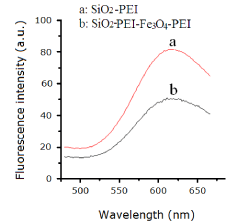
The size of carboxyl-modified FMNPs was measured using a transmission electron microscope (TEM). As shown in Figure 4, the carboxyl-modified FMNPs were uniform with average diameter ~100 nm. We can see that the surface of silica NPs is evenly coated by a layer of Fe3O4. Dye-doped silica NPs take advantages of resistance to photo bleaching and fluorescence signal amplification. In the process of preparing carboxyl-modified FMNPs, the surface of RuBpy-doped silica NPs was coated with PEI and Fe3O4 based on the self-assembly method. PEI is a polymer with low molecular weight, which has no impact on fluorescence signals of RuBpy dyes entrapped inside the silica matrix. However, RuBpy-doped silica NPs were coated by Fe3O4 nanoparticles with diameter of ~10 nm, which may reduce the fluorescence signals of RuBpy, dyes. In order to further verify this inference, the fluorescence spectra of RuBpy-doped silica NPs coated only by Fe3O4 and RuBpy-doped silica NPs coated only by PEI were studied. Compared with the fluorescence intensity of RuBpy-doped silica NPs coated only by PEI, the fluorescence intensity of RuBpy-doped silica NPs coated only by Fe3O4 decreased significantly (~50%) under the same excitation conditions (Figure 5). So we conclude that Fe3O4 NPs occupy the aperture on the surface of silica NPs and shield the excitation light resulting in decreased fluorescence intensity. In addition, the prepared biofunctional NPs were imaged using fluorescence microscopy. Carboxyl-modified FMNPs display red fluorescence with the excitation at 360 nm and good mono dispersion. In addition, the VSM curve shows that carboxyl-modified FMNPs have a good superparamagnetism (data not show). In order to verify the magnetism of carboxyl-modified FMNPs, carboxyl-modified FMNPs were dispersed in deionized water and then placed on a magnetic frame. After 1 min, carboxyl-modified FMNPs can be captured absolutely by the magnetic frame.
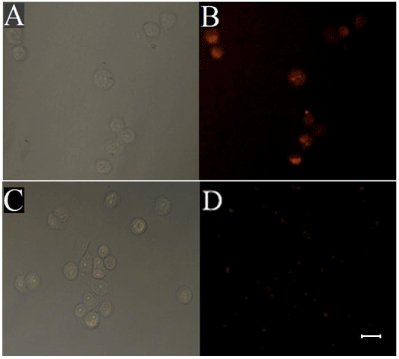
Wheat germ agglutinin (WGA) is glycoprotein synthesized from plant cells, which selectively binds to glycosyl groups of glycoproteins on cell membrane of cancer cells [28, 29]. To demonstrate the biological application of as-prepared fluorescent-magnetic-bifunctional NPs (FMNPs), WGA-conjugated FMNPs (FMNPs-WGA) were used for recognition and separation of human lung cancer A549 cells. A549 cells were incubated with WGA-conjugated FMNPs and separated by a magnetic field and then imaged using fluorescence microscopy. The control group was performed corresponding to experimental group except that the carboxyl-modified FMNPs (FMNPs-COOH) were used instead of WGA-conjugated FMNPs (FMNPs-WGA). As shown in Figure 6, after the A549 cells were incubated with carboxyl-modified FMNPs (FMNPs-COOH), only dispersed red fluorescent NPs are exhibited and no cells were observed in the view field, indicating that there is no nonspecific binding between carboxyl-modified FMNPs (FMNPs-COOH) and A549 cells. While A549 cells were incubated with WGA-modified FMNPs (FMNPs-WGA), red fluorescence of RuBpy on the surface of cells can be observed. Therefore, we believe that FMNPs-WGA can be used as an effective probe for recognition and separation of cancer cells. However, weak red fluorescence was observed either in experimental or control group. As discussed above, RuBpy-doped silica NPs are coated by Fe3O4, which may reduce the fluorescence signals of RuBpy dyes entrapped inside the silica matrix. Subsequent silica coating may further reduce the signals from the fluorescent core of the NPs.
We have prepared RuBpy-doped silica nanoparticles (~60 nm) and Fe3O4 nanoparticles (~10 nm) using the reverse micro emulsion method (W/O) and the high temperature oil cracking method, respectively. The carboxyl-modified fluorescent-magnetic silica nanoparticles (FMNPs-COOH) are uniform with average diameter ~100 nm. Carboxyl-modified fluorescent-magnetic silica nanoparticles (FMNPs-COOH) and WGA-conjugated fluorescent-magnetic silica nanoparticles (FMNPs-WGA) are incubated with A549 cells and separated by a magnetic field and then imaged using fluorescence microscopy, respectively. There is no nonspecific binding between FMNPs-COOH and A549 cells. While A549 cells incubated with FMNPs-WGA, exhibit red fluorescence of RuBpy on the surface of cells. We believe that FMNPs-WGA as effective probes can be used for recognition and separation of cancer cells.
This work was supported by the National Basic Research Program of China (973 Program, Nos. 2011CB933600 and 2006CB933100), the Science Fund for Creative Research Groups of NSFC (Nos. 20621502 and 20921062), the National Natural Science Foundation of China (21275110), and the Fundamental Research Funds for the Central Universities (2042014kf0246).
TEM images of carboxyl-modified RuBpy-doped silica nanoparticles.
(A) TEM image of Fe3O4 nanoparticles. (B) XRD patterns of Fe3O4. (C) XRD patterns of α-Fe3O4.
Fabrication of fluorescent-magnetic-bifunctional nanoparticles with coating method using fluorescent dye-doped silica nanoparticles and magnetic nanoparticles.
TEM image of carboxyl-modified fluorescent-magnetic-bifunctional silica nanoparticles.
Effect of the presence of Fe3O4 on the fluorescent signal of RuBpydoped silica nanoaprticles.
Fluorescence images of A549 cells upon incubation with WGAconjugated fluorescent-magnetic-bifunctional nanoparticles (FMNPs-WGA) (A, B) and carboxyl-modified fluorescent-magnetic-bifunctional nanoparticles (FMNPs-COOH) (C, D). (A, C) bright-field image; (B, D) fluorescence image. Scale bar 25 μm.