
Case Series
Austin J Allergy. 2022; 8(1): 1042.
Complementary Treatment of Allergic Conjunctivitis: The Role of Quercetin
Mazzolani F¹, Togni S², Petrangolini G², Allegrini P² and Riva A²*
¹Private Practice, Centro Oculistico Bergamasco, Bergamo, Italy
²Research and Development Department, Indena SpA, Viale Ortles 12 - 20139 Milan, Italy
*Correspoing author: Riva A, Research and Development Department, Indena SpA, Viale Ortles 12 - 20139 Milan, Italy
Received: October 10, 2022; Accepted: November 04, 2022; Published: November 11, 2022
Abstract
Quercetin offers interesting beneficial options in clinical practice by providing anti-allergic and anti-inflammatory activities. This article reports and discusses the effectiveness of oral administration of quercetin Phytosome™ in adults presenting allergic conjunctivitis. The analysis was conducted by evaluating both subjective symptoms reported by individuals; and objective analysis of tear film instability due to allergic conjunctivitis inflammation using a non-invasive and computerized evaluation model. Outcomes provided evidence that quercetin Phytosome may significantly be a natural effort to avoid clinical worsening when supplemented as add-on to continuous anti-histaminic treatment and also prevent clinical exacerbations.
Keywords: Allergic conjunctivitis; Quercetin; Tear film non-invasive BUT; Inflammation; Phytosome
Introduction
Quercetin, a natural substance belonging to the family of bioflavonoids, is present in fruits (grapefruit, apples, berries), vegetables (beans, onions, capers and broccoli), as well as red wine and black and green tea [1]. Due to its inhibitory activity on enzymes like lipoxygenase and peroxidase, and its ability to suppress proinflammatory mediators release such as interleukin 4, quercetin possesses anti-inflammatory and immunomodulating properties [2- 5].
Outdoor and indoor allergens exposure can cause allergic conjunctivitis, a disorder affecting the quality of life of many people. Current therapies range from drugs (i.e. antihistaminic, mast cell stabilizers, anti-inflammatory, corticosteroids and immunomodulators) both systemically and topically administered, to natural substances that can be of support [6]. Benefits of quercetin in allergic conditions have been recently reviewed [7], where the effects of quercetin in vitro and in vivo models in allergic asthma, allergic rhinitis and atopic dermatitis are fully summarized and shown.
Therefore, these outcomes confirm and underline quercetin anti-allergic benefits and the potential indication as a natural help in Allergic Conjunctivitis (AC). This clinical study was performed to evaluate the efficacy of 500 mg/day for 2 weeks of oral consumption of bioavailable quercetin (quercetin Phytosome, Quercefit™) for the relief of ocular symptoms, improvement stability of tear film and reduction of anti-allergic medications use in adults with allergic conjunctivitis.
Material and Methods
30 Eyes of 15 subjects (12 women, mean age 42 years, and 3 men, mean age 38 years) were considered. Subjects were followed according to standard clinical practice, using standard diagnostic and intervention procedures, without any foreseeable risk for the evaluated subjects in line with the practice of our Center. A complete eye examination was performed and in all cases an analysis using a new Dry Eye module was performed (VX210®Visionix Medical Italy). Placido-disc technology allows advanced analysis of precorneal tear film structure and stability. The concentric ring pattern is projected onto cornea and subject is asked to blink. The rings will be distorted as soon as the cornea becomes dry. The time interval between last blink and the appearance of a distorted ring pattern gives the measurement of Non Invasive First Breakup Time (NIBUT).
Subjects were visited at baseline before the treatment and after 15 days of supplementation with Quercefit™ (a solid dispersion of quercetin in phospholipids, Indena SpA) at the dosage of 2 tablets/ day (500 mg total).
The only local therapy used was Olopatadine, 0.1% solution (Alcon, US), one drop in each affected eye twice per day for 2 weeks. No topical antibiotic or corticosteroid therapy was allowed.
After 15 days, the topical therapy was suspended, while quercetin Phytosome was continued for further 2 weeks, in order to monitor potential relapses.
The primary efficacy endpoint was the change in mean Total Ocular Symptom Score (TOSS) from baseline after 2 weeks of study supplementation. Ocular signs and symptoms were recorded using the TOSS scale (where 0 = no symptoms; 1 = mild symptoms; 2 = moderate symptoms and 3 = severe symptoms) for the following ocular signs and symptoms: itching, lacrimation, conjunctival congestion, hyperemia and photophobia at baseline and after 15 days.
The TOSS score as sum of the single parameters was calculated, according to literature [8,9] at time 0 and time 15days.
The secondary efficacy endpoint was tear break-up time (NIBUT) improvement after 2 weeks of supplementation using non-invasive Scheimpflug Camer examination (VX210® Visionix Medical, Italy).
Several non-invasive instruments are used for automatic Non- Invasive Tear Breakup Time (NIBUT) measurements [10]. In noninvasive procedure, a grid or concentric ring pattern is projected onto cornea and subject is asked to blink; the rings will appear distorted when cornea becomes dry. Generally, >10 seconds is thought to be normal [11-13] 5 to 10 seconds, borderline, and < 5 seconds is considered low.
NIF-BUT (Non Invasive First Breakup Time) and NIAvg-BUT (Non Invasive Average Breakup Time) data were collected.
NIF-BUT indicates, in seconds, the time that elapses at the opening of the eye following one or more blinks and the instant in which the first tear film break occurs or the moment in which a further blink occurs.
NIAvg-BUT indicates, in seconds, the average tear film breakup time calculated by considering the average of all corneal sectors during the entire measurement.
Statistical analysis of TOSS data at T15 vs T0 was performed by Wilcoxon test (two tails). The Student’s t-test was used to assess the statistical significance of changes in non-invasive tear breakup time (NIF-BUT and NIAvg-BUT) and p< 0.05 was considered statistically significant.
Results
All subjects reported improvement in primary and secondary endpoints. As described in Figure 1, the 100% of subjects reported almost complete restoring (p<0.0001) after supplementation in TOSS score, considered as sum of the individual symptoms (Figure 1, left) and as individual fifteen T0-T15 values (Figure 1, right).
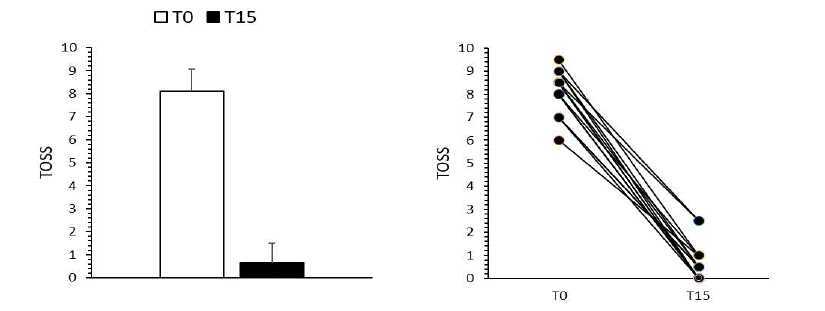
Figure 1: TOSS score. In panel left the sum of individual symptoms is reported; in panel right the individual fifteen T0-T15 values are plotted. Results are expressed as Mean ± S.D. *P<0.0001 vs T0, Wilcoxon test
Furthermore, both NIF-BUT and NIAvg-BUT showed statistically significant improvement (p<0.0001 by Student’s t-test) in terms of tear stability and the corneal grid after supplementation, giving a restoration of a normal tear film (Figure 2).
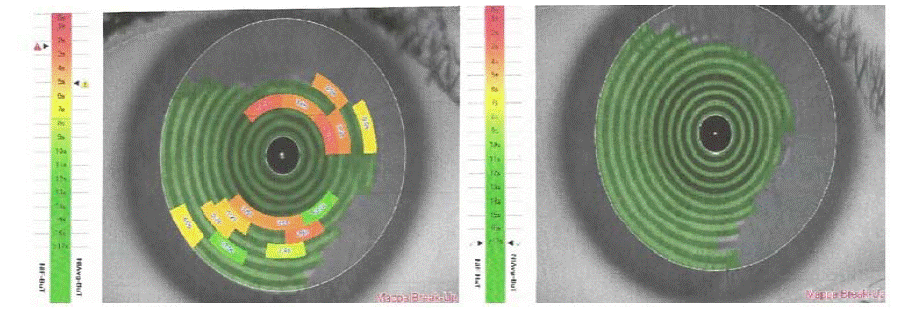
Figure 2a: Corneal concentric ring pattern before (left) and after 15 days (right) supplementation.
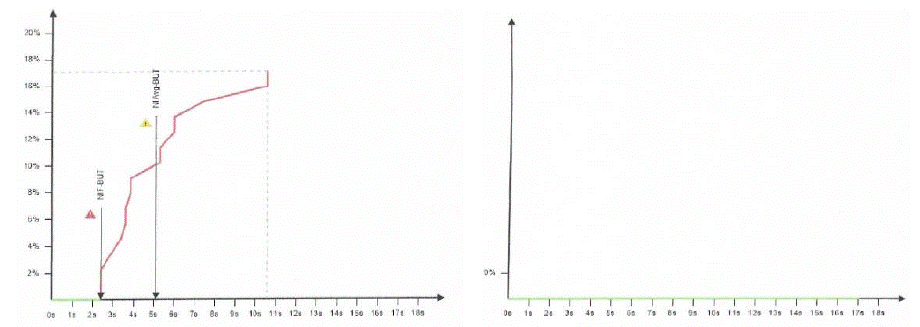
Figure 2b: NIF-BUT and NIAvg-BUT before (left) and after 15 days (right) supplementation.
Tear film analysis was chosen as a method for evaluating the efficacy of oral supplementation with quercetin Phytosome because it is also indicative of inflammatory states that alter the correct homeostasis of the tear film. Measurements obtained clearly point out beneficial increase in tear breakup time associated with a clinical improvement of tear film stability.
Discussion
The present research demonstrated that quercetin Phytosome™ may potentially be a natural aid to subjects with allergic conjunctivitis by avoiding clinical worsening when supplemented as add-on to continuous anti-allergic medications and also by preventing clinical exacerbations. Clinical effects of anti-histamine and corticosteroids often could be very short-lived after suspension, such as in a few days symptoms and inflammation reappear promptly [14,15]. Despite the fact that several efforts have recently been made to develop new compounds with longer duration of action [16], classical antihistamines and corticosteroids may be unable to completely inhibit allergic reaction especially after intense allergen exposure, or interfering disorders in highly allergic subjects. Therefore, the use of add-on supplementation could be fruitful in such situations.
In the present study, the TOSS, a questionnaire used by allergists to assess eye symptoms in allergic conjunctivitis subjects, was chosen because it is simple to understand and sufficiently precise for our purpose to analyze tear film quality after quercetin Phytosome supplementation in allergic conjunctivitis subjects. Actually, the favorable effect exerted by bioavailable quercetin in the first phase of supplementation was even evident in the second part of supplementation, such as between the third and the fourth week, when subjects discontinued topical treatment with Olopatadine, while the supplementation with quercetin Phytosome was maintained (data not shown). Another important aspect concerns the tolerability. In this regard, no subjects experienced treatment-emergent adverse events.
Since another interesting novelty of this study is the use of a non-invasive technique for Tear Breakup Time (TBUT, recorded as the number of seconds that elapse between the last blink and the appearance of the first dry spot in the tear film). The traditional method reproducibility is low, therefore numerous non-invasive techniques have been developed to avoid instillation of fluorescein and contact between the measuring instrument and the eye or eyelids. Noninvasive testing is considered more precise, repeatable and therefore preferable to invasive testing in tear film stability assessment.
Thanks to its original formulation, quercetin Phytosome can hit the target much more efficiently compared to unformulated quercetin, since quercetin Phytosome shows optimized bioabsorption and solubility and solubility, which makes it usable at lower dosages retaining efficacy with no undesirable effects [17].
Therefore, the results obtained in the present study are in agreement with previous evidences observed in a placebo-controlled randomized study [18], whereas 4 week oral supplementation with quercetin Phytosome was effective in reducing subjective symptoms related to allergic reactions to allergens in Japanese adults.
Also in a pilot study in asthmatic subjects, quercetin Phytosome supplementation showed to be effective in preventing and reducing allergic symptoms [19]. Recent evidences on healthy subjects suggested that quercetin Phytosome produced a dose-related reduction in the wheal response to histamine effects, with a potential preventive role in conditions associated with altered histamine release and in edema prevention [20].
Dietary supplements will play a relevant role in the future treatment of allergic conjunctivitis, thanks to the proved evidence of their effectiveness and safety. In particular, there is evidence that supplementation with quercetin Phytosome may be favorably used to prevent clinical worsening as add-on strategy and to reduce clinical exacerbations as mere preventive approach.
Our study has several limitations, such as the small sample size, the lack of a placebo group and the use of just NITBUT test for the assessment of anterior segment inflammation due to allergic conjunctivitis.
However, our findings may be considered promising; future investigative studies should be performed by evaluating quercetin supplementation in a placebo-controlled study involving a larger number of individuals and by utilizing different morphometric tests.
Acknowledgement
We would like to express our gratitude to Dr. Paola Misiano for her valuable editorial support and Dr. Mauro A.M Carai for statistical analysis.
References
- Shaik YB, Castellani ML, Perrella A, Conti F, Salini V, et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J Biol Regul Homeost Agents. 2006; 20: 47-52.
- Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets. 2010; 9: 263-85.
- Mlcek J, Jurikova T, Skrovankova S, Sochor J. Quercetin and Its Anti-Allergic Immune Response. Molecules. 2016; 21: 623.
- Ebihara N, Takahashi K, Takemura H, Akanuma Y, Asano K, et al. Suppressive Effect of Quercetin on Nitric Oxide Production from Nasal Epithelial Cells. Evid Based Complement Alternat Med. 2018; 2018: 6097625.
- Sakai-Kashiwabara M, Asano K. Inhibitory action of quercetin on eosinophil activation in vitro. Evid Based Complement Alternat Med. 2013; 2013: 127105.
- Labib BA, DI Chigbu. Therapeutic Targets in Allergic Conjunctivitis. Pharmaceuticals (Basel). 2022; 15: 547.
- Jafarinia M, Hosseini MS, Kasiri N, Fazel N, Fathi F, et al. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin Immunol. 2020; 16: 36.
- Bielory L, MM Hom, A Nguyen. Total Ocular Symptom Score (TOSS) and Dry Eyes. 02/2013. p. AB1-AB332.
- Singh N, Nagpure PS, Yadav MK, Chavan S, Manpe S, et al. Effectiveness of oral antihistamine and intranasal steroid spray in relieving ocular symptoms in allergic rhintiis using total ocular symptom score. Otorhinolaryngology Clinics: An International Journal. 2016; 8: 45-50.
- Lan W, Lin L, Yang X, Yu M. Automatic noninvasive tear breakup time (TBUT) and conventional fluorescent TBUT. Optom Vis Sci. 2014; 91: 1412-8.
- Lee JH, CW Kee. The significance of tear film break-up time in the diagnosis of dry eye syndrome. Korean J Ophthalmol. 1988; 2: 69-71.
- Abelson MB, Ousler GW, Nally LA, Welch D, Krenzer K, et al. Alternative reference values for tear film break up time in normal and dry eye populations. Adv Exp Med Biol. 2002; 506: 1121-5.
- Nichols JJ, Nichols KK, Pauent B, Saracino M, Mitchell GL. Evaluation of tear film interference patterns and measures of tear break-up time. Optom Vis Sci. 2002; 79: 363-9.
- Portnoy JM, C Dinakar. Review of cetirizine hydrochloride for the treatment of allergic disorders. Expert Opin Pharmacother. 2004; 5: 125-35.
- Ciprandi G, Ricca V, Paola F, Alessandra B, Riccio AM, et al. Duration of antiinflammatory and symptomatic effects after suspension of intranasal corticosteroid in persistent allergic rhinitis. Eur Ann Allergy Clin Immunol. 2004; 36: 63-6.
- Bosma R, den Bor JV, Vischer HF, Labeage L, Leurs R, et al. The long duration of action of the second generation antihistamine bilastine coincides with its long residence time at the histamine H. Eur J Pharmacol. 2018; 838: 107-111.
- Riva A, Ronchi M, Petrangolini G, Bosisio S, Allegrini P, et al. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur J Drug Metab Pharmacokinet. 2019; 44: 169-177.
- Yamada S, Shirai M, Inaba Y, Takara T. Effects of repeated oral intake of a quercetin-containing supplement on allergic reaction: a randomized, placebocontrolled, double-blind parallel-group study. Eur Rev Med Pharmacol Sci. 2022; 26: 4331-4345.
- Cesarone MR, Belcaro G, Hu Shu, Dugall M, Hosoi M, et al. Supplementary prevention and management of asthma with quercetin phytosome: a pilot registry. Minerva Med. 2019; 110: 524-529.
- Belcaro G, Cesarone MR, Scipione C, Scipione V, Dugall M, et al. Quercetin Phytosome reduces the wheal response to histamine injection. Esperienze Dermatologiche - Dermatological Experiences- Minerva Medica. 2020; 22: 5-9.