
Research Article
Ann Agric Crop Sci. 2022; 7(4): 1122.
To Study the Culturable Bacterial Endophytes Community Diversity and Abundance Associated with Chrysanthemum (Dendranthema Grandiflora Tzvelev) Plant Grown Under Organic and Commercial Practices
Shilpa S¹, Anjali C²* and Rajesh K³
¹Department of Basic Sciences, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, India
²Department of Soil Science and Water Management, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, India
³Department of Soil Science and Water Management, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, India
*Corresponding author: Chauhan Anjali, Department of Soil Science and Water Management, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Nauni, Solan - 173 230, H.P, India
Received: September 15, 2022; Accepted: October 25, 2022; Published: November 01, 2022
Abstract
Chrysanthemum (Dendranthema grandiflora Tzvelev) belongs to family Asteraceae and is a popular flower crop suitable for both pot culture and bedding purposes. The quality of flowers is greatly influenced by the quantity as well as sources of nutrients. Presently, these nutrients are supplied through chemical fertilizers. The escalating prices of chemical fertilizers and their indiscriminate use has not only adversely affects the soil health and environment but also reduces the productivity of crops. The situation emphasized the need for developing alternate production system that is eco-friendly and is more judicious in maintaining soil health. So, the present investigations were carried out to characterize and evaluate the effects of PGPB isolated from chrysanthemum plant (roots, stem and leaf) samples. Out of 143 purified isolates, a total of forty four (16 organic and 28 inorganic) morphologically distinct isolates with dominant PGP traits, isolated from different plant samples collected from different districts of Himachal Pradesh were selected for further screening for P-solubilization efficiency, siderophore, IAA, HCN, ammonia, lytic enzyme production and antagonism against Pythium ultimum, Rhizoctonia solani and Fusarium oxysporum under laboratory conditions. These selected forty four isolates were then assessed and compared to study the genetic diversity of culturable bacterial endophytes of chrysanthemum.
Keywords: Chrysanthemum; Plant Growth Promoting Bacteria (PGPB); P-solubilization; Siderophore; IAA; Biocontrol; Genectic diversity
Abbreviations
PGPB: Plant Growth Promoting Bacteria; PGP: Plant Growth Promoting, IAA: Indole Acetic Acid; HCN: Hydrogen Cyanide; PGPR: Plant Growth Promoting Rhizobacteria; cfug-1: Colony Forming Unit Per Gram; PVK: Pikovskaya; PCR: Polymerase Chain Reaction; dNTPs: Deoxynucleotide Triphosphates; DNA: Deoxyribonucleic Acid; TAE: Tris Acetate; EDTA: Ethylenediamine tetra-acetic acid
Introduction
Chrysanthemum (Dendranthema grandiflora Tzvelev) popularly known as ‘Guldaudi’ or ‘mums’ a member of the family Asteraceae [1], are herbaceous perennial plants or subshrubs, occupies a prominent place in ornamental horticulture is one of the commercially exploited flower crops [2]. Chrysanthemums are one of the prettiest varieties of perennials and also known as favorite flower for the month of November. It is mainly grown for cut and loose flowers used for decoration, hair adornments, making garlands and religious function. Chrysanthemum is not only being used for its flowers but also for essential oils, sesquiterpenoids, medicinal herb (i.e. powerful anti-microbial, anti-inflammatory, immuno-modulatory, and neuro-protective effects), insecticides, etc. The quality of flowers is greatly influenced by the quantity as well as sources of nutrients. Presently, these nutrients are supplied through chemical fertilizers. The escalating prices of chemical fertilizers and their indiscriminate use has not only adversely affects the soil health and environment but also reduces the productivity of crops. The situation emphasized the need for developing alternate production system that is eco-friendly and is more judicious in maintaining soil health. So, the present investigations were carried out to characterize and evaluate the effects of Plant Growth-Promoting Rhizobacteria (PGPR) isolated from rhizosphere and roots of chrysanthemum. Plant Growth-Promoting Rhizobacteria (PGPR) are free-living soil bacteria that aggressively colonize the rhizosphere/endorhizosphere, enhance the growth and yield of plants when applied to seed or crops [3]. In recent years, much attention has been paid to natural methods of crop growing in expectation n of moving toward agriculturally and environmentally sustainable development. Plant Growth Promoting Rhizobacteria (PGPR) are considered as a biological fertilizer, one of the most important requirements to protect environment from pollution, a cheap alternative that replaces expensive chemical fertilizers as they can contribute to mobilization, mineralization and recycling of nutrients in an effective manner [4] and provides a safe and clean product [5]. The use of microbial technologies is increasing day by day in agriculture [6] to reduce the impacts on human health and environment, development of resistance in plant pests, etc. A number of soil bacteria which flourish in plant rhizosphere and roots stimulate plant growth by different mechanisms and are collectively known as Plant Growth Promoting Rhizobacteria (PGPR). Endophytic bacteria from leaf, stem and root are known to enhance plant growth in nonleguminous crops and improve their nutrition through nitrogen fixation, phosphate solubilisation or siderophore production (iron chelation). Besides biofertilization, endophytic bacteria are also reported to promote plant growth and yield through direct production of phytohormones, or enzymes, or indirectly through biological control of plant pests and diseases or induced resistance response (biotization). In return, the plant protects endophytes and provides them with nutrients in form of photosynthates. Endophytes are increasingly gaining scientific and commercial interest because of this potential to improve plant quality and growth and their close association with internal tissues of host plant. The direct mechanisms include atmospheric nitrogen fixation, phosphate solubilization, siderophore production and secretion of plant growth promoting hormones [7]. The indirect mechanisms include biological control of phytopathogens/deleterious microbes through antibiotic production, lytic enzymes, siderophore and HCN secretion. These mechanisms remarkably improve plant health and promotes growth and yield of the crop [8,9]. PGPR includes the genera Acinetobacter, Alcaligenes, Arthrobacter, Azospirillum, Azotobacter, Bacillus, Beijerinckia, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Rhizobium and Serratia (Dursan et al. 2008). The predominant PGPR’s belong to genera Pseudomonas and Bacillus because of their association with soil organic matter, nutritional diversity and rapid growth rate [11]. It have been reported that specific micro-organisms improve growth and yield of crop. Thus, inoculation with specific bacteria (PGPR) may enhance the health and fertility of the soil that contributes and leads to the production of higher value sustainable products with good quality. The proposed research work was aimed to study culturable endophytes community diversity and abundance associated with chrysanthemum plant grown under organic and commercial practices and development of efficient biofertilizer/plant growth promoting bacteria with multiple Plant Growth Promoting (PGP) traits.
Materials and Methods
Collection of Plant Samples
The plant samples (root, stem and leaf) of chrysanthemum (Dendranthema grandiflora Tzvelev) were collected from Solan, Sirmour and Hamirpur districts of Himachal Pradesh. A total of 48 samples i.e. 24 organic and 24 inorganic plant (leaf, stem and roots) samples were collected from selected locations. In each district, two locations were selected and under each location two sites were selected for collection of samples. From each site two samples were collected i.e. one organic and one inorganic. The samples were placed in plastic bags and stored in Soil Microbiology Laboratory for further isolation and analysis work.
Isolation and Enumeration of Microbial Population
The plant (leaf, stem and root) samples were washed under running tap water, surface sterilized with 70 per cent ethanol for 45 seconds and 2.0 per cent sodium hypochlorite for 4-5 minutes followed by repeated 5-6 times washing in sterilized distilled water. The surface sterility of plant samples was cross checked by incubating the sterilized nutrient agar medium plates containing 0.1ml of final wash as control for 48 h at 28±2oC. One gram of surface sterilized plant sample was crushed in 9 ml of sterilized distilled water to produce slurry using pestle and mortar under aseptic conditions. A known amount (0.1ml) of serially diluted suspension was spread on pre-poured solid agar medium viz., nutrient agar medium [12], tryptic soy agar and King’s B medium with the help of glass spreader under aseptic conditions. Plates were incubated in inverted position at 28±2oC for 24 to 48 h. After the incubation period, the microbial count was expressed as colony forming unit per gram of plant sample (cfug-1 plant sample).
Screening for Multifarious Plant Growth Promoting Traits
Selected bacterial endophytes were screened for Phosphate solubilizing Pikovskaya’s (PVK) agar plate as per the method of Pikovskaya [13] and noted for clear yellow zone around the colony, Nitrogen fixing activity on Jensen’s medium [14], Siderophore production using blue agar plates containing chrome azurol S [15], IAA production in Luria Bertani broth (amended with 5 mM L-tryptophan, 0.065% sodium dodecyl sulfate and 1% glycerol), Hydrogen cyanide production on King’s B agar medium with 4.4 g glycine/l [16], lytic enzyme production and antifungal activity against different fungal pathogens viz., Rhizoctonia solani, Fusarium oxysporum and Pythium ultimum on potato dextrose agar medium and percent growth inhibition was calculated [17].
Biochemical and Molecular Identification of Bacterial Isolates
Morphological characteristics of isolates including colony morphology, Gram’s reaction, cell shape and presence of spores were investigated. Colony morphology and cell morphology were observed on nutrient agar medium and nutrient broth, respectively. The biochemical characterization of the isolate was done using commercial kits (KB009 Hi carbohydrate TM kit) [18].
PCR Amplification of Bacterial 16S rDNA, Sequencing and Phylogenetic Analysis
PCR reaction was carried out using universal 16S rRNA gene primers in 20 μl reaction mixture. It contained ~50ng of template DNA, 20 pmoles of each primer, 0.2 mM dNTPs and 1 U Taq polymerase (Genei, Banglore) in 1xPCR buffer. Reaction were cycled 35 times at 94°C for 30 s, 58°C for 30 s, 72°C for 1 min 30 s followed by final extension at 72°C for 10 min. The PCR products were analyzed on 1% agarose gel in 1xTAE buffer, run at 100V for 1 h. Gel was stained with ethidium bromide and photographed. The amplified PCR product was excised from the gel and purified using gel/PCR extraction kit (RBC’s Real genomics). The comparison of sequence was performed via the internet at National Center for Biotechnology Information (NCBI) database by employing BLAST algorithm [19]. Multiple alignments were generated by the MULTALIN program from the web site: http://prodes.toulouse.inra.fr/multialin/multialin. html [20]. Phylogenetic relatedness of isolates was drawn using neighbour joining phylogenetic tree using Mega 6 software. The gene sequence has been submitted under Accession No.-KF560310 in NCBI GenBank database.
Genetic Diversity of Selected Bacterial Endophytes
To assess and compare the genetic diversity of predominant bacterial endophyte isolates from roots, stem and leaves of chrysanthemum, DNA sequence analysis of 16S rRNA gene was conducted. The amplification of gene encoding 16S rDNA of bacterial endophyte isolates was done using standard PCR reaction employing universal primer set ‘16S-1375’ (16S-1375F: 5’GCAAGTCGAGCGGACAGATGGGAGC3’ and 16S-1375R: 5’ AACTCTCGTGGTGTGACGGGCGGTG3’). PCR reactions were performed in a 25 μL volume containin 2 μL MgSO4, 2 μL dNTPs (10mM each), 0.3 μL Taq polymerase and 1 μL each of forward and reverse primers. Amplifications were run under the following cycling conditions: initial denaturation at 95°C, followed by 30 cycles of denaturing at 94°C for 30 seconds, annealing at 54°C for 30 seconds, extension at 72°C for 1 min 30 seconds followed by final extension at 72°C for 10 min.
Statistical Analysis
The data were statistically analyzed as described by Gomez and Gomez [21].
Results and Discussion
Isolation and Enumeration of Bacterial Endophytes
Isolation of microorganisms was carried out from the leaf, stem and roots of the chrysanthemum (Dendranthema grandiflora Tzvelev) collected from different locations/sites/subsites of Solan (Nauni and Deothi), Sirmour (Rajgarh and Sargaon) and Hamirpur (Neri and Didwi Tikker) districts of Himachal Pradesh. The population capable of growth on different media was counted and reported as cfu/g sample.
Microbial population in the organic samples of chrysanthemum plants
A summary of endophytic microorganisms in organic plant sample (roots, stem and leaf) of chrysanthemum at different districts located in Himachal Pradesh is presented in (Table 1) and Plate 1. Among different plant samples, maximum (74.05×10² cfu/g roots) viable count was recorded for root samples, which was found to be significantly more than stem (57.59×10² cfu/g stem) and leaf samples (52.75×10² cfu/g leaf). However, the maximum (68.97×10² cfu/g sample) count was recorded for Nauni (Solan) location which was statistically at par with (68.34×10² cfu/g sample) Deothi (Solan) location, whereas, minimum (55.02×10² cfu/g sample) for Neri (Hamirpur) location. Among different media used for isolation of bacterial endophytes, maximum (72.45×10² cfu/g sample) viable count was registered for tryptic soya agar medium, which was statistically at par with nutrient agar medium (72.13×10² cfu/g sample) while minimum (39.49×10² cfu/g sample) was recorded for King’s B medium.
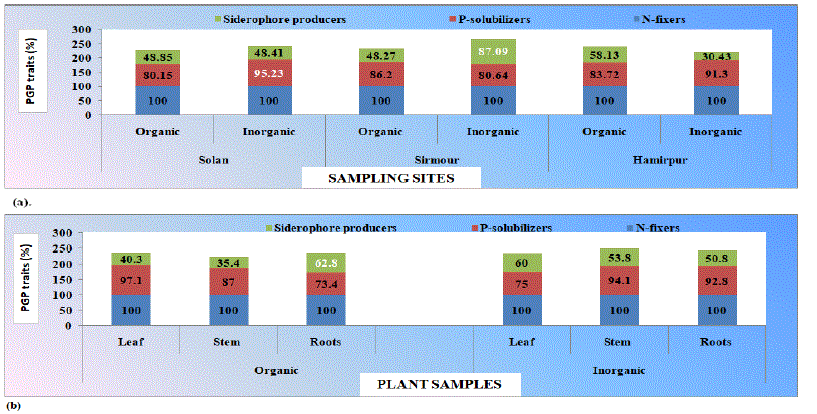
Figure 1: Characterization of bacterial endophytes isolated from (a). Different sites of sampling and (b). Different plant parts of organic and inorganic samples for
phosphate solubilization, siderophore production and ability to fix nitrogen.
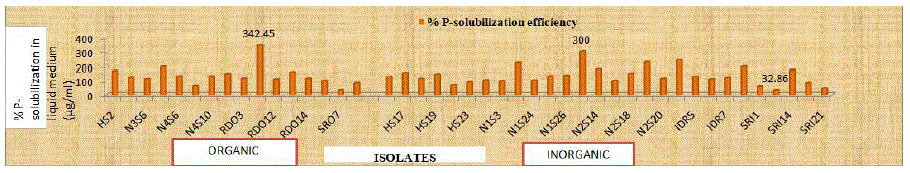
Figure 2: Percent P-solubilization efficiency (% SE) of selected bacterial endophytes on solid medium.
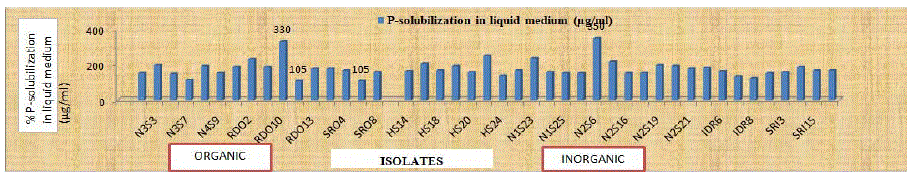
Figure 3: P-solubilization efficiency (μg/ml) of selected bacterial endophytes in liquid medium.
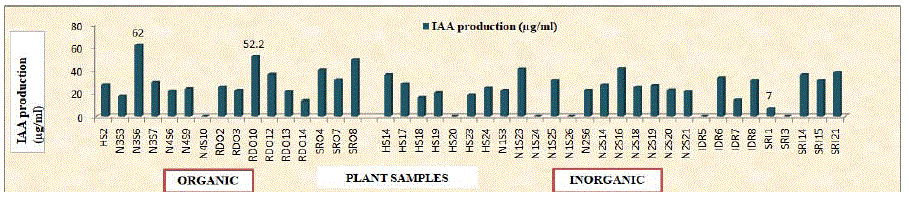
Figure 4: IAA production by selected bacterial endophytic isolates in Luria Bertani broth.
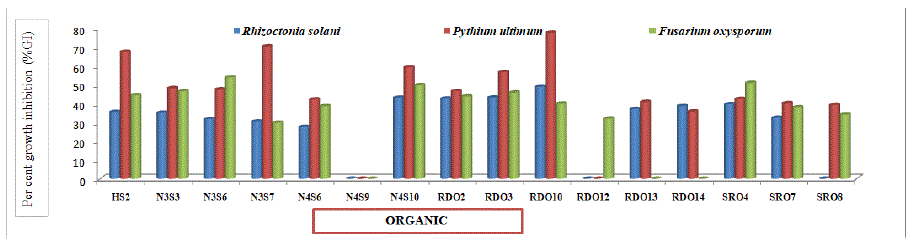
Figure 5a: Percent growth inhibition by selected bacterial isolates isolated from organic plant samples of Chrysanthemum against Rhizoctonia solani, Pythium ultimum and Fusarium oxysporum.
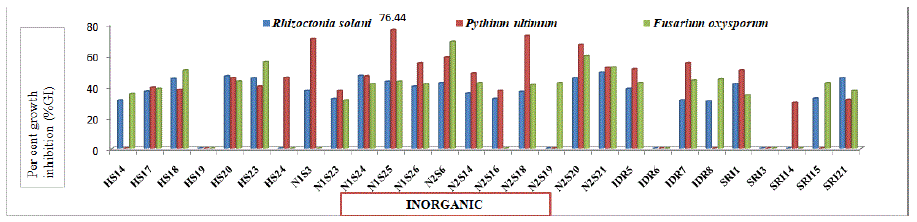
Figure 5b: Percent growth inhibition by selected bacterial isolates isolated from inorganic plant samples of Chrysanthemum against Rhizoctonia solani, Pythium ultimum and Fusarium oxysporum.
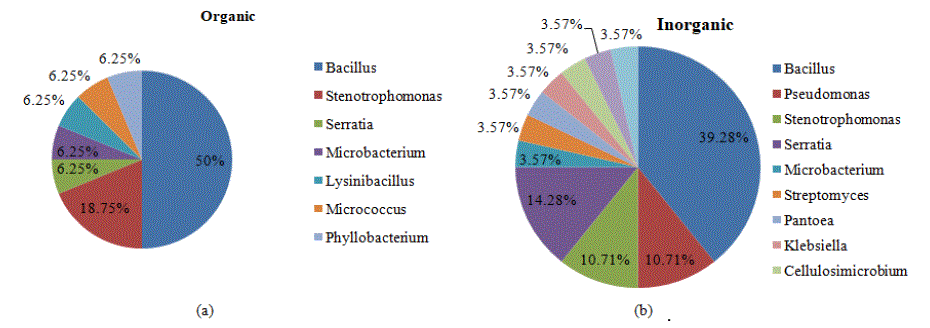
Figure 6a: Relative abundance of endophytic bacteria at genus level associated with (a) organic plant samples and (b) inorganic plant samples.
Location
Sites
Endophytic count (102 cfu/g plant sample)
Nutrient Agar
Tryptic Soy Agar
King’s Medium
Mean
Roots
Stem
Leaf
Mean
Roots
Stem
Leaf
Mean
Roots
Stem
Leaf
Mean
Roots
Stem
Leaf
Mean
Solan
Nauni
93.33
80.33
72.33
81.99
94.00
77.56
69.23
80.26
58.67
41.67
33.67
43.00
82.00
66.52
58.41
68.97
Deothi
96.33
83.33
75.33
84.99
98.67
75.67
63.67
79.33
51.00
34.67
36.44
39.07
82.00
64.55
58.48
68.34
Sirmour
Rajgarh
83.00
62.21
67.00
70.73
89.67
72.67
63.67
75.33
47.67
30.67
32.67
40.33
73.44
55.18
54.44
61.02
Sargaon
77.67
54.67
56.67
63.00
88.00
71.00
61.00
73.33
51.67
34.67
32.27
39.77
72.44
53.44
49.98
58.62
Hamirpur
Neri
75.00
62.21
54.00
63.73
78.67
61.67
54.67
65.00
44.00
33.45
31.56
34.97
65.89
52.44
46.74
55.02
Didwi Tikker
79.67
66.67
58.67
68.33
75.00
58.67
50.67
61.44
51.00
34.98
36.00
39.81
68.55
53.44
48.44
56.81
Mean
84.16
68.23
64.00
72.13
87.33
69.54
60.48
72.45
50.66
35.01
33.76
39.49
74.05
57.59
52.75
CD0.05 for
Plant parts (P)=1.76
Media(M)= 1.75
Interaction P X M=3.04
Intraction P X S X M=NS
Sites (S)=2.48
Interaction P X S=NS
Interaction S X M=4.30
Table 1: Enumeration of bacterial endophytes associated with chrysanthemum under organic cultivation.
Data presented in (Table 2) revealed that inorganic plant sample (roots, stem and leaf) of chrysanthemum collected from different locations harboured variable number of bacteria. Among different plant samples, maximum (65.87×10² cfu/g roots) viable count was recorded for root samples and minimum (50.11×10² cfu/g leaf) for leaf samples. For different sites, the maximum (66.66×10² cfu/g sample) count was recorded for Nauni (Solan) location which was at par with (65.64×10² cfu/g sample) Deothi (Solan) location and minimum (49.06×10² cfu/g sample) for Neri (Hamirpur) location. Among different media used for isolation of bacterial endophytes, maximum (6.315×10² cfu/g sample) viable count was registered for both nutrient agar and tryptic soya agar medium and minimum (39.49×10² cfu/g sample) was recorded for King’s B medium. The endophytic bacterium actually resides within apoplastic spaces inside the host plant and there is only some evidence of endophytes occupying intracellular spaces [22]. The internal tissues of plants provide relatively uniform and protected environment when compared with rhizosphere and rhizoplane [23,24]. Reported that variation of microbial diversity depends much on soil chemical, physical and biological properties. Gupta [25] also reported that the population of phosphate solubilizing microorganisms varied from 20-24 per cent of the total population and in some soils it may be up to 85 per cent of the total population. The solubilization of phosphorus in the rhizosphere and endorhizosphere is the most common mode of action implicated in PGPB that increase nutrient availability to host plants [26,27]. The variation in the endophytic bacterial population may be attributed to location, variety, time of sampling, physic-chemical properties of soil and environmental conditions of the location. The results are in confirmation with those of Sharma [28] and Kaushal (2011) who has also reported significant variation in microbial population with respect to location/plant parts used for the isolation.
Location
Sites
Endophytic count (102 cfu/g plant sample)
Nutrient Agar
Tryptic Soy Agar
King’s Medium
Mean
Roots
Stem
Leaf
Mean
Roots
Stem
Leaf
Mean
Roots
Stem
Leaf
Mean
Roots
Stem
Leaf
Mean
Solan
Nauni
90.33
79.33
72.33
80.66
86.67
74.67
67.67
76.33
51.00
40.45
37.56
43.00
76.00
64.81
59.18
66.66
Deothi
87.00
77.56
69.00
77.85
89.67
78.67
71.67
80.00
45.00
34.78
37.44
39.07
73.89
63.67
59.37
65.64
Sirmour
Rajgarh
67.67
61.66
49.67
59.66
70.00
59.67
52.45
60.70
46.00
36.78
38.21
40.33
61.22
52.70
46.77
53.56
Sargaon
69.67
58.67
51.67
60.00
72.00
61.56
54.87
62.81
45.66
37.67
36.00
39.77
62.44
52.63
47.51
54.19
Hamirpur
Neri
66.00
57.67
48.00
57.22
64.67
53.67
46.67
55.00
42.67
31.66
30.60
34.97
57.78
47.66
41.75
49.06
Didwi Tikker
71.00
63.29
53.21
62.50
72.67
61.67
54.67
63.00
48.00
41.00
30.45
39.81
63.89
55.32
46.11
55.10
Mean
75.27
66.36
57.31
66.31
75.94
64.98
58.00
66.31
46.38
37.05
35.04
39.49
65.87
56.13
50.11
CD0.05 for
Plant parts (P)=1.31
Media (M)=1.32
Interaction P X M=2.27
Intraction P X S X M=NS
Sites (S)=1.85
Interaction P X S=NS
Interaction S X M=3.21
Table 2: Enumeration of bacterial endophytes associated with chrysanthemum under inorganic cultivation.
Screening of Bacterial Endophytes on The Basis of Phenotypic Characterization and Multifarious Plant Growth Promoting Traits
All the bacterial endophytes isolated from organic and inorganic plant sample of chrysanthemum collected from different locations were nitrogen fixers. Maximum siderophore producers (87.09 per cent) were recorded for inorganic samples collected from Sirmour district, whereas, minimum (30.43 per cent) were recorded for inorganic samples collected from Hamirpur district. Maximum P-solubilizers (95.23 per cent) were observed for inorganic plant samples collected from district Solan and minimum (80.15 per cent) for organic plant samples collected from district Solan. For organic plant samples, (97.12, 87.09 and 73.45) per cent isolates were P-solubilizers and (40.30, 35.48 and 62.83) per cent isolates were siderophore producers isolated from leaf, stem and roots, respectively. Similarly, for inorganic plant samples (75.00, 94.15 and 92.85) per cent isolates were P-solubilizers and (60.00, 53.84 and 50.89) per cent isolates were siderophore producers isolated from leaf, stem and roots, respectively. Out of total isolated bacterial endophytes, 143 bacterial endophytes (51 organic and 92 inorganic) were selected on the basis of predominant growth, phenotypic characterization and possessing triple plant growth promoting traits viz. P-solubilization, ability to fix nitrogen and siderophore production efficiency on different media. All the isolates exhibited variation in performance of different plant growth promoting traits. All the 143 selected bacterial isolates were P-solubilizers, nitrogen fixers and siderophore producers. Also the data in the tables depicts the colony morphology, Gram’s reaction and cell shape of selected isolates. The isolates showed variation w.r.t. Gram’s reaction (+ve and -ve) and were rods, cocci and coccobacilli in shape. From the tables, it is revealed that all the isolates possess variable morphological features with respect to their form, elevation, margin, pigment. All the selected isolates from organic and inorganic plant samples showed morphologically different colonies. Out of total 56.86 (29/51) per cent and 51.08 (47/92) per cent endophytic bacteria were Gram’s negative for organic and inorganic samples, respectively.
Characterization of Selected Bacterial Endophytes
A total of 44 (16 organic and 28 inorganic) morphologically distinct isolates with dominant PGP traits, isolated from different plant samples collected from different districts of Himachal Pradesh, were selected for further characterization. All the 44 bacterial endophytic isolates were screened for the solubilization of Tri-Calcium Phosphate (TCP) and were able to solubilize TCP in Pikovskaya’s agar. Data presented in (Table 3) revealed that within isolates of organic samples, the maximum (4.45) Phosphate Solubilizing Index (PSI) was recorded with isolate RDO10 and minimum (1.33) PSI was recorded with isolate SRO7. While, within isolates of inorganic samples, the maximum (4.00) Phosphate Solubilizing Index (PSI) was recorded with isolate N2S6 and minimum (1.32) PSI was recorded with isolate SRI3. The P-solubilizing activities of selected bacterial endophytes were compared on the basis of per cent P-Solubilization Efficiency (%SE) on PVK agar medium and P-solubilization in PVK broth. The results revealed that within isolates of organic samples, the isolate RDO10 had highest (342.45 per cent) P-solubilization efficiency, however, the lowest (33.33 per cent) phosphate Solubilizing Efficiency (%SE) was recorded with isolate SRO7. Whereas, within isolates of inorganic samples, the isolate N2S6 had highest (300.00 per cent) P-solubilization efficiency, however, the lowest (32.86 per cent) phosphate Solubilizing Efficiency (%SE) was recorded with isolate SRI3. Whereas, no significant difference was found in PSI and %SE between isolates from organic and inorganic plant samples. The quantitative results revealed significant variation among the isolates to solubilize the insoluble Tri-Calcium Phosphate (TCP) in liquid medium (Table 3). Within isolates from organic samples, the maximum (330.00 μg/ml) P-solubilization was recorded for RDO10 isolate, whereas minimum (105 μg/ml) was recorded for RDO12 and SRO7 isolates. However, within isolates from inorganic samples, the maximum (350.00 μg/ml) P-solubilization was recorded for N2S6 isolate, whereas minimum (120 μg/ml) was recorded for IDR8 isolate. Also the viable count after 72 h of incubation varied from (44×106 to 92×106 cfu/ml) and (37×106 to 104×106 cfu/ml) for isolates from organic and inorganic plant samples, respectively. Phosphorus and nitrogen are among the essential nutrients of the plants. Phosphorus is available to plants in the form of phosphate anions, which are mostly trapped by precipitation with cations such as Mg2+, Ca2+, Al3+ and Fe3+, so become insoluble and unavailable to plants in these forms. Bhattacharya and Jha [29] reported that endophytes have the capacity to mineralize and solubilize the inorganic as well as organic insoluble complex forms of phosphorus by releasing organic acids or extracellular hydrolytic enzymes and hence improve the accessibility of nutrients to plants. Phosphorus is one of the essential macronutrient required for biological growth and development of the plants [30,31]. Most of the phosphorus present in the soil is in the form of insoluble phosphates and hence unavailable to plants. Plant growth promoting bacteria are able to solubilize and make them available to the plants. Thus, P-solubilization is considered as one of the most important attribute of the PGPB [32]. The siderophore production efficiency of selected isolates was confirmed using the Chrome Azuerol Sulphate (CAS) assay. Table 4 revealed that great variation was observed in colony size (0.27 to 1.23 mm and 0.20 to 1.89 mm), zone size (0.47 to 1.60 mm and 0.47 to 2.35 mm) for isolates from organic and inorganic plant samples, respectively. Only two types of siderophores were produced. i.e. carboxylate (40.90 percent) and hydroxymate (59.09 per cent), whereas, catecholate type of siderophores were not observed for any of the selected isolates. Within isolates of organic samples, the isolate RDO10 had highest (114 per cent) siderophore production efficiency and (210.08 %SU) siderophore production. Whereas, within isolates of inorganic samples, the highest (200.00 per cent) siderophore production efficiency was recorded with isolate SRI3 and maximum (186.23 %SU) siderophore production was recorded with isolate IDR5. Whereas, isolates from inorganic plant samples showed maximum siderophore production efficiency (85.27 per cent) and (89.24 %SU) siderophore production than isolates from organic samples. Significant difference was found in PSI and %SE between isolates from organic and inorganic plant samples. The present results are in confirmation with [33]. Variation in final pH of supernatant ranged from 4.89 to 5.96. The present findings are in line with those of Shyam [34] who reported a wide range (19.42 to 68.07 %SU) with CAS liquid assay. The results are also in confirmation with Kirti [35].
Isolates
P-solubilization in solid medium
Viable Count
(106 × cfu /ml)P-solubilization in liquid medium (μg/ml)
Final pH of supernatant
Phosphate solubilization
index (PSI)(%) P-solubilization efficiency (%SE)
ISOLATES FROM ORGANIC PLANT SAMPLES
HS2
2.65
165.00
82.00
150
5.62
N3S3
2.21
121.67
57.00
195
5.31
N3S6
2.01
112.33
44.00
145
5.84
N3S7
2.93
193.94
92.00
110
5.74
N4S6
2.29
129.88
49.00
190
5.99
N4S9
1.64
64.15
67.00
150
5.53
N4S10
1.98
129.85
71.00
185
5.71
RDO2
2.46
146.57
74.00
230
5.37
RDO3
2.15
115.05
65.00
185
5.42
RDO10
4.45
342.45
92.00
330
4.34
RDO12
2.09
109.59
47.00
105
5.50
RDO13
2.58
158.21
71.00
175
5.99
RDO14
2.14
114.28
82.00
175
5.31
SRO4
2.00
100.00
65.00
165
5.32
SRO7
1.33
33.33
64.00
105
5.56
SRO8
1.86
86.00
55.00
155
5.32
Mean
2.29
132.64
67.31
171.87
5.49
ISOLATES FROM INORGANIC PLANT SAMPLES
HS14
2.25
125.00
80.00
160
4.79
HS17
2.53
153.97
82.00
205
5.12
HS18
2.13
113.21
104.00
165
5.41
HS19
2.41
141.67
86.00
190
5.23
HS20
1.71
71.67
58.00
155
5.03
HS23
1.90
90.00
65.00
250
4.65
HS24
2.03
103.03
69.00
135
3.65
N1S3
1.97
97.87
54.00
165
4.02
N1S23
3.20
220.00
79.00
235
5.41
N1S24
2.01
101.89
45.00
155
5.41
N1S25
2.30
130.43
87.00
150
4.09
N1S26
2.33
133.77
57.00
150
4.79
N2S6
4.00
300.00
71.00
350
4.19
N2S14
2.63
175.44
65.00
215
5.52
N2S16
2.00
100.00
37.00
150
4.79
N2S18
1.79
145.61
40.00
150
3.35
N2S19
3.24
224.24
42.00
195
5.41
N2S20
2.14
114.00
71.00
190
5.02
N2S21
3.40
240.00
82.00
175
5.11
IDR5
2.17
127.00
68.00
180
5.01
IDR6
2.10
110.83
70.00
160
5.11
IDR7
2.21
121.21
81.00
130
4.93
IDR8
2.95
195.35
45.00
120
4.80
SRI1
1.60
60.00
85.00
150
5.03
SRI3
1.32
32.86
80.00
155
4.71
SRI14
2.70
170.00
47.00
185
4.93
SRI15
1.85
85.00
65.00
165
5.71
SRI21
1.45
45.98
54.00
165
5.21
Mean
2.29
133.21
66.75
176.78
4.87
CD0.05
OvsINO
NS
NS
NS
3.00
0.13
WOR
0.92
13.08
12.59
13.54
0.60
WINO
0.92
13.08
12.59
13.54
0.60
Table 3: Qualitative and Quantitative estimation of tri calcium phosphate solubilization by selected bacterial endophytes.
Isolates
Siderophore estimation on solid medium
Final pH of supernatant
Quantitative estimation
(Per cent siderophore unit)Colony size
(mm)Zone size
(mm)Siderophore production efficiency (%SE)
Siderophore type
ISOLATES FROM ORGANIC PLANT SAMPLES
HS2
0.60
0.97
61.67
Hydroxymate
5.21
57.84
N3S3
0.47
0.83
76.59
Hydroxymate
5.87
69.21
N3S6
0.33
0.53
60.60
Carboxylate
5.67
51.23
N3S7
1.23
1.53
24.39
Hydroxymate
5.01
51.22
N4S6
1.10
1.53
39.09
Carboxylate
5.21
61.37
N4S9
0.27
0.47
74.07
Hydroxymate
4.99
58.82
N4S10
0.27
0.53
96.29
Carboxylate
5.04
146.08
RDO2
1.20
1.60
33.33
Hydroxymate
6.02
34.50
RDO3
1.23
1.55
26.01
Hydroxymate
5.23
58.82
RDO10
0.50
1.07
114.00
Hydroxymate
5.02
210.08
RDO12
0.75
1.15
53.33
Hydroxymate
5.34
62.35
RDO13
0.95
1.25
31.57
Hydroxymate
4.89
75.49
RDO14
0.67
1.13
68.66
Hydroxymate
5.45
76.78
SRO4
1.20
1.57
30.83
Hydroxymate
5.96
51.37
SRO7
1.00
1.27
27.00
Hydroxymate
5.34
35.49
SRO8
0.60
1.00
66.67
Carboxylate
5.82
78.82
Mean
0.77
1.12
55.25
5.37
73.71
ISOLATES FROM INORGANIC PLANT SAMPLES
HS14
0.67
1.13
68.66
Hydroxymate
5.45
76.78
HS17
0.77
1.47
90.90
Hydroxymate
5.34
97.98
HS18
0.73
1.17
60.27
Carboxylate
5.34
66.86
HS19
0.70
1.40
100.00
Hydroxymate
5.56
121.21
HS20
0.53
1.13
113.21
Carboxylate
5.84
83.72
HS23
0.60
1.33
121.67
Hydroxymate
5.35
116.27
HS24
1.89
2.35
24.59
Carboxylate
5.45
41.18
N1S3
0.23
0.53
130.43
Hydroxymate
5.54
136.27
N1S23
0.40
0.83
107.50
Carboxylate
5.45
101.37
N1S24
0.60
1.20
100.00
Carboxylate
5.12
86.47
N1S25
0.47
0.90
91.49
Hydroxymate
5.35
82.75
N1S26
1.87
2.33
24.59
Carboxylate
5.45
41.18
N2S6
0.60
1.40
133.33
Carboxylate
5.45
140.18
N2S14
1.40
2.10
50.00
Hydroxymate
5.67
56.09
N2S16
0.95
1.50
57.89
Hydroxymate
5.43
49.67
N2S18
0.76
1.46
90.90
Hydroxymate
5.34
97.98
N2S19
0.60
1.40
133.33
Carboxylate
5.34
140.18
N2S20
0.71
1.41
100.00
Hydroxymate
5.56
121.21
N2S21
0.53
1.13
113.21
Carboxylate
5.84
83.72
IDR5
0.20
0.47
135.00
Carboxylate
5.46
186.23
IDR6
1.00
1.27
27.00
Hydroxymate
5.67
36.47
IDR7
0.31
0.54
76.67
Carboxylate
5.67
83.33
IDR8
0.52
0.75
46.00
Hydroxymate
5.34
50.98
SRI1
1.30
1.87
43.85
Carboxylate
5.13
46.37
SRI3
0.20
0.60
200.00
Hydroxymate
5.05
178.82
SRI14
0.30
0.53
76.67
Carboxylate
5.67
83.33
SRI15
0.50
0.73
46.00
Hydroxymate
5.34
50.98
SRI21
1.87
2.33
24.59
Carboxylate
5.45
41.18
Mean
0.75
1.25
85.27
5.45
89.24
CD0.05
OvsINO
NS
0.03
1.51
NS
1.55
WOR
0.20
0.15
6.81
0.33
7.01
WINO
0.20
0.15
6.81
0.33
7.01
ND= not detected
**Initial pH =7.0; ***Per cent Siderophore unit (%SU)=where, Ar= Absorbance of reference (control) at 630 nm As= Absorbance of reference test at 630 nm.
Table 4: Qualitative and Quantitative estimation of siderophore production efficiency by selected bacterial endophytes of chrysanthemum (Dendranthema grandiflora Tzvelev).
Table 5 revealed that 86.36 (38/44) per cent isolates had the ability to produce IAA from tryptophan. IAA production by selected bacterial endophytes from organic and inorganic plant samples ranged from (13.30 to 62.00 μg/ml) and (7.00 to 56.00 μg/ml). Within isolates from organic plant samples, maximum (62.00 μg/ml) IAA production was recorded with isolate N3S6 which is statistically higher than that of all other isolates and minimum (13.30 μg/ml) IAA production was recorded with isolate RDO14. Similarly, within isolates from inorganic plant samples, maximum (56.00 μg/ml) IAA production was recorded with isolate N2S6 and minimum (7.00 μg/ ml) IAA production was recorded with isolate SRI1. Viable count after 72 h of incubation varies from (32.00×106 to 47.80×106 cfu/ml) and (23.67×106 to 55.60×106 cfu/ml) fror isolates from organic and inorganic plant samples, respectively. Final pH of the supernatant ranges from 4.99 to 6.01. IAA has been implicated in virtually every aspect of plant growth and development, as well as defense responses [36]. IAA is one of the physiologically most active auxins which is a common product of L-tryptophan metabolism of several plant growth promoting microorganisms [37]. Production of HCN and ammonia by microorganisms has been suggested as an important biofertilizer and biocontrol feature to enhance the plant growth. Selected forty four bacterial endophytes were screened for HCN and ammonia production on King’s B medium and peptone broth, respectively. Only 65.90 (29/44) per cent isolates were able to produce ammonia and 36.36 (16/44) per cent isolates were HCN producers (Table 6). Data in Table 6 also revealed that selected isolates were screened for chitinase, protease and amylase enzyme activities. Out of total selected isolates, thirty seven (84.09 per cent) showed chitinase activity with Enzyme Index (EI) ranging from 1.19 to 2.64. Maximum (2.64) EI was recorded for isolate RDO10, whereas, minimum (1.19) was recorded with isolate IDR8. Thirty two (72.72 per cent) and thirty eight (86.36 per cent) isolates exhibited protease and amylase activity with EI ranging from (1.45 to 3.66) and (1.17 to 2.55), respectively. Maximum (3.66) EI for protease enzyme activity was noted with isolate N1S25 and minimum (1.45) with isolate HS18. Similarly, maximum (2.55) EI for amylase enzyme activity was noted with isolate N1S25 and minimum (1.17) with isolate IDR8. Bacterial endophytes protects the plants from the fungal cell wall or cell membrane degradation caused by fungi and insects, by degrading cell membrane proteins or extracellular virulence factors, or by stimulating systemic resistance in plants [38]. HCN is recognized as a biocontrol agent, based on its ascribed toxicity against plant pathogens [39]. The level of HCN produced by bacteria in vitro is not only correlated with biocontrol activity but also indirectly increase the availability of phosphate. Ammonia productionis responsible for the indirect plant growth promotion and can serve as a triggering factor by suppressing plant pathogens [40]. The production of lytic enzyme has been considered with defence related mechanisms which has been documented by Jetiyanon [41] who found that a mixture of B. amyloliquefaciens strain IN937a and B. pumilus strain IN937b induced the peoduction of defence related enzymes against the pathogen. Extracellular enzyme production like chitinase, lipase, protease, amylase contributed to the ability of bacteria isolated from Valeriana officinalis to suppress the fungal diseases and thus demonstrated the potential of PGPR for biological control [42].
Isolates
Viable Count (106 × cfu/ml)
Indole-3-acetic acid (μg/ml)
Final pH of supernatant
ISOLATES FROM ORGANIC PLANT SAMPLES
HS2
35.60
27.20
5.43
N3S3
39.30
17.00
5.38
N3S6
39.40
62.00
5.84
N3S7
47.80
29.30
5.46
N4S6
47.50
21.50
5.13
N4S9
39.50
23.40
5.67
N4S10
44.50
ND
5.56
RDO2
45.80
25.00
5.87
RDO3
35.00
22.00
5.46
RDO10
41.00
52.20
5.05
RDO12
45.20
36.30
5.67
RDO13
43.40
21.10
5.34
RDO14
33.50
13.30
5.45
SRO4
35.00
40.00
5.52
SRO7
32.00
31.50
5.31
SRO8
47.10
49.20
5.13
Mean
40.72
29.43
5.45
ISOLATES FROM INORGANIC PLANT SAMPLES
HS14
41.33
36.00
5.32
HS17
52.33
28.00
5.67
HS18
55.60
16.00
5.34
HS19
46.79
20.10
5.37
HS20
38.90
ND
5.24
HS23
47.89
18.20
5.78
HS24
34.50
24.20
5.38
N1S3
42.30
22.00
5.16
N1S23
33.67
41.00
5.43
N1S24
36.79
ND
5.95
N1S25
32.90
31.00
5.77
N1S26
34.90
ND
5.94
N2S6
42.32
56.00
5.52
N2S14
43.45
27.00
5.21
N2S16
31.34
41.50
6.01
N2S18
47.89
25.00
5.53
N2S19
43.40
26.30
5.93
N2S20
39.90
22.40
5.44
N2S21
45.60
21.00
5.84
IDR5
44.67
ND
5.92
IDR6
38.78
33.00
5.37
IDR7
43.40
14.00
5.95
IDR8
46.78
31.00
5.61
SRI1
45.67
7.00
5.62
SRI3
23.67
ND
5.47
SRI14
33.40
36.00
5.31
SRI15
39.20
31.00
4.99
SRI21
38.90
38.00
5.84
Mean
40.93
23.06
5.56
CD0.05
OvsINO
NS
1.29
0.08
WOR
7.83
5.84
0.37
WINO
7.83
5.84
0.37
ND= not detected
Table 5: Quantification of IAA production (μg/ml) by different bacterial endophytes of chrysanthemum (Dendranthema grandiflora Tzvelev).
Isolates
Chitinase
Protease
Amylase
HCN production
Ammonia production
Zone size
(mm)*E.I.
Zone size
(mm)*E.I.
Zone size
(mm)*E.I.
ISOLATES FROM ORGANIC PLANT SAMPLES
HS2
9.00
1.50
5.00
2.50
3.00
1.50
-
++
N3S3
9.50
1.90
5.50
1.89
-
-
+
-
N3S6
-
-
8.00
1.86
6.00
1.39
-
+++
N3S7
19.00
1.65
-
-
13.00
1.73
+
+++
N4S6
16.00
2.05
9.00
2.36
-
-
-
+
N4S9
18.50
1.85
-
-
11.00
1.83
+
+
N4S10
8.50
2.12
3.50
1.67
2.50
1.66
-
++
RDO2
-
-
6.00
2.22
4.00
1.48
-
+++
RDO3
-
-
7.00
3.04
5.00
2.17
-
-
RDO10
17.50
2.64
4.50
3.00
1.50
2.14
+
+++
RDO12
18.00
2.50
4.40
2.44
2.40
1.33
-
-
RDO13
-
-
5.50
2.50
3.50
1.59
+
++
RDO14
25.00
2.27
9.50
1.90
7.50
1.50
-
+
SRO4
27.00
1.80
-
-
1.70
2.42
-
+++
SRO7
17.00
1.54
-
-
1.10
1.37
+
-
SRO8
21.00
1.40
-
-
1.50
1.36
-
-
ISOLATES FROM INORGANIC PLANT SAMPLES
HS14
14.50
1.76
4.50
3.75
2.50
2.08
+
+++
HS17
12.00
1.93
-
-
2.60
2.36
-
+
HS18
6.50
1.71
3.00
1.45
-
-
-
+
HS19
8.00
2.05
4.00
3.07
2.00
1.53
-
+++
HS20
10.00
1.66
6.00
3.00
4.00
2.00
+
-
HS23
27.00
1.58
13.50
1.60
11.50
1.36
-
+
HS24
20.00
1.33
-
-
4.00
1.81
-
-
N1S3
12.00
1.34
8.00
3.07
6.00
2.30
-
++
N1S23
13.00
1.30
-
-
7.00
2.33
+
+++
N1S24
-
-
4.50
2.09
2.50
2.27
-
-
N1S25
17.50
1.40
6.60
3.66
4.60
2.55
-
++
N1S26
-
-
10.00
2.70
8.00
2.16
-
-
N2S6
15.00
1.97
11.00
1.83
9.00
1.50
+
+++
N2S14
16.50
1.43
-
-
10.50
1.40
-
++
N2S16
29.00
1.28
4.40
2.93
2.40
1.60
-
++
N2S18
11.00
1.61
7.00
2.50
-
-
+
-
N2S19
10.00
1.33
-
-
2.70
1.20
-
+
N2S20
7.00
1.40
-
-
1.70
2.42
-
++
N2S21
13.00
1.32
9.00
1.55
7.00
1.20
+
-
IDR5
10.50
1.34
6.50
1.71
4.50
1.18
+
-
IDR6
22.00
1.18
3.80
2.71
1.80
1.28
-
+++
IDR7
12.00
1.30
4.20
3.00
-
-
-
+
IDR8
26.00
1.19
4.00
2.35
2.00
1.17
-
-
SRI1
8.00
1.29
4.00
2.50
2.00
1.25
-
-
SRI3
-
-
3.90
3.54
1.90
1.72
+
+++
SRI14
9.50
1.37
5.50
1.89
-
-
+
-
SRI15
14.00
1.41
10.00
1.69
8.00
1.35
+
++
SRI21
11.00
1.44
-
-
5.00
1.66
-
++
ND= not detected
*Enzyme index (E.I.) = A/B Where, A= Halozone diameter+Colony diameter; B= Colony diameter;
**HCN = Change in colour of filter paper from yellow to brown (+) and (-) no change
***Ammonia production= fair (+); Good (++); Very good (+++) ammonia producers; no activity (-)
Table 6: In vitro screening of selected bacterial endophytes for antagonistic traits of plant growth promotion.
In vitro antifungal activity (Table 7) of all the selected forty four endophytic isolates was tested against phytopathogenic fungi viz. Rhizoctonia solani, Pythium ultimum and Fusarium oxysporum. Bacterial isolates showed variation in antifungal activity against the tested fungal pathogens. Data in present table revealed that thirty six (81.81 per cent), thirty seven (84.09 per cent) and thirty four (77.27 per cent) isolates showed percent Growth Inhibition (%GI) against Rhizoctonia solani, Pythium ultimum and Fusarium oxysporum, respectively. Among isolates obtained from organic plant samples, maximum (48.89 and 77.78 per cent) growth inhibition was recorded with isolates RDO10 against Rhizoctonia solani and Pythium ultimum while minimum (27.56 and 35.78 per cent) growth inhibition was shown by isolate N4S6 and RDO14 against Rhizoctonia solani and Pythium ultimum, respectively. Also, maximum (54.00 per cent) growth inhibition was observed with isolates N3S6. against Fusarium oxysporum, respectively. The minimum (29.78 per cent) growth inhibition was observed with isolates N3S7 against Fusarium oxysporum. Similarly, among isolates obtained from inorganic plant samples, maximum (48.89, 76.44 and 68.89 per cent) growth inhibition was recorded with isolates N2S21, N1S25 and N2S6 against Rhizoctonia solani, Pythium ultimum and Fusarium oxysporum, respectively, while minimum (30.44, 29.38 and 31.11 per cent) growth inhibition was shown by isolate IDR8, SRI14 and N1S23 against Rhizoctonia solani, Pythium ultimum and Fusarium oxysporum, respectively. Whereas, between isolates from organic and inorganic plant samples, maximum (44.70) per cent growth inhibition against Pythium ultimum was shown by isolates from organic plant samples which is statistically higher than isolates from inorganic plant samples (37.37 per cent). However, no significant difference was recorded in per cent growth inhibition against Rhizoctonia solani and Fusarium oxysporum by isolates from organic and inorganic plant samples. The results are in line with Sharma et al. [36] who reported maximum per cent growth inhibition i.e. 76.12 per cent against Pythium ultimum with SJ6 isolate, 42.22 per cent against Rhizoctonia solani with SR5 isolate and 75.44 per cent against Fusarium oxysporum with SN1 isolate. Biological control using microorganisms has been studied intensively by many researchers as an effective alternative to control pests/diseases [43,44]. The formation of zone is due to the secretion of antifungal substances that might have diffused in the medium and resulted in the fungal growth inhibition.
Solates
Per cent growth inhibition (%GI) against
Rhizoctonia solani
Pythium ultimum
Fusarium oxysporum
ISOLATES FROM ORGANIC PLANT SAMPLES
HS2
35.56
67.56
44.44
N3S3
35.12
48.22
46.67
N3S6
31.78
47.56
54.00
N3S7
30.44
70.44
29.78
N4S6
27.56
42.22
38.67
N4S9
ND
ND
ND
N4S10
43.11
59.33
49.78
RDO2
42.67
46.67
43.78
RDO3
43.23
56.78
45.89
RDO10
48.89
77.78
40.00
RDO12
CI
ND
32.00
RDO13
37.11
40.89
ND
RDO14
38.67
35.78
ND
SRO4
39.67
42.45
51.00
SRO7
32.45
40.21
38.00
SRO8
ND
39.33
34.22
Mean
30.39
44.70
34.26
ISOLATES FROM INORGANIC PLANT SAMPLES
HS14
31.11
ND
35.22
HS17
36.67
39.33
38.67
HS18
45.00
38.00
50.44
HS19
ND
ND
ND
HS20
46.67
45.50
43.25
HS23
45.33
40.00
55.56
HS24
ND
45.67
ND
N1S3
37.11
70.44
ND
N1S23
32.00
37.11
31.11
N1S24
46.89
46.67
41.56
N1S25
43.11
76.44
43.11
N1S26
40.00
54.88
41.56
N2S6
42.22
58.67
68.89
N2S14
35.56
48.22
42.22
N2S16
32.00
37.11
ND
N2S18
36.44
72.66
40.89
N2S19
ND
ND
42.22
N2S20
45.33
66.67
59.33
N2S21
48.89
52.00
52.19
IDR5
38.67
51.11
42.22
IDR6
ND
ND
ND
IDR7
31.11
54.88
43.78
IDR8
30.44
CI
44.46
SRI1
41.56
50.44
34.22
SRI3
ND
ND
ND
SRI14
ND
29.38
ND
SRI15
32.33
CI
42.22
SRI21
45.33
31.45
37.11
Mean
30.84
37.37
33.22
CD0.05
OvsINO
NS
0.81
NS
WOR
3.45
3.68
10.44
WINO
3.45
3.68
10.44
ND= not detected, CI= contact inhibition
*Per cent growth inhibition (%GI) =×100 , Where, C: growth of fungus in control; T: Growth of fungus in test.
Table 7: Percent Growth inhibition of test fungus by selected bacterial endophytes of chrysanthemum (Dendranthema grandiflora Tzvelev).
Biochemical Characterization of Selected Bacterial Endophytes
Morphological and biochemical characterization were used to identify the isolated bacterial endophytes upto genus level as per Bergey’s Manual of Determinative Bacteriology. (Table 8) revealed that out of total forty four isolates, only nineteen (43.18 per cent) isolates were positive for indole test, fourteen (31.81 per cent) isolates showed positive response for methyl red test, twenty six (59.09 per cent) isolates were positive for Voges Proskauer test, twenty three (52.27 per cent) were able to utilize citrate. Hydrogen sulfide production was observed with only twelve (27.27 per cent) isolates. Number of bacterial isolates that showed positive results for different biochemical tests varied as thirty two (72.72 per cent) for catalase, twenty three (52.27 per cent) for oxidase, twenty eight (63.63 per cent) for lipase production. Twenty four (54.54 per cent) isolates were able to hydrolyse gelatin, while twenty eight (63.63 per cent) were able to hydolyse starch. Whereas, twenty seven (61.36 per cent), thirty three (75.00 per cent) and twenty nine (65.90 per cent) isolates were able to ferment dextrose, lactose and sucrose, respectively. The results of the present study are in line with that of Ghani et al. and Sharma [45,46].
Endophytes
Base pairs
Accession number
Closest relative
Per cent similarity BLASTn
Phylogenetic group
Strain designation
ISOLATES FROM ORGANIC PLANT SAMPLES
HS2
1025
MN186788
Stenotrophomonas maltophilia strain ATCC 13637
97.54
Gammaproteobacteria
Stenotrophomonas maltophilia strain HS2
N3S3
555
MN186799
Bacillus velezensis strain CBMB205
97.10
Firmicutes
Bacillus velezensis strain N3S3
N3S6
856
MN186803
Bacillus amyloliquefaciens strain MPA 1034
99.18
Firmicutes
Bacillus amyloliquefaciens strain N3S6
N3S7
708
MN242732
Lysinibacillus pakistanensis strain NCCP-54
98.15
Firmicutes
Lysinibacillus pakistanensis strain N3S7
N4S6
944
MN186795
Bacillus subtilis strain IAM 12118
98.06
Firmicutes
Bacillus subtilis strain N4S6
N4S9
871
MN186793
Micrococcus luteus strain NCTC 2665
99.66
Actinobacteria
Micrococcus luteus strain N4S9
N4S10
922
MN186783
Bacillus licheniformis strain DSM 13
99.56
Firmicutes
Bacillus licheniformis strain N4S10
RDO2
1174
MN186791
Bacillus wiedmannii strain FSL W8-0169
97.08
Firmicutes
Bacillus wiedmannii strain RDO2
RDO3
863
MN186796
Phyllobacterium ifriqiyense strain STM 370
99.77
Alphaproteobacteria
Phyllobacterium ifriqiyense strain RDO3
RDO10
916
MN186774
Bacillus subtilis strain IAM 12118
98.91
Firmicutes
Bacillus subtilis strain RDO10
RDO12
723
MN242729
Bacillus aryabhattai B8W22
99.17
Firmicutes
Bacillus aryabhattai strain RDO12
RDO13
1115
MN186787
Serratia nematodiphila strain NBRC 102204
96.45
Gammaproteobacteria
Serratia nematodiphila strain RDO13
RDO14
1058
MN186808
Stenotrophomonas maltophilia strain IAM 12423
99.33
Gammaproteobacteria
Stenotrophomonas maltophilia strain RDO14
SRO4
791
MN186789
Microbacterium testaceum strain DSM 20166
98.48
Actinobacteria
Microbacterium testaceum strain SRO4
SRO7
925
MN186797
Bacillus toyonensis strain BCT-7112
99.67
Firmicutes
Bacillus toyonensis strain SRO7
SRO8
654
MN242742
Stenotrophomonas pavanii strain LMG25348
99.24
Gammaproteobacteria
Stenotrophomonas pavanii strain SRO8
ISOLATES FROM INORGANIC PLANT SAMPLES
HS14
995
MN186781
Bacillus mojavensis strain ifo 15718
99.09
Firmicutes
Bacillus mojavensis strain HS14
HS17
797
MN242733
Stenotrophomonas bentonitica strain BII-R7
90.59
Gammaproteobacteria
Stenotrophomonas bentonitica strain HS17
HS18
867
MN186805
Stenotrophomonas rhizophila strain e-p10
93.22
Gammaproteobacteria
Stenotrophomonas rhizophila strain HS18
HS19
750
MN186806
Stenotrophomonas bentonitica strain BII-R7
95.97
Gammaproteobacteria
Stenotrophomonas bentonitica strain HS19
HS20
1024
MN186802
Cellulosimicrobium funkei strain W6122
98.91
Actinobacteria
Cellulosimicrobium funkei strain HS20
HS23
926
MN186786
Pseudomonas aeruginosa strain NRBC 12689
99.57
Gammaproteobacteria
Pseudomonas aeruginosa strain HS23
HS24
633
MN186784
[Pseudomonas] hibiscicola strain ATCC 19867
95.55
Gammaproteobacteria
[Pseudomonas] hibiscicola strain HS24
N1S3
962
MN186794
Bacillus halotolerans strain DSM 2802
98.86
Firmicutes
Bacillus halotolerans strain N1S3
N1S23
900
MN242728
Serratia nematodiphila DZ0503SBS1
99.67
Gammaproteobacteria
Serratia nematodiphila strain N1S23
N1S24
787
MN186780
Bacillus tequilensis strain 10b
98.05
Firmicutes
Bacillus tequilensis strain N1S24
N1S25
1227
MN186776
Bacillus subtilis strain JCM 1465
95.11
Firmicutes
Bacillus subtilis strain N1S25
N1S26
1014
MN186807
Streptomyces rubiginosohelvolus strain NBRC 12912
99.01
Actinobacteria
Streptomyces rubiginosohelvolus strain N1S26
N2S6
360
MN186777
Pseudomonas aeruginosa strain DSM 50071
96.30
Gammaproteobacteria
Pseudomonas aeruginosa strain N2S6
N2S14
1226
MN186775
Serratia marcescens strain NBRC 102204
97.58
Gammaproteobacteria
Serratia marcescens strain N2S14
N2S16
638
MN186800
Serratia nematodiphila strain DZ0503SBS1
99.06
Gammaproteobacteria
Serratia nematodiphila strain N2S16
N2S18
994
MN186778
Bacillus subtilis strain NRBC 13719
99.69
Firmicutes
Bacillus subtilis strain N2S18
N2S19
999
MN186798
Serratia marcescens strain NBRC 102204
98.15
Gammaproteobacteria
Serratia marcescens strain N2S19
N2S20
983
MN186779
Bacillus aryabhattai B8W22
98.88
Firmicutes
Bacillus aryabhattai strain N2S20
N2S21
1216
MN186792
Klebsiella grimontii strain SB73
94.22
Gammaproteobacteria
Klebsiella grimontii strain N2S21
IDR5
1003
MN186801
Bacillus subtilis strain IAM 12118
98.60
Firmicutes
Bacillus subtilis strain IDR5
IDR6
189
MN242731
Pantoea ananatis strain 1846
97.27
Gammaproteobacteria
Pantoea ananatis strain IDR6
IDR7
927
MN186810
Arthrobacter globiformis strain JCM 1332
97.51
Actinobacteria
Arthrobacter globiformis strain IDR7
IDR8
922
MN186804
Microbacterium trichothecenolyticum strain DSM 8608
98.80
Actinobacteria
Microbacterium trichothecenolyticum strain IDR8
SRI1
968
MN186785
Bacillus subtilis strain BRCC 10255
97.83
Firmicutes
Bacillus subtilis strain SRI1
SRI3
930
MN186790
Bacillus pseudomycoides strain NBRC 101232
99.68
Firmicutes
Bacillus pseudomycoides strain SRI3
SRI14
870
MN186782
Staphylococcus sciuri strain DSM 20345
99.31
Firmicutes
Staphylococcus sciuri strain SRI14
SRI15
879
MN186809
Bacillus megaterium strain ATCC 14581
99.54
Firmicutes
Bacillus megaterium strain SRI15
SRI21
304
MN242730
Bacillus flexus strain SBMP3
99.34
Firmicutes
Bacillus flexus strain SRI21
Table 8: Genetic diversity of selected bacterial isolates on the basis of phylogenetic analysis.
Genetic Diversity of The Selected Bacterial Endophytic Isolate(S) Associated With Chrysanthemum (Dendranthema Grandiflora Tzvelev) By 16S rDNA Sequencing
To assess and compare the genetic diversity of culturable bacterial endophytes of chrysanthemum (Dendranthema grandiflora Tzvelev) isolated from organic and inorganic samples collected from different districts of Himachal Pradesh, sequence analysis of 16S rDNA gene was conducted. Sequence analysis of forty four isolates, based on BLASTn search revealed the presence of bacteria belonging to 14 different genus Bacillus, Pseudomonas, Stenotrophomonas, Lysinibacillus, Micrococcus, Streptomyces, Pantoea, Klebsiella, Phyllobacterium, Serratia, Microbacterium, Cellulosimicrobium, Arthrobacter and Staphylococcus. The isolates exhibited nucleotide similarity with the nearest relatives in the NCBI GenBank database ranging from 90.59 to 99.77 per cent. Among endophytic bacteria Bacillus has been reported as most dominant genera [47,48] which support our findings. In general, the Phylum Proteobacteria, including the Classes a, β and γ-Proteobacteria, were reported to be dominant in diversity analysis of endophytes, although members of the Firmicutes are also among the classes most consistently found as endophytes.
Conclusion
Our research effort are towards helping the poor farmers as the focus of this study is on isolation, screening and characterization of plant growth promoting bacteria and their role in plant growth promotion. A pool of promising PGPB was screened for their plant growth promoting properties. The differences in plant growth promotion among the isolates were attributed to their individual competencies. On the basis of results of different PGP activities and their biocontrol ability, we suggested that these strains of PGPB have potential to be used as biofertilizers as well as bioprotectant agents having the potential to supplement the chemical fertilizers and pesticides. From the present investigation it is clear that selected isolates have potential to act as biofertilizer, biostimulant and bioprotectant.
Acknowledgments
Financial support from DBT scheme, Government of India, New Delhi, India is duly acknowledged.
References
- Anderson R L. Reclassification of genus chrysanthemum. Hort Science. 1987; 22: 313.
- Kumari A, Goyal RK, Choudhary M, Sindhu SS. Response of single and co-inoculation of plant growth promoting rhizobacteria on growth, flowering and nutrient content of chrysanthemum. African Journal of Microbiology Research. 2015; 9: 1896-1906.
- Kumar A, Maurya BR, Raghuwanshi R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatalysis and agricultural biotechnology. 2014; 3: 121-128.
- Prasanna R, Kanchan A, Kaur S, Ramakrishnan B, Ranjan K, Singh MC, et al. Chrysanthemum Growth Gains from Beneficial Microbial Interactions and Fertility Improvements in Soil Under Protected Cultivation. Horticultural Plant Journal. 2016; 2: 229-239.
- Barea JM. Future challenges and perspectives for applying microbial biotechnology in sustainable agriculture based on a better understanding of plant-microbiome interactions. Journal of Soil Science and Plant Nutrition. 2015; 15: 0-0.
- Rascovan N, Carbonetto B, Perrig D, Díaz M, Canciani W, Abalo M, et al. Integrated analysis of root microbiomes of soybean and wheat from agricultural fields. Scientific Reports. 2016; 6.
- Bhardwaj S, Dipta B, Kirti S, Kaushal R. Screening of efficient rhizobacteria associated with cauliflower (Brassica oleraceavar. botrytis L.) for plant growth promoting traits. Journal of Applied and Natural Science. 2017; 9: 167-172.
- Gholami A, Shahsavani S, Nezarat S. The Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Germination, Seedling Growth and Yield of Maize. World Academy of Science, Engineering and Technology, International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering. 2009; 3: 9-14.
- Kaushal M, Kaushal R. Plant growth promoting rhizobacteria- impacts on cauliflower yield and soil health. The Bioscan. 2013; 8: 549-552.
- Dursan A, Ekinci M, Donmez M F. Effects of inoculation bacteria on chemical content, yield and growth in rocket (Eruca vesicaria subsp. Sativa). Asian Journal of Chemistry. 2008; 20: 3197-3202.
- Egamberdiyeva D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Applied Soil Ecology. 2007; 36: 184-189.
- Subba Rao N S. Soil microorganisms and plant growth. Oxford and IBH Publishing Company, New Delhi. 1999; 250.
- Pikovskaya R I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya. 1948; 7: 362-370.
- Jensen E S. Inoculation of pea by application of Rhizobium in planting furrow. Plant and Soil. 1987; 97: 63-70
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Analytical biochemistry. 1987; 160: 47-56.
- Bakker AW, Schippers B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biology & Biochemistry. 1987; 19: 451-457.
- Vincent J M. Distortion of fungal hyphae in the presence of certain inhibitors. Nature. 1947; 150: 158-850.
- Holt J G, Krieg N R, Sneathm P H A, Staley J T, Williams S T. Bergey’s manual of determinative bacteriology, 9th edn.1994
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic acids research. 1997; 25: 3389-3402.
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic acids research. 1988; 16: 10881-10890.
- Gomez K A, Gomez A A. Statistical procedure for agriculture research. 2nd ed. John wiley and Sons, New York. pp. 1984; 427-357.
- An Q L, Yang X L, Dong Y M, Feng L J, Kuang B J, Li J D. Using confocal laser scanning microscope to visualize the infection of rice roots by GFPlabelled Klebsiella oxytoca SAZ, an endophytic diazotroph. Acta Botanica Sinica. 2001; 43: 558-564.
- Sharma PK, Sarita S, Prell J. Isolation and characterization of an endophytic bacterium related to Rhizobium/agrobacterium from wheat (Triticum aestivum L.) roots. Current Science. 2005; 89: 608-610.
- Wieland G, Neumann R, Backhaus H. Variation of Microbial Communities in Soil, Rhizosphere, and Rhizoplane in Response to Crop Species, Soil Type, and Crop Development. Applied and Environmental Microbiology. 2001; 67: 5849-5854.
- Gupta S. Studies on selected plant growth promoting rhizobacteria on growth and yield of capsicum (Capsicum annuum L.). M.Sc. Thesis submitted to Dr Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP). 2012.
- Richardson A E. Prospects for using soil microorganisms to improve the acquisitions of phosphorus by plants. Australian Journal of Plant Physiology. 2001; 28: 897-906.
- Thakur D. Preparation of bioactive phosphocompost and its effect on soil properties and crop yield. Ph.D. Thesis submitted to Dr Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP). 2014.
- Sharma K. Selection and Characterization of Endophytic and Rhizospheric Microorganisms of Chrysanthemum (Dendranthema grandiflora Tzvelve). 2009.
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World Journal of Microbiology and Biotechnology. 2012; 28: 1327-1350.
- Mehta P, Chauhan A, Mahajan R, Mahajan P K, Shirkot C K. Strain of Bacillus circulans isolated from apple rhizosphere showing plant growth promoting potential. Current Science. 2010; 98: 538-542.
- Gupta S. Impact of indigenous plant growth promoting rhizobacteria and chemical fertilizers on soil health and productivity of capsicum (Capsicum annuum L.). Ph.D. Thesis submitted to Dr Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP). 2016.
- Patel DK, Archana G, Kumar GN. Variation in the Nature of Organic Acid Secretion and Mineral Phosphate Solubilization by Citrobacter sp. DHRSS in the Presence of Different Sugars. Current Microbiology. 2007; 56: 168-174.
- Sharma B C, Subba R, Saha A. In vitro solubilization of tricalcium phosphate and production of IAA by phosphate solubilizing bacteria isolated from tea rhizosphere of Darjeeling Himalaya. Plant Sciences Feed. 2012; 2: 96-99.
- Shyam V. Selection and characterization of rhizospheric and endophytic microorganisms of Prunus avium. M.Sc. Thesis. Dr YS Parmar University of Horticulture and Forestry, Nauni, Solan (HP). 2010.
- Kirti S. Characterization of plant growth promoting rhizobacteria and arbuscular mycorrhizae from cherry (Prunus avium L.). M.Sc. Thesis submitted to Dr Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP). 2013.
- Sharma S, Minakshi, Kaushal R, Chauhan A. Characterization of efficient plant growth promoting rhizobacteria associated with chrysanthemum (Dendranthema grandiflora Tzvelev). Journal of Pharmacology and Phytochemistry. 2018; 7: 1547-1554.
- Ghosh S, Basu PS. Production and metabolism of indole acetic acid in roots and root nodules of Phaseolus mungo. Microbiological research. 2006; 161: 362-366.
- Egamberdieva D, Wirth S, Behrendt U, Ahmad P, Berg G. Antimicrobial Activity of Medicinal Plants Correlates with the Proportion of Antagonistic Endophytes. Frontiers in Microbiology. 2017; 8.
- Rijavec T, Lapanje A. Hydrogen Cyanide in the Rhizosphere: Not Suppressing Plant Pathogens, but Rather Regulating Availability of Phosphate. Frontiers in Microbiology. 2016; 7.
- Minaxi, Saxena J, Chandra S, Nain L. Synergistic effect of phosphate solubilizing rhizobacteria and arbuscular mycorrhiza on growth and yield of wheat plants. Journal of Soil Science and Plant Nutrition. 2013; 13: 0-0.
- Jetiyanon K. Defensive-related enzyme response in plants treated with a mixture of Bacillus strains (IN937a and IN937b) against different pathogens. Biological Control. 2007; 42: 178-185.
- Ghodsalavi B, Ahmadzadeh M, Soleimani M, Madloo PB, Taghizad-Farid R. Isolation and characterization of rhizobacteria and their effects on root extracts of Valeriana officinalis. Australian Journal of Crop Science. 2013; 7: 338-344.
- Duffy B, Keel C, Défago G. Potential Role of Pathogen Signaling in Multitrophic Plant-Microbe Interactions Involved in Disease Protection. Applied and Environmental Microbiology. 2004; 70: 1836-1842.
- Ko W, Tsou Y, Lin M, Chern L. Activity and characterization of secondary metabolites produced by a new microorganism for control of plant diseases. New biotechnology. 2010; 27: 397-402.
- Sharma S. Effect of plant growth promoting rhizobacteria and chemical fertilizers on growth and flowering of chrysanthemum (Dendranthema grandiflora tzvelve). M.Sc. Thesis submitted to Dr Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP). 2015.
- Ghani M, Ansari A, Aman A, Zohra RR, Siddiqui NN, Qader SAU. Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pakistan journal of pharmaceutical sciences. 2013; 26: 691-7.
- Rosenblueth M, Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Molecular plant-microbe interactions : MPMI. 2006; 19: 827-837.
- Shi Y, Yang H, Zhang T, Sun J, Lou K. Illumina-based analysis ofendophytic bacterial diversity and space-time dynamics in sugar beet on thenorth slope of Tianshan mountain. Applied Microbiology and Biotechnology. 2014; 98: 6375–6385.