
Research Article
Ann Agric Crop Sci. 2025; 10(1): 1176.
Study on the Effects of Bacillus Thuringiensis H-01-14 on The Growth and Development of Spodoptera Litura
Hu J1,2‡, Zhao Y1‡, Yu S1‡, Chen Y1, Ma R1, Yang C1 and Chen H1*
¹College of Agronomy, Sichuan Agricultural University, Chengdu, 611130, China
²Technical Center of Wenshan, Yunnan Tobacco Company, Wenshan, 663000, China
‡These three authors contributed equally to this work.
*Corresponding author: Huabao Chen, College of Agronomy, Sichuan Agricultural University, Chengdu, 611130, China Tel: 13084306538; Email: chenhuabao@sicau.edu.cn
Received: February 12, 2025; Accepted: March 10, 2025; Published: March 13, 2025;
Abstract
Bacillus thuringiensis (Bt) is an important microbial resource for pest control. Previous studies have shown that Bt strain H-01-14 exhibits certain insecticidal effects against Spodoptera litura. This study systematically investigates the effects of strain H-01-14 on the growth, development, and reproduction of S.litura. Histological methods were employed to compare the effects of strain H-01-14 on the testes, ovaries, and midgut of adult S.litura. Growth and development indicators demonstrated that strain H-01-14 extended the larval, pupal, adult, and eclosion periods of S.litura by 2.47 days, 1.71 days, 1.68 days, respectively. Pupal and eclosion rates decreased by 23.34% and 40.34%, respectively. Furthermore, the fecundity per female decreased by 49.75%, and hatching rate decreased by 68.08%. However, there was no significant effect observed on the growth and development of subsequent generations (F1) of S.litura. Histological observations revealed significant pathological changes in the ovarian germ cells of S.litura treated with strain H-01-14, characterized by uneven cytoplasmic structure and vacuolation, while no significant effects were observed in the midgut and testes. RNA-seq analysis identified 279 differentially expressed genes (DEGs), including 198 up-regulated and 81 down-regulated genes. GO and DEGs enrichment analysis indicated that strain H-01-14 mainly affected genes related to chitinase, phospholipase, trypsin, and aldo-keto reductase, thereby influencing the growth, development, immune function, and detoxification metabolism of S.litura. The isolation of this strain holds practical significance for the green control and resistance management of S.litura and potentially other pests.
Keywords: Bacillus thuringiensis; Growth and development; Spodoptera litura; Transcriptome sequencing
Introduction
Spodoptera litura (Fabricius) is a significant agricultural pest that causes substantial economic losses to a wide range of crops globally, causing significant economic losses to a variety of crops. Its host range is extensive, including over 300 species across approximately 100 plant families, including both crops and ornamental plants. In recent years, with the continuous adjustment of agricultural planting structures in China and the increase of agricultural replanting index, the severity of S.litura damage has been escalating annually [1], especially posing a serious threat to the safe production of vegetables [2]. Historically, chemical control has been the main method for managing S.litura, leading to increased resistance and reduced control efficacy. In pursuit of sustainable pest management, biological control has been actively promoted and applied as an effective approach in China.
During the formation of spores, Bacillus thuringiensis produces crystalline proteins at one or both ends of its bacterial cells. These crystalline proteins exhibit significant insecticidal effects against various pests such as Lepidoptera and Coleoptera, and they are widely utilized in agricultural and sanitary pest control, thereby significantly reducing the use of chemical insecticides [3]. Studies have shown that different strains of B. thuringiensis vary in their toxicity to Lepidoptera larvae. Due to insect avoidance or inhibition of toxin proteins, insects may not ingest sufficient quantities of toxin proteins, hence the lethality of these proteins to insects is not as high as originally thought. However, even small amounts of toxin proteins can sufficiently disrupt normal insect growth and development, leading to abnormalities or even death. Research indicates that B. thuringiensis insecticides not only kill target pests but also inhibit, impede, and prolong the growth, development, and reproduction of pests [4,5]. Yu's study [6] found that sublethal concentrations of strain Bt 46 adversely affect the growth and development of Mythimna separata Walker, particularly reducing their reproductive capacity and the hatchability and survival rates of their offspring, consistent with findings on sublethal concentration treatments of contemporary populations using chemical pesticides [7,8].
Building upon prior research isolating the pathogenic Bt strain H-01-14 against S. litura, this study extensively investigates its impact on the growth, development, and reproduction of S.litura. The aim is to enhance the reservoir of wild B. thuringiensis strains for biological insecticides, enrich the theoretical foundation of biological control, provide new strategies and methods for the control of S.litura, and serve as a reference for biological control to other pests.
Materials and Methods
Materials
S. litura: 2nd instar larvae, bred indoors through artificial rearing; strain H-01-14 isolated and preserved by the Agrochemicals Laboratory of Sichuan Agricultural University.
Stomach Poison Activity Assay
The biological assay method for S. litura utilized a feed mixing technique: Healthy 2nd instar larvae of uniform size were selected as test insects. Artificial feed was prepared according to a specified formula and preparation method. A mixture of 200 mg/mL spore crystal suspension was added, mixed thoroughly, allowed to solidify, and cut into suitable pieces, which were then placed into bioassay trays. Each treatment involved 10 larvae per replicate, with 3 replicates per sample, and larvae were reared at 28 °C, 80% humidity, and 12 h light/12 h dark cycle conditions. The control group was treated with sterile water under similar conditions. After 72 hours of cultivation, dead larvae were recorded to calculate corrected mortality rates [9].
Morphological Observation and 16S rDNA Sequence Determination
Morphological identification of colony and cell morphology of bacterial strains followed the the methodology described by Ammons et al. (2002) [10].
Total DNA of the tested strains was extracted using a bacterial genomic DNA kit. PCR amplification was performed using universal bacterial primers 27F-1492R: 1 μl cDNA, 1 μl upstream primer F, 1 μl downstream primer R, 12.5 μl 2×Taq Master Mix, and 9.5 μl ddH2O. The PCR reaction program included initial denaturation at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 54°C for 30 s, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. The quality of PCR products was checked by 2% agarose gel electrophoresis. PCR products were sequenced by Sangon Biotech (Shanghai, China). Sequences were compared with NCBI database for homology and a phylogenetic tree was constructed using MEGA 7.0 software to determine their taxonomic status.
Effects of H-01-14 on Growth and Development of S. litura
A mixture of 200 mg/mL spore crystal suspension of strain H-01- 14 was added to artificial feed. The control group received artificial feed containing an equivalent volume of sterile water. Each treatment involved 100 larvae with 3 replicates per treatment. After 5 days of feeding, larvae were observed daily for mortality and developmental stage changes until pupation and eclosion. Adults were provided with 10% honey water post-eclosion. Male and female individuals were paired in a 1:1 ratio and placed in rectangular disposable boxes (10.0 cm×10.0 cm×5.0 cm) with 10-20 biological replicates per treatment. Nutrition was supplemented with cotton balls dipped in 10% honey water, with daily changes of cotton balls and oviposition papers. Eggs were collected until adult death. Eggs collected were placed in an artificial climate incubator, and eggs from the F0 generation (S. litura fed for 5 days) were transferred to the next generation (F1) to investigate whether strain H-01-14 affects the growth and development of S. litura. Finally, statistics were conducted on larval period, pupal period, pupation period, adult period, eclosion period, as well as the number of F1 and F2 larvae hatched and unhatched egg masses to calculate hatchability.
Histological Observation of the effect of H-01-14 Strain on S. litura Ovary, Oocyte, and Midgut
The method for handling S. litura larvae was the same as above. Within 0-12 hours post-eclosion, 10 male and 10 female S. litura adults from generations F0 and F1 were respectively selected. The reproductive organs (testes, ovaries) of treatment and control groups of S. litura adults (F0, F1) and midgut tissues of fifth instar larvae were dissected using dissecting scissors. Paraffin sections were prepared according to modified Lee's methods [11], including embedding, sectioning, staining, and observation using an optical microscope. Morphological changes in reproductive organs of S. litura adults after treatment with strain H-01-14 were observed with reference to related literature [12-15].
Transcriptome Analysis of Stomach Poison Action of S. litura after Treatment with H-01-14 Strain
Sample collection: Samples were collected when S. litura reached the 6th instar after stomach poison treatment. Samples from control and experimental groups were frozen rapidly in liquid nitrogen and stored at -80°C until further experiments for RNA extraction and sequencing conducted by Shanghai Ouiyi Biotechnology Co., Ltd.
RNA Extraction, cDNA Library Construction and Sequencing
During the library construction phase, total RNA of S. litura male and female adults was extracted using the Trizol method. RNA sample purity, concentration, and integrity were assessed to obtain highquality RNA for constructing cDNA libraries. Subsequently, mRNA was enriched from eukaryotic mRNAs, which were fragmented using ultrasound. cDNA synthesis involved first-strand cDNA synthesis using fragmented mRNA and random hexamers, followed by second-strand cDNA synthesis using RNAseH and dNTPs under DNA polymerase I system. Purified cDNA was subjected to end repair and A-tailing, followed by adapter ligation. cDNA fragments of approximately 200-300 bp were selected using AMPure XP beads, PCR amplified, and purified again using AMPure XP beads to evaluate library quality and make sure the cDNA fragments are of moderate length and the library content is sufficient. Finally, sequencing was performed on the Illumina Hiseq Nova 6000 platform by Shanghai Ouiyi Biotechnology Co., Ltd.
Transcriptome Splicing, Assembly, and Annotation of Gene Function
Raw reads were processed using fastp for quality control to remove adapter sequences and low-quality reads, resulting in clean reads. Trinity software was used for transcriptome assembly to obtain a high-quality unigene library of S. litura adults. The assembled unigene sequences were functionally annotated using BLAST software (E-value = 10-5) against seven databases: Nr (https://blast. ncbi.nlm.nih.gov/Blast.cgi), Nt (https://blast.ncbi.nlm.nih.gov/Blast. cgi), Swiss-Prot (https://www.uniprot.org), GO (http://geneontology. org), KO (https://www.genome.jp/kegg), KOG/COG (https://www. ncbi.nlm.nih.gov/COG), and Pfam (http://pfam.xfam.org) [16-18].
Results and Analysis
Insecticidal Activity Assay Results
Using indoor-reared fall armyworms as test insects, the biological activity of the parasporal crystal mixture from 143 strains of Bacillus thuringiensis was assessed. The results are shown in Table 1. Strains H-02-05, K-03-02, P-11, M-02-04, and H-01-14 exhibited insecticidal effects against fall armyworm larvae, with significant toxicity observed for strain H-01-14, achieving a corrected mortality rate of 46.67% at 72 hours.
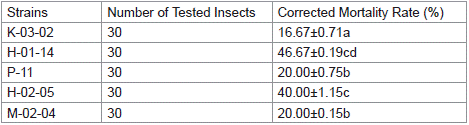
Table 1: Biological activity screening of partial isolates.
Strain Identification
Strain H-01-14 was morphologically characterized under a microscope (Figure 1a), exhibiting irregular circular, flattened white colonies with slight elevation in the center, dry surface, and incomplete edges; parasporal crystal morphology appeared circular and rhomboid (Figure 1b).
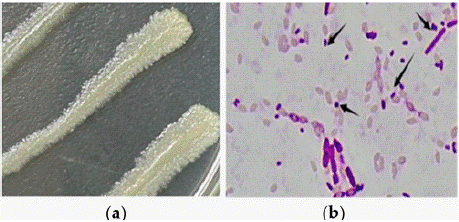
Figure 1: (a) Colonies of strain H-01-14 under the microscope (b) Crystals
of strain H-01-14 under the microscope.
The 16S rRNA PCR product sequence of strain H-01-14 was compared for homology with sequences in the GenBank nucleotide database, confirming its identity as Bacillus thuringiensis subsp. kurstaki H-01-14, as depicted in the phylogenetic tree constructed using MEGA7.0 software (Figure 2).

Figure 2: Constructing a phylogenetic tree based on 16S r DNA sequences.
Effect of Strain H-01-14 Treatment on S. litura Growth and Development
Starting from second-instar larvae, S. litura were fed with strain H-01-14 for 5 days, and the developmental stages from F0 to F1 pupation and eclosion were recorded (Table 2 and Figure 3). Results indicated that compared to the control, strain H-01-14 treatment significantly prolonged the larval, pupal, adult, and eclosion periods of F0 generation S. litura by 2.47 d, 1.71 d, 1.68 d, and shortened adult lifespan significantly by 4.51 d, with no significant effect on pupation period. For the F1 generation under continuous culture, strain H-01-14 prolonged eclosion and pupal periods by 0.67 d and 2.00 d, respectively, with no significant effects on larval and pupation periods. Strain H-01-14 also significantly inhibited pupation rate, reducing pupation by 23.34% and eclosion rate by 40.34% for F0 generation S. litura.
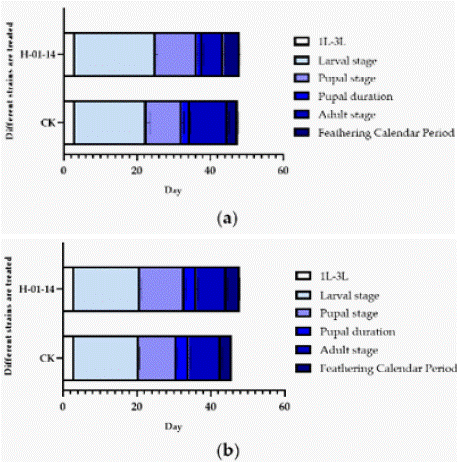
Figure 3: (a) Effects of different strains on the development duration of F0;
(b) Effects of different strains on the development duration of S. litura.
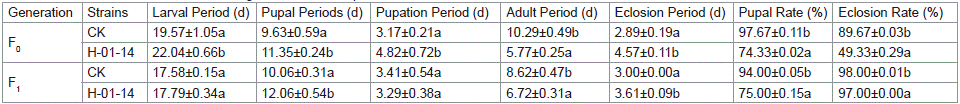
Table 2: Effects of different strains on the growth and development of F0 and F1 of S. litura.
Effect of Strains H-02-05 and H-01-14 on S. litura Oviposition and Hatchability
S. litura second-instar larvae were treated with strain H-01-14, and oviposition of F0 and F1 generations was recorded (Figure 4). Compared to the control, strain H-01-14 significantly reduced egg production by single-headed females in F0 by 49.75% and F1 by 2.84%.
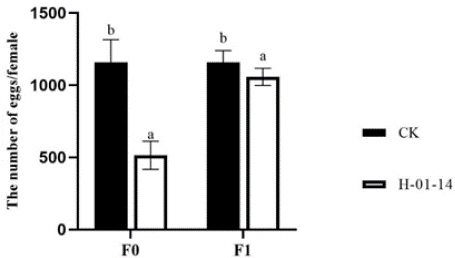
Figure 4: Effect of different strain treatments on the spawning of different
S. litura generations.
Hatchability rates of F1 and F2 generations were also assessed following treatment with strain H-01-14 (Figure 5), revealing significant reductions of 13.86% and 68.08%, respectively, compared to controls.
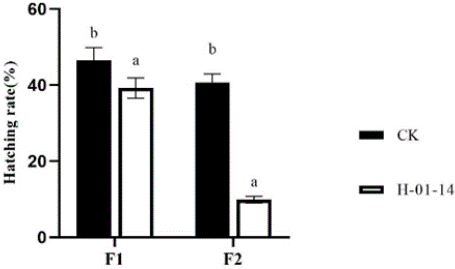
Figure 5: Effect of different strain treatments on hatching rate of different
S. litura generation.
The effect of H-01-14 strain on the spermatozonal and ovarian tissues of S. litura
To further understand the effects of strain H-01-14 on the reproductive system of S. litura, this experiment conducted paraffin section observations of the testes, ovaries, and midgut. Ten slides per organ were examined, selecting fields with a reproducibility rate exceeding 80% for photographic analysis, as shown in Figure 6.
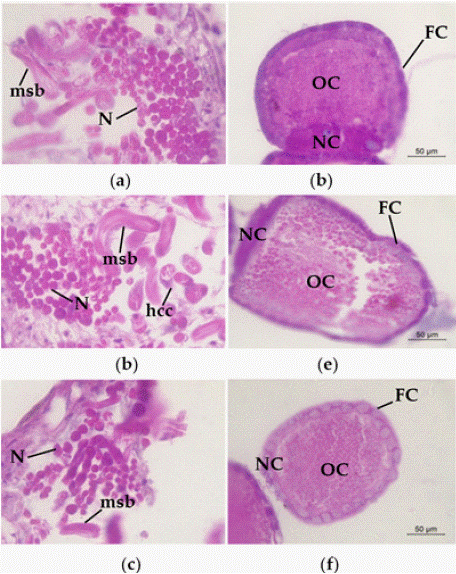
Figure 6: Pathological changes of H-01-14 strain on the spermatozonal and
ovarian tissues of S. litura. (a) nest of CK; (b) fine nest of F0 generation;
(c) nest of F1 generation. (d) ovaries of CK; (e) ovaries of F0 generation. (f)
ovaries of F1 generation. MSB: mature sperm bundle; N: nucleus; hcc: apical
sac cell; OC: oocyte; NC: trophoblast; FC: follicular cells.
Observations of testis sections revealed no significant pathological changes in the testes of S. litura F0 and F1 compared to the control following treatment with strain H-01-14 (Figure 6 a-c).
Examination of ovary sections showed marked pathological changes in the ovarian tissue of adult S. litura F0 treated with strain H-01-14, characterized by uneven cytoplasmic structure and the presence of vacuoles. Follicular cell rupture and irregular structure were observed, whereas no significant pathological changes were noted in the F1 generation (Figure 6).
Observation of midgut sections revealed regular epithelial cell arrangement with numerous microvilli in the normal midgut of S.litura larvae. Treatment with strain H-01-14 did not induce significant pathological changes in the midgut of fifth-instar larvae compared to the control (Figure 7).
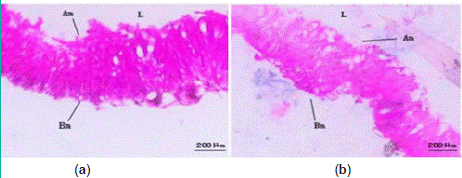
Figure 7: Pathological changes of strain H-01-14 on midgut tissue of
S. litura. (a) CK midgut; (b) H-01-14 midgut; Am: apical membrane; Bm:
basement membrane; L: lumen.
Differentially Expressed Genes Related to Insect Growth and Development
Chitinases, phospholipases, aldo-keto reductases, and serine proteases play significant roles in the overall growth and development of insects. This study identified 12 relevant genes from the transcriptomes of S. litura treated with gastric toxins from strain H-01-14 and the control group, including 1 chitinase gene (chitinase 10), 1 phospholipase gene (phospholipase A2-like), 1 trypsin inhibitor-like gene, 4 trypsin genes (trypsin alpha-3-like, trypsin CFT- 1-like), 2 chymotrypsin genes (chymotrypsin-1-like), and 1 aldo-keto reductase gene (aldo-keto reductase AKR2E4-like), as shown in Table 3 and Figure 10.
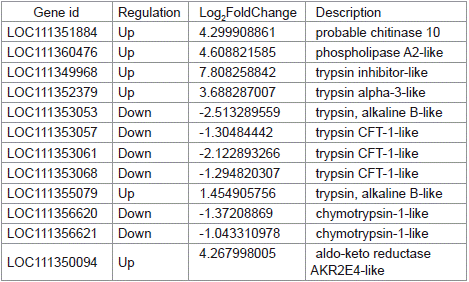
Table 3: Statistical table of differentially expressed genes related to insect
growth and development.
Transcriptome Analysis of S. litura after Treatment with Strain H-01-14
Number of Differentially Expressed Genes: DEseq2 method was used to analyze differentially expressed genes (DEGs) obtained from transcriptome data. The results indicated a total of 279 DEGs between control and treated samples, with 198 upregulated genes and 81 downregulated genes. The overall distribution of DEGs is shown in Figure 8.
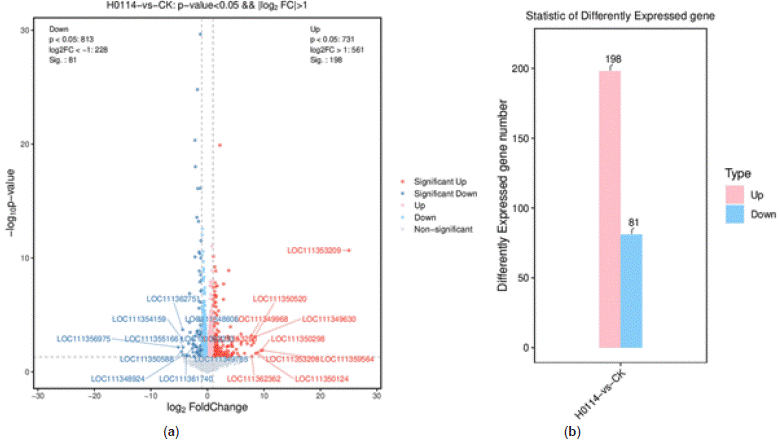
Figure 8: Holistic distribution diagram of differential genes. (a) Volcano map of holistic
distribution diagram of differential genes; (b) bar plot of holistic distribution diagram of differential genes.
GO and KEGG Enrichment Analysis of Differentially Expressed Genes
Analysis of differentially expressed genes in S. litura after treatment with strain H-01-14 revealed enrichment in GO pathways primarily associated with serine-type endopeptidase activity, phospholipases, and chitin-based cuticle development, as depicted in Figure 9a. The top 20 pathways with the smallest p-values were selected for significant enrichment analysis in bubble plots. KEGG pathway enrichment analysis (Figure 9b) indicated significant enrichment in glycine, serine, and threonine metabolism, pancreatic secretion, and protein digestion and absorption pathways.
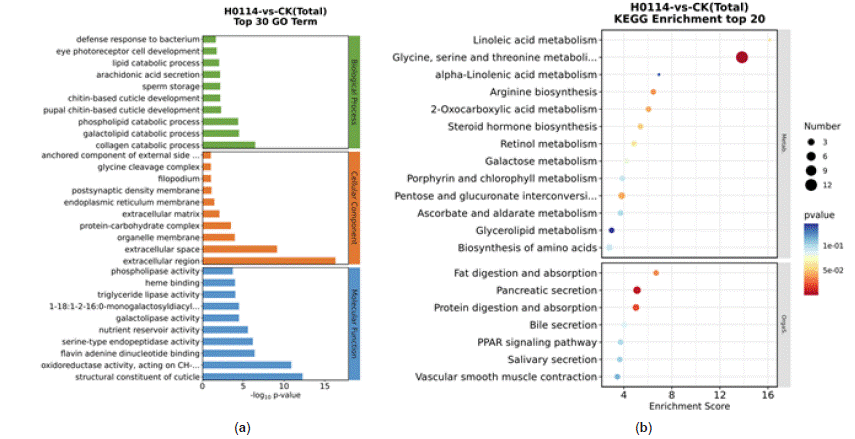
Figure 9: GO classification map of DEGs (a), Enrichment bubble map of KEGG pathway of DEGs (b).
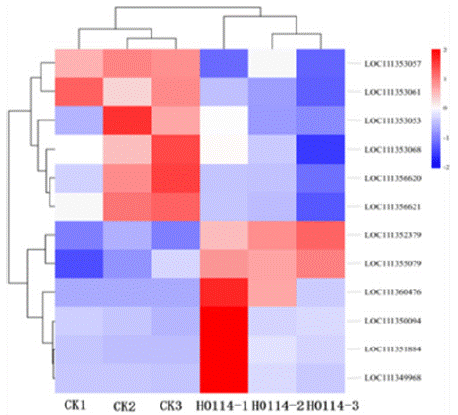
Figure 10: Heatmap of differentially expressed genes associated with
insect growth and developments.
Summary and Discussion
In this study, we found that Bacillus thuringiensis strain H-01-14 significantly prolonged the larval, pupal, and eclosion periods of S. litura, while also shortening their adult lifespan. It also significantly affected oviposition and offspring hatchability, and disrupted ovarian tissue of S. litura. Transcriptomic sequencing revealed that strain H-01-14 primarily influences the expression of genes related to chitinase, phospholipase, trypsin, and aldo-keto reductase, thereby affecting the growth, development, immune function, and detoxification metabolism of S. litura.
The study demonstrates that Bt insecticides not only kill target pests but also inhibit, hinder, and prolong pest growth, development, and reproduction [19]. We found that extending the developmental period of surviving larvae can correspondingly reduce the number of pest generations, thereby mitigating crop damage and preventing unnecessary losses [5]. Song [20] observed pathological changes in the midgut tissues of third-instar S. litura fed with Vip3Aa protein for 24 hours, showing epithelial swelling, cytoplasmic vacuolation, and shortened, deformed, and detached microvilli. GO enrichment analysis of transcriptomic results revealed that genes differentially expressed in S. litura larvae treated with strain H-01-14 were mainly enriched in pathways such as serine-type endopeptidase activity, phospholipase, and chitin-based cuticle development. The significant upregulation of the differential genes, including chitinase 2 and phospholipase A2, aligns with previous studies on their impact on fall armyworm growth and development [21]. In earlier reports [20,22], it was found that the chitinase 2 gene plays a crucial role in insect growth, development, and molting processes, regulating growth and reproduction. The significant upregulation of this differential gene in our study is consistent with previous research on its impact on fall armyworm growth and development. Phospholipase is an important enzyme present in organisms, playing a significant role in cell membrane structure formation, maintenance, and breakdown, as well as in phospholipid metabolism, hormone signal transduction, and biological membrane stability. The gene significantly associated with this experiment is phospholipase A2. This enzyme has been studied in several insects, including Drosophila melanogaster, red flour beetle, and tobacco budworm [23-26]. Research has shown that targeted inhibition of sPLA2 weakens the immune response of various lepidopteran insects [27]. KEGG pathway analysis in our study revealed that differentially expressed genes were mainly enriched in pathways such as pentose and glucuronate interconversions, galactose metabolism, glycerolipid metabolism, pancreatic secretion, protein digestion, and absorption. Serine proteases in lepidopteran insect intestines are involved in Bt protoxin activation and toxin degradation processes. Resistance of insects to Bt may depend on changes in the expression levels or types of serine proteases (e.g., trypsin and chymotrypsin) in the insect gut [28-31], Rodriguez [32] identified a crucial molecular gene called serine protease-like serine protease, which encodes a protein that confers resistance to Bt Cry1Ca1 protoxin in the fall armyworm Spodoptera frugiperda. Further studies have shown that RNA interference technology can significantly reduce the sensitivity of S. frugiperda to Bt Cry1Ca1 protoxin by silencing this gene. Aldo-keto reductase family genes participate in the ecdysteroid metabolism of lepidopteran insects. Yamamoto et al. [33] discovered another aldo-keto reductase AKR2E8 in silkworm Bombyx mori, which is expressed in the testes, ovaries, and fat bodies of B. mori, with the highest expression in the midgut.
Author Contributions
J.H., C.Y. and H.C. conceived and designed the research. Y.Z. and S.Y. conducted the experiments. Y.Z., Y.C., R.M. analyzed the data. Y.Z. and S.Y wrote the manuscript. All authors read and approved the manuscript.
Funding
This research was funded by Science and Technology Plan General Project of Wenshan Tobacco Company of Yunnan Province (No.20235326001).
Data Availability Statement: Data are contained within the article.
References
- Ming L, Du YW, Yuan GG. Spodoptera litura larvae are attracted by HvAV- 3h-infected S. litura larvae-damaged pepper leaves. Pest Manag. Sci. 2023; 79: 2713-2724.
- Un-Nisa E, Ahmad M, Sheikh U. Lethal and sublethal effects of flubendiamide and spirotetramat against the leaf worm, Spodoptera litura (fabricius) under laboratory conditions. PeerJ. 2023; 11: e15745.
- Schnepf E, Crickmore N, Van Rie J. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998; 62: 775-806.
- Bel Y, Ferre J, Hernandez-Martinez P. Bacillus thuringiensis toxins: functional characterization and mechanism of action. Toxins. 2020; 12: 785.
- Maeda M, Mizuki E, Nakamura Y. Recovery of Bacillus thuringiensis from marine sediments of japan. Curr. Microbiol. 2000; 40: 418-422.
- Yu H, Wang Y, Sun L. Effects of Bacillus thuringiensis on pathogenicity and growing development of Mythimna separata (walker). Journal of Northeast Agricultural University. 2021; 52: 21-28.
- Jingjing H, Changzhong L, Yinfeng M. Sublethal effects of imidacloprid to acy rthosiphon pisum. Plant Protection. 2009; 35: 86-88.
- Zhongfang L, Yuntao F, Yue G. Effects of sublethal dose of imidacloprid on life table of experimental populations of lacewing Chrysoperla nipponensis (okamoto) (Neuroptera: chrysopidae). Journal of Plant Protection. 2016; 43: 1014-1019.
- Sauka DH, Peralta C, Perez MP. Bacillus thuringiensis Bt_UNVM-84, a novel strain showing insecticidal activity against Anthonomus grandis Boheman (Coleoptera: curculionidae). Toxins. 2023; 16: 4.
- Ammons D, Rampersad J, Khan A. Usefulness of staining parasporal bodies when screening for Bacillus thuringiensis. J. Invertebr. Pathol. 2002; 79: 203- 204.
- Lee J, Jeong B, Kim J. Specialized digestive mechanism for an insectbacterium gut symbiosis. The ISME journal. 2024; 18.
- Sun R, Cui G, Chen Y. Proteomic profiling analysis of male infertility in Spodoptera litura larvae challenged with azadirachtin and its potentialregulated pathways in the following stages. Proteomics. 2018; 18: e1800192.
- Liang W, Feng C, Yaqing C. The testis development and spermatogenesis in Spodoptera litura (Lepidoptera: noctuidae). Journal of South China Normal University (Natural Science Edition). 2019; 51: 47-56.
- Bo X, Guanghong L, Fanghai W. Research on the development of prothoracic glands of Spodoptera litura. Acta Scientiarum Naturalium Universitatis Sunyatseni. 2012; 51: 97-100.
- Yinghua S, Yacai Y, Guren Z. Comparative study on zn2+ accumulation in different parts of gut from Spodoptera litura fabricius (Lepidoptera: noctuidae). Journal of Environmental Entomology. 2010; 32: 338-346.
- Chen S, Zhou Y, Chen Y. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018; 34: i884-i890.
- Grabherr MG, Haas BJ, Yassour M. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011; 29: 644- 652.
- Altschul SF, Madden TL, Schaffer AA. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic. Acids. Res. 1997; 25: 3389-3402.
- Zhu Q, Arakane Y, Banerjee D. Domain organization and phylogenetic analysis of the chitinase-like family of proteins in three species of insects. Insect. Biochem. Mol. Biol. 2008; 38: 452-466.
- Song F, Chen C, Wu S. Transcriptional profiling analysis of Spodoptera litura larvae challenged with Vip3Aa toxin and possible involvement of trypsin in the toxin activation. Sci. Rep. 2016; 6: 23861.
- Xiaohui Z, Ziniu Y, Cui H. Effect of cry1c toxin from Bacillus thuringiensis on growth, survival and feeding behavior of beet army worm larva. Journal of Zhejiang Agricultural University. 1999; 64-68.
- Ganbaatar O, Cao B, Zhang Y. Knockdown of Mythimna separata chitinase genes via bacterial expression and oral delivery of RNAi effectors. BMC Biotechnol. 2017; 17: 9.
- Karumbaiah L, Oppert B, Jurat-Fuentes JL. Analysis of midgut proteinases from Bacillus thuringiensis-susceptible and -resistant Heliothis virescens (Lepidoptera: noctuidae). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2007; 146: 139-146.
- Shrestha S, Park Y, Stanley D. Genes encoding phospholipases A2 mediate insect nodulation reactions to bacterial challenge. J. Insect Physiol. 2010; 56: 324-332.
- Su C, Tu G, Huang S. Genome-wide analysis of chitinase genes and their varied functions in larval moult, pupation and eclosion in the rice striped stem borer, Chilo suppressalis. Insect Mol. Biol. 2016; 25: 401-412.
- Ryu Y, Oh Y, Yoon J. Molecular characterization of a gene encoding the Drosophila melanogaster phospholipase A2. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 2003; 1628: 206-210.
- Lyu B, Li J, Niemeyer B. Identification, structural modeling, gene expression analysis and RNAi effect of putative phospholipase A2 in the lone star tick Amblyomma americanum. Ticks Tick-Borne Dis. 2024; 15: 102256.
- Li H, Oppert B, Higgins RA. Comparative analysis of proteinase activities of Bacillus thuringiensis-resistant and -susceptible Ostrinia nubilalis (Lepidoptera: crambidae). Insect. Biochem. Mol. Biol. 2004; 34: 753-762.
- Quan PQ, Li JR, Liu XD. Glucose dehydrogenases-mediated acclimation of an important rice pest to global warming. Int. J. Mol. Sci. 2023; 24: 10146.
- Oppert B, Kramer KJ, Johnson D. Luminal proteinases from Plodia interpunctella and the hydrolysis of Bacillus thuringiensis CryIA(c) protoxin. Insect. Biochem. Mol. Biol. 1996; 26: 571-583.
- Oppert B, Kramer KJ, Beeman RW. Proteinase-mediated insect resistance to Bacillus thuringiensis toxins. J. Biol. Chem. 1997; 272: 23473-23476.
- Rodriguez-Cabrera L, Trujillo-Bacallao D, Borras-Hidalgo O. RNAi-mediated knockdown of a Spodoptera frugiperda trypsin-like serine-protease gene reduces susceptibility to a Bacillus thuringiensis cry1ca1 protoxin. Environ. Microbiol. 2010; 12: 2894-2903.
- Yamamoto K, Endo S. Bombyx mori-derived aldo-keto reductase AKR2e8 detoxifies aldehydes present in mulberry leaves. Chem. Biol. Interact. 2022; 351: 109717.