
Review Article
Austin Alzheimers J Parkinsons Dis. 2025; 8(1): 1043.
Current Target Development and Future Prospects of Alzheimer’s Disease
Snehal N Tayade and Saurabh Maru*
Department of Pharmacology, SVKM’s NMIMS School of Pharmacy and Technology Management, Babulde, Shirpur, 425405 Maharashtra, India
*Corresponding author: Mr. Saurabh Maru, Assistant Professor, SPTM Shirpur, NMIMS Deemed to be University, India Email: skmaru@gmail.com
Received: May 06, 2025 Accepted: June 10, 2025 Published: June 12, 2025
Abstract
Alzheimer’s is a leading neurodegenerative disease, but we’re still searching for treatments that can truly stop or reverse it. The good news is, we’re getting better at spotting it early, even before symptoms show up, thanks to advances in brain imaging. While the “amyloid hypothesis” (targeting amyloid plaques in the brain) has been a major focus for drug development, these antiamyloid drugs haven’t delivered the hoped-for clinical benefits. It’s becoming clear that other factors, like individual differences in brain resilience (“cognitive reserve”), also play a role in how the disease progresses. Newer research is exploring other avenues, like targeting tau protein, inflammation in the brain, and problems with how brain cells connect. But there are challenges, like the high cost of developing brain drugs and the possibility that some people are naturally resistant to cognitive decline (“anti-CDR”). To make real progress against Alzheimer’s, we need researchers in universities and companies to work together, sharing a common goal of better diagnosis and innovative treatments.
Keywords: Alzheimer’s Disease; Pathophysiology; Clinical trial; Biomarkers; TAU; Neuroinflammation; Synaptic dysfunction; Anti-CDR; CDRenablement
Introduction
Alzheimer’s disease (AD) is a progressive and irreversible neurodegenerative disease clinically characterized by impaired memory and cognitive function [1]. Despite recent advancements in medical care, therapeutic strategies, and dementia research, the currently approved treatment can only mitigate the symptoms [2]. In AD, a decrease in the level of neurotransmitters such as acetylcholine and a rapid loss of neurons localizing in the hippocampal region are observed [3]. As AD progresses, coroner plaques composed of amyloid β-protein are observed in the brain, and the amounts of taucontaining neurofibrillary tangles also increase [4]. Research aimed at understanding the mechanism of development has been actively conducted in various fields, including immunology and statistics [5]. Therefore, treatment methods targeting the disease marker protein, amyloid, and immune cells essential for plaque clearance have been developed [6]. These methods have been introduced as treatment methods and drug candidates, but they have not been approved for clinical application because an appropriate neuroprotective effect, a suitable patient selection method, and a target molecule for AD have not been established [7]. The exact cause of AD is still unknown [7]. The participation of several factors, including mitochondrial abnormalities, protein denaturation and aggregation, and metabolic disturbance, is also considered as causative factors of AD [8]. Moreover, as well as environmental factors, genetic factors related to presenilin 1, APP, and apolipoprotein E are considered as trigger factors for the onset of AD [8]. Because numerous factors are considered to be participating factors in developing AD, understanding the mechanism of onset and development is considered to be the cornerstone for establishing an effective treatment or a diagnosis allowing its detection at the very early stage [9]. Besides, in addition to understanding AD, it is also necessary to establish patient care systems [10]. AD is a degenerative brain disease that leads to steady exacerbation and has a considerably adverse effect on the patient’s medical and nursing needs [11]. Early detection methods and improvements in the patient’s quality of life must be developed to promote early care, and it is essential to develop medical systems that consider dementia care and nursing care together [12].
Overview of Alzheimer’s Disease
Currently, Alzheimer’s disease (AD) is one of the most important diseases in developed countries (Figure 1) [13]. AD is pathologically characterized by progressive and specific degeneration of the central nervous system, which preferentially attacks discrete subsets of cortical and limbic system neurons [13]. It was clearly admitted that amyloid-β (Aβ) deposition, tau-containing neurofibrillary tangles produced by the hyperphosphorylation of tau, and neuronal/ synapse loss lead to the pathophysiology of the disease with varying contributions in distinct disease stages[14]. The current treatment to improve cognitive symptoms is mainly based on acetylcholine, either enhancing acetylcholine levels or reducing the breakdown of acetylcholine [15]. Other drugs enable cognitive and behavioral treatment [16]. Although several disease-modifying drugs that start from newly clarified mechanisms of the disease or refinement of past strategies have been expected to be therapeutic, most have failed in their late-stage trials so far [17]. Accumulated evidence over the last two decades strongly suggests that the oligomeric forms of Aβ, rather than Aβ monomers, are the major toxic diffusible species and the key etiologic culprit of cognitive decline in AD [18]. On the other hand, in tauopathy, tau oligomers and PHF are considered toxic themselves, which also spread the pathology [19]. Physiologically, Aβ peptides, especially Aβ 1-40, are released from plasma membrane neuronal and non-neuronal cells via proteolytic cleavage of amyloid precursor protein by secretases [20].
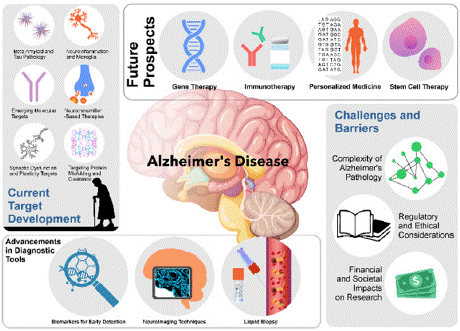
Figure 1: Alzheimer’s Disease.
Prevalence and Societal Impact
With the rapid growth of aged populations in many countries, dementia—of which Alzheimer's disease is the leading cause—has become a problem [21]. In Japan, which is the most rapidly aging country in the world, it is estimated that 4.6 million people had dementia in 2012, and the number is expected to increase to 7 million in 2025 [22]. Added to that, the testimony of the seriousness of the problem of dementia, the age-specific disability-adjusted life years (DALY) of women aged 50–89 as caused by Alzheimer's disease was 657 years in 2010, and it is projected to increase to 9,610 years in 2060 in Japan, far outstripping the years of DALY of other major diseases, such as cerebrovascular disease, cancer, heart disease, diabetes mellitus, and osteoporosis [23]. Alzheimer's disease is the most feared disease in the aging society because it deprives people of their dignity and autonomy through progressive cognitive decline to the degree that they can no longer lead normal lives, while at the same time it has a profound, overwhelming effect on their family, the healthcare system, and society [24].
Current Understanding of Alzheimer’s Pathophysiology
Current understanding of Alzheimer’s development has been built and updated through further studies using various model systems and experimental approaches [25]. To date, the widely accepted hypothesis for Alzheimer’s is the amyloid cascade: the formation of Aβ peptide produced by the cleavage of APP by β- and γ-secretases [26]. Although this hypothesis is still valid, an increasing number of reports have shown results that do not fit well with the ‘amyloid hypothesis’ [27]. Only a small number of Aβ 40/42 positive deposits have been detected in the brains of dysmorphic Down syndrome patients rather than the widely background Aβ oligomer in sporadic Alzheimer’s patients [28]. In addition, a polymorphism of α-secretase gene activity shortens Aβ peptide formation, reducing the risk of developing late-onset Alzheimer’s disease [29].
Considering these findings, several other hypotheses have been raised to cover these results, such as mitochondrial dysfunction, cholinergic neuron degeneration, and decreased neuron proliferation and differentiation [30]. Other observational factors include the observation that Alzheimer’s patients suffer from dysregulated energy utilization, resulting in brain weight loss, and that insulin receptor substrate in human brain tissue is cross-linked to Aβ, which is a key finding in type II diabetes [31]. Altered cell cycle control, as a result of intracellular neuronal Aβ accumulation, is also involved in the pathogenesis [32]. Furthermore, several types of prion diseases share a common molecular mechanism with the generation of Aβ oligomers, specifically in several kinds of early-onset Alzheimer’s disease and amyloidogenic Aβ peptide mutants with prion protein interaction [33]. Collectively, true understanding and a cure for this disease must derive from the combined knowledge of all of these factors [34].
Current Target Development for Alzheimer's Disease
Since 2000, initially targeting AChE and Aβ and changing to multi-targeting AD drugs, more than 10 drugs completed clinical trials; however, no drug has been registered in the market to date. After 2010, pioglitazone and insulin, anti-diabetic drugs, were developed for Alzheimer’s disease, showing a symptomatic effect [35]. Insulin is essential for memory and learning behaviour and has been shown that insulin levels decreased in AD brains from 50 years old due to central insulin resistance [36]. Human brain insulin absorption is very poor due to peptide molecular weight, but insulin nasal spray is effective in some people [37]. Currently, intranasal insulin is expected to be a curative drug for Alzheimer’s disease [38]. Clinical trials of PPAR γ agonist anti-diabetics such as rosiglitazone, pioglitazone, and fenofibrate are in progress in phase 3 clinical trials [39].
NMDA or MTHFR/CBS-CTH function, ADEIH serum biomarkers are involved in Alzheimer’s disease as therapeutic targets, and NMDA inhibition is a cure for glutamatergic excitotoxicity (Figure 2) [40]. Bupropion is also an attractive compound that has been developed into an NMDA antagonist [41]. Inhibition of homocysteine formation in the methyl group cycle for blood vessels and the central lower seating pathway of the nerve reduces the amount of cysteine available as a GSH precursor and thus increases the speed of the GSH peak after exercise [40]. Formed H2S itself is a neurotransmitter and has been proposed as a possible risk factor for Alzheimer’s disease by damaging exercise-induced GSH sulfide levels [42]. Recently, ROS scavenging of reduced GSH has been reported to prevent the neurotoxic effect resulting from enhanced oxidative stress [42]. In this review, we discuss the current target development for Alzheimer’s disease and the future prospects.
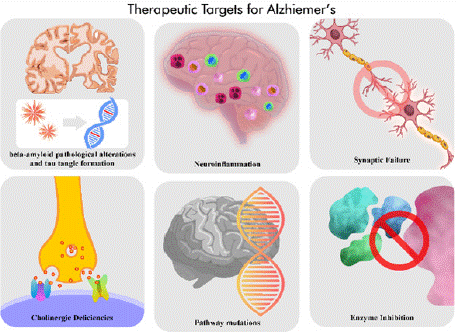
Figure 2: Alzheimer’s disease as therapeutic targets.
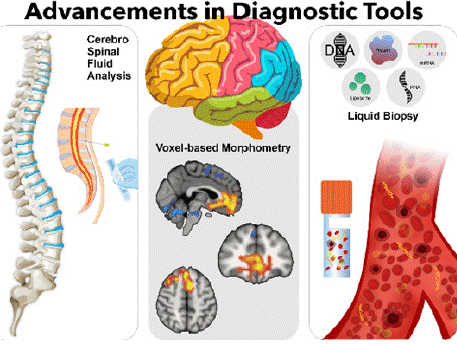
Figure 3: Alzheimer’s pathology imaging techniques.
Beta-Amyloid and Tau Pathology
Basic studies of beta-amyloid pathological alterations and tau tangle formation greatly aided in the identification of the connection between beta-amyloid and presenilin gene mutations in Alzheimer's disease [43]. In recent years, human-induced pluripotent stem cells have become important research and development tools, especially for the study of neurodegenerative diseases [44].
Numerous discussions have also been held regarding the development of tau disease after beta-amyloid pathology [45]. Numerous therapeutic trials have failed despite the emphasis on beta-amyloid-targeting therapy, underscoring the complexity of Alzheimer's disease as an illness with various aetiologies [46]. Both underlying aetiologies and environmental factors must be taken into account in order to properly treat beta-amyloid and tau disease [47]. Early-onset Alzheimer's disease was first identified by the presence of neurofibrillary tangles and amyloid plaques in the brains of affected individuals [48].
The genetic basis of the disease has been investigated extensively through the use of positional cloning techniques [49]. The 1989 discovery of the amyloid precursor protein gene and the 1986 identification of beta-amyloid as a primary constituent of amyloid plaques are significant turning points in the study of Alzheimer's disease [50]. Researchers tried to create animal models to investigate the function of tau protein, a crucial component of tangles, since it is connected to microtubule formation [51]. Tau, presenilin 1, and presenilin 2 are among the genes linked to early-onset Alzheimer's disease that were discovered between 1995 and 2000 [52].
Neuroinflammation and Microglia
Alzheimer's disease (AD) is influenced by neuroinflammation [53]. Activation of several immune cells in the brain, including microglia, which results in inflammatory reactions that are triggered by the production of cytokines and neurotransmitters are various symptoms and factors that can be observed[54].
It has been proposed for more than a century that inflammation plays a part in AD pathophysiology, but only recently has it drawn international scientific attention [55]. Amyloid plaques are usually surrounded by active microglia in the postmortem brains of AD patients [56]. Microglia can be good or bad, depending on their activation state [57]. In general, chronic excessive inflammation is detrimental and is believed to have a role in AD pathogenesis by causing an overabundance of different cytokines to be released [58]. Recent research has connected the development of AD to gene loci related to microglial activity [59]. In both the developing and adult brain, microglia also contribute to synaptic pruning by engulfing undesirable connections [60]. However, multiple independent investigations have shown that neurotoxic proteins released by AD neurones might result in increased synapse engulfment activity, which accelerates synaptic loss [61].
Neurotransmitter-Based Therapies
Targeting synaptic dysfunction in Alzheimer’s disease (AD) is a vital approach to tackle its complex pathogenesis [62]. The cholinergic hypothesis posits that cholinergic deficiencies are the primary cause of disrupted synaptic information processing [63]. To mitigate early‑stage AD symptoms such as mood disturbances and cognitive decline, pharmacological agents like donepezil, a reversible cholinesterase inhibitor, have been developed to enhance receptor sensitivity, reduce acetylcholine release, or inhibit its hydrolysis [67] [68]. Promising compounds identified in preclinical studies have made synaptic plasticity an attractive target for intervention [65]. Research has shown that overexpression of Aβ impairs paired‑pulse facilitation (PPF), long‑term potentiation (LTP), and micro excitatory postsynaptic current (mEPSC), thereby offering innovative strategies for addressing synaptic dysfunction [69]. Early‑stage therapies, including anti‑Tau antibodies and direct Aβ inhibitors, have demonstrated potential in altering biochemical pathways and decelerating disease progression [66]. However, challenges related to blood‑brain barrier permeability and dose sensitivity persist, although advancements such as in‑silico LTP models suggest possible solutions.
Synaptic Dysfunction and Plasticity Targets
According to the cholinergic hypothesis; Cholinergic deficiencies are the main reason for impaired synaptic information processing [77]. Donepezil, a reversible cholinesterase inhibitor, targets acetylcholine release, improve receptor sensitivity, or inhibit acetylcholine hydrolysis. Through this, it reduces early-stage AD signs such as including mood problems and cognitive loss [78].
It has been discovered that Aβ when overexpressed inhibits paired-pulse facilitation (PPF), long-term potentiation (LTP), and micro excitatory postsynaptic current (mEPSC), creating new strategies for treating synaptic dysfunction [80]. Anti-Tau antibodies and direct Aβ inhibitors are examples of early-stage medications that have shown promise in modifying biochemical pathways and slowing the progression of disease [81]. There are still issues with blood-brain barrier permeability and dose sensitivity, but developments like insilico LTP models point to possible fixes [82]. The combination of RGS4 and mGlu allosteric modulators has demonstrated potential for improving cognitive performance, whereas PRC2 inhibitors have been created to control pyramidal engram activity and restore phosphatase levels [83]. To completely implement these treatment approaches, more molecular and pathway target refinement is required [84].
Emerging Molecular Targets
Recent investigations have uncovered a wide array of compounds that play a role in the pathology of Alzheimer's disease (AD) [85]. Some of these compounds are linked to inflammatory conditions that are particularly characteristic of AD, potentially acting as surrogate markers for the disease's fundamental mechanisms [86]. Through various pathways (both direct and indirect), other compounds influence tau and Aβ levels [87]. The importance of these molecules in the pathogenesis of AD demands a more thorough evaluation, because the differing phenotypes of familial AD—arising from PSEN1 mutations—alongside the diverse embryonic expression patterns of presenilin (PSEN) and its genetic profile, indicate that the underlying pathophysiology of familial AD cases might indeed be distinct [88]. A continual focus on biogenesis and its neurotoxic potential has emerged in the field of AD research; however, a range of methodologies aimed at addressing AD is also being developed [89].
An abundance of novel molecular targets has been demonstrated to affect the etiology of Alzheimer's Disease (AD); however, the precise function of supplementary molecules remains ambiguous [90]. Treatments are presently evolving into a new phase of drug repositioning (1), which modifies strategies to incorporate the reduction of β-amyloid precursor protein (APP) synthesis, the minimization of neuroinflammation and the enhancement of the neuronal microenvironment—this occurs concurrently with Aβ reduction therapy [91]. Future therapeutic targets are anticipated to diversify even more, because they will emphasize inflammation suppression, microenvironment enhancement and synapse protection, marking a considerable shift in the methodology for managing AD [92].
Targeting Protein Misfolding and Clearance
Proteinopathy represent a hallmark of neurodegeneration, particularly in the context of Alzheimer’s disease, where the misfolding and aggregation of proteins can propagate beyond the initially affected cells [93]. These processes frequently result in largescale deposition of misfolded proteins, which are visible at the tissue level [94]. Addressing the root cause of the disease has proven to be challenging (due to limitations in both conventional and innovative drug approaches), as well as ethical and legal concerns [95]. Using large molecules to target protein misfolding and aggregation may, however, be counterproductive because of a lack of selectivity for diseased cells over healthy ones [96]. Although immunotherapeutic strategies— including specific-targeting active and passive approaches—remain promising, advances such as an anti-Tau vaccine and monoclonal antibodies designed for micro-dosing have shown potential [97]. Targeting nitrated or peroxidised monomeric Tau, which contributes to oligomer formation, has also led to the identification of new drug candidates [98]. Future strategies should focus on minimizing crossreactivity of therapeutic agents while concentrating on intraneuronal proteinopathy proteins [99].
Inhibiting enzymes that are responsible for these (pathological) processes could potentially thwart the onset, spread and progression of proteinopathy [100]. This includes the seeding of such conditions [101]. Promising drug characteristics and innovative approaches should, however, continue to be explored and developed, because they hold significant potential [102]. Although challenges remain, the pursuit of effective solutions is essential [103].
Advancements in Diagnostic Tools
Histopathological verification frequently (and often) overestimates the sensitivity and specificity of diagnostic tests; this tendency has led numerous researchers to concentrate on cerebrospinal fluid (CSF) biomarkers [104]. The biomarkers that have been most extensively studied include beta-amyloid 42, total tau and tau phosphorylated at threonine 181 (Table 1) [105]. CSF beta-amyloid 42 levels have been reported to correlate with brain extracellular beta-amyloid plaque levels [106]. However, a dependable individual patientbased assay to confirm that peripheral blood tests accurately predict brain pathology remains underdeveloped [107]. Efforts to identify plasma biomarkers have explored several options: beta-amyloid 42, discloid-1, cerebrospinal and brain-enriched A4 proteins and even ventricular enlargement with increasing PL2/PL1 ratios [108]. Complement receptor 1 variants, isoforms, protein levels and RhD protein concentrations have also been linked to early cognitive impairment of unknown origin [109]. Although combining plasma biomarkers remains underutilized, current approaches barely tap into the wealth of data generated by cognitive assessments and clinical evaluations [110].
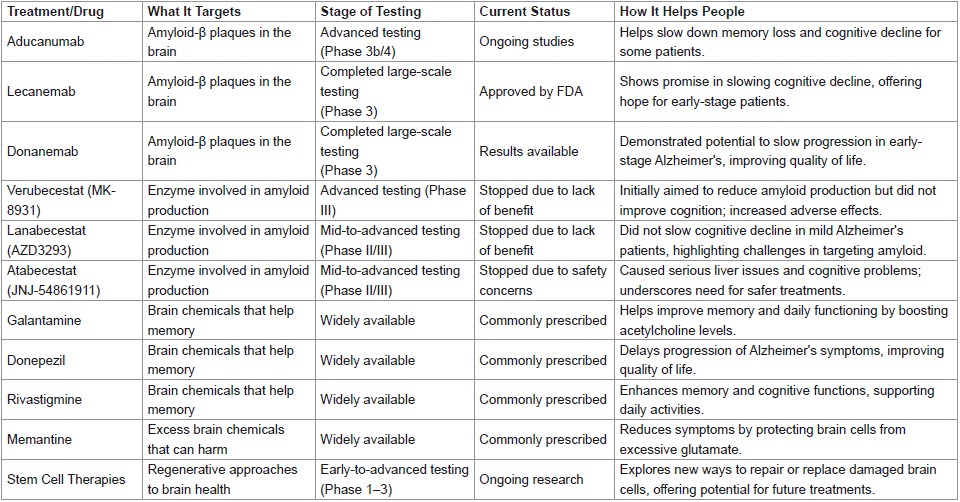
Table 1: Clinical Trials and Drug Development.
Various technical challenges have impeded the utilization of Alzheimer’s pathology imaging techniques. Some of the techniques utilised are positron emission tomography (PET) and single-photon emission computed tomography (SPECT), for diagnostic purposes [111]. These technologies regardless, have proven to be essential the diagnosis of the disease[112].
Biomarkers for Early Detection
Alzheimer's disease is a prevailing cause of dementia, underscoring the urgent need for extensive research aimed at identifying effective biomarkers for diagnosis and treatment. [114] Recent progress in neuroimaging, along with the discovery of cerebrospinal fluid (CSF) and blood biomarkers, has significantly enhanced the early detection of AD with remarkable specificity. [115] Notably, alterations in brain MRI can be detected prior to the manifestation of symptoms in AD patients; however, mild cognitive impairment (MCI) is acknowledged as a precursor to dementia.
The examination of CSF offers a crucial opportunity for identifying prion-related amyloidopathy, as well as a newly recognized condition known as primary age-related tauopathy. While CSF-based tests show potential, blood-based tests are more convenient to administer and have advanced notably, particularly with the development of DNA methylation-based biomarkers. Additionally, a decline in olfactory function has been identified as a significant early marker of AD. Moreover, biopsy samples from regions such as the skin, eyes, and nasal mucosa can provide valuable diagnostic insights for individuals at risk of developing AD. This comprehensive review emphasizes the importance of current biomarkers for preclinical Alzheimer's disease (AD) and explores the potential advancements they may bring to AD research.
Nevertheless, the implications of these findings are far-reaching, despite the challenges that remain. A deeper understanding of biomarkers is crucial, as it could significantly influence future therapeutic strategies.
Neuroimaging Techniques
Alzheimer's disease (AD) is marked by the accumulation of amyloid plaques and neurofibrillary tangles, accompanied by synaptic and neuronal degeneration. To effectively identify individuals with AD, it is essential to utilize reliable biomarkers that accurately reflect the underlying neuropathology in a non-invasive manner.
Among the various neuroimaging techniques available, MRI has demonstrated particular efficacy. In patients diagnosed with AD, notable atrophy is evident in both the hippocampus and entorhinal cortex. Consequently, accelerated rates of atrophy are typically regarded as necessary criteria for clinical trials related to AD. By evaluating atrophy in participants over time, researchers have found that the percentage change in medial temporal lobe (MTL) volume over a one-year span may provide a significant metric for gathering clinical data concerning this specific alteration. Nevertheless, additional research is needed to substantiate these findings, as a comprehensive understanding of the complexities of AD is vital for the development of effective interventions.
Another approach to identifying the neuropathology associated with AD involves employing voxel-based morphometry (VBM) to analyze variations in grey matter. A common method is to compare differences between groups with AD, mild cognitive impairment (MCI), and normal controls. Diffusion tensor imaging (DTI) serves as another technique for detecting alterations in white matter. Recent studies have documented a marked reduction in AD-related fractional anisotropy (FA) in the entorhinal cortex and hippocampal regions. Future research utilizing DTI will be necessary to explore this early stage further.
Liquid Biopsy for Alzheimer’s
The progressive dementia known as Alzheimer's disease (AD) is characterised by neurofibrillary tangles and amyloid plaques [136]. AD is diagnosed using these two characteristics [137]. Researchers use tau tracers, amyloid positron emission tomography, and cerebrospinal fluid biomarkers to evaluate new medications in clinical trials and comprehend the course of AD [138]. The disease progresses to further stages as it gets worse [139]. Early detection is therefore essential [140]. Especially in the preclinical phases, the development of useful liquid biopsies for early AD identification is essential for prompt therapy [140].
According to recent research, biomarkers such total and phosphorylated tau, neurofilament light chain, Aβ1–40, and Aβ1–42 may offer a viable substitute method for quickly evaluating AD patients [141]. Additionally, liquid biopsy can be used to detect and track a biomarker's response to treatment and can be developed as a drugfree method in clinical practice [142]. Analysing blood-based protein, RNA, miRNA, and DNA levels can greatly aid in the formulation of clinical trials because blood is rich in sensitive information [143]. Furthermore, AD patients have a low level of EVs [144]. It should be enhanced by customising the immune system for pathology, staging, early diagnosis, and an accurate survey to evaluate nerve activation and function [145]. After that, circulating DNA samples, EV components, and miRNA should be examined to determine the effectiveness of current pharmacological therapies [146].
Clinical Trials and Drug Development
The high number of drug failures in Alzheimer’s disease (AD), along with the slow and costly drug development process, has led to fewer diverse treatment options and less involvement from pharmaceutical companies [147]. But these failures haven’t been for nothing—they've provided valuable lessons for researchers and the industry [148]. For a long time, most drug pipelines have focused on the amyloid-β peptide as a target, but after so many setbacks, there’s now a growing appreciation for other approaches, like targeting extracellular tau and exploring alternative pathways [149]. Scientists are realizing that looking beyond amyloid mechanisms might be the key to real progress [150] (Table 2).
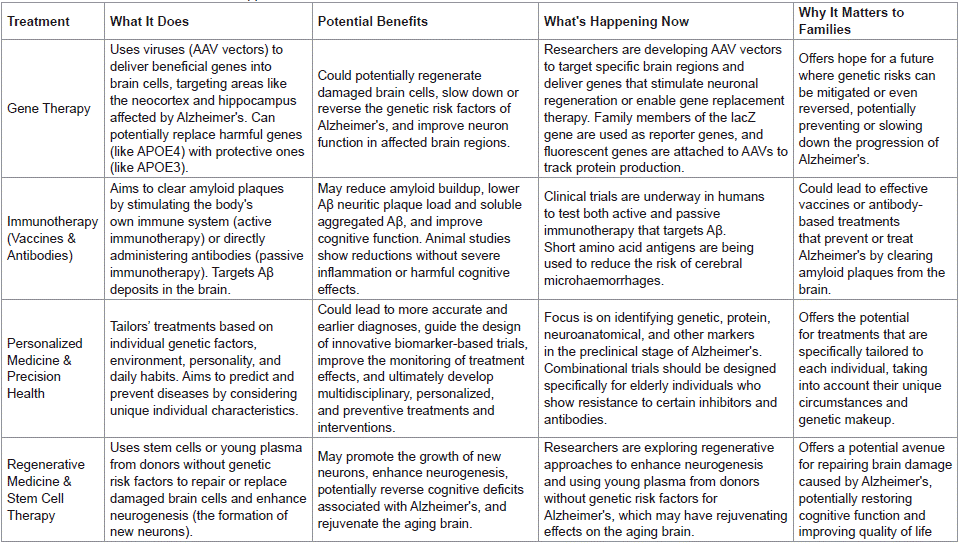
Table 2: Alzheimer’s Disease Treatment Approach.
As our understanding of AD deepens, it’s becoming clearer that the rising levels of Aβ and other protein buildups in aging brains might share a common root cause—a problem with the brain’s ability to properly clear out these proteins [151]. This could be due to issues with the body's natural clearance and degradation systems, or even factors like coexisting conditions and epigenetic susceptibilities [152]. Data from both animal studies and human brain imaging strongly link dysfunctional elimination pathways to the protein deposits seen in AD [153]. But there’s still debate—does a reduced ability to clear proteins actually cause these buildups, especially at lower levels before the disease fully develops [154]. There are still many unanswered questions [155]. What’s clear is that deficiencies in these elimination pathways leave gaps in our understanding of the early amyloidogenic state that drives AD [156].
Successes and Failures of Current Drug Candidates
The enormous effort to develop new drugs for Alzheimer’s disease (AD) has resulted in a vast and complex collection of clinical trial data, covering both symptom-relieving and disease-modifying treatments [157]. There have been a few positive trials, mostly using anti-amyloid secretase modulators, which supports the idea that the earliest detectable changes in neurodegenerative diseases come from the faulty processing of proteins [158]. However, many other types of drugs have been tested, and most have failed to show benefits [159]. Despite these setbacks, the number of potential drug targets for AD continues to grow [160]. Researchers are now also looking at broader, systems-level treatments that could be useful across different neurological diseases [161]. The few successful trials have been extremely valuable in guiding research, reinforcing the focus on Aβ as a target [162]. At the same time, many other drug candidates that previously failed are being revisited, now with stronger scientific foundations [163]. The lessons learned from clinical trials are crucial when planning future dementia treatments [164]. But there’s a real challenge—repeated failures have discouraged some funding bodies and investors [165]. This makes it harder for new drug candidates to progress, especially when compared to other central nervous system (CNS) diseases like diabetes, inflammation, and pain, where drugs that initially failed in AD later found success in treating other neurological conditions [166].
Key Challenges in Drug Development for Alzheimer’s
A total of 14 drugs have been approved for Alzheimer’s disease treatment, but none of them can modify the disease process or provide a cure [167]. This is because several challenges make drug development difficult [168]. Here, we look at three major obstacles. First, for a drug to be effective, it needs to reach the site where plaques and neurofibrillary tangles form in the brain [169]. But the brain has a blood-brain barrier, which can make it difficult for therapeutic drugs to pass through and reach their target [170]. Second, many Aβ immunotherapies have been developed to reduce Aβ peptide expression [171]. However, a phase 3 trial of passive Aβ immunotherapy had to be discontinued because most participants developed amyloid-related imaging abnormalities [172]. On the other hand, multiple active Aβ immunotherapies have been tested, and so far, no clinical trial has reported this as a major adverse reaction [173]. Third, drugs designed to suppress tau protein tend to have long-term effects, requiring careful dosing and prolonged administration [174]. The best time for treatment is believed to be during the early stages of mild cognitive impairment, but this is still debated [175].
The long and silent phase of drug development extends the time it takes for a treatment to reach the market [176]. This, combined with the complex regulations surrounding dementia treatments, makes the process both difficult and time-consuming [177]. When it comes to the amyloid hypothesis, many clinical trials have reported negative results, even though drug development has closely followed pharmacokinetic and pharmacodynamic principles [178]. This has been discouraging for some researchers, but it also signals a shift in the approach to amyloid clinical trials [179]. To overcome this impasse, changes are being made to clinical trial strategies [180]. These include adjusting research populations and incorporating additional dementia diagnostics [181]. Some of these diagnostics use molecules directly linked to neurodegenerative disorders, while others rely on data about synaptic failure from cytoplasmic proteins or commercially available markers to better study drug effects [182].
Innovative Trial Designs and Adaptive Methodologies
The traditional randomized controlled clinical trial remains the gold standard for evaluating Alzheimer’s disease treatments [183]. However, running these trials under current regulatory guidelines is extremely costly [184]. To make them viable for investors and other stakeholders, phase III trials must be large in size and scope [185]. These large trials typically focus on patients in the later stages of the disease, often in the mild to moderate dementia phase [186]. To ensure the presence of amyloid pathology, researchers use amyloid PET scans or CSF Aβ42 testing to select participants [187]. The problem is that by the time the disease has progressed enough for enrolment, it may already be too late for a treatment to make a meaningful difference due to the extensive damage that has occurred [188]. So far, more than 99% of anti-amyloid treatment trials have failed to show any significant cognitive benefits, with failure rates exceeding 99% in advanced-stage trials [189]. In the case of beta-secretase inhibitors, phase III studies even showed an increase in the rate of cognitive decline [190]. These failures highlight a crucial lesson—patients must be enrolled at a stage where they can truly benefit from the treatment’s mechanism of action [191]. This is essential not only for scientific success but also for securing regulatory approval [192].
Concepts such as the assertion that 'the sole factor of significance in a clinical trial is the patient' contribute minimally to accelerating the process of innovative drug discovery. The primary challenge for a competent research team lies in the meticulous selection of welldefined, homogeneous molecular populations and the identification of appropriate patients—those who are most likely to experience substantial treatment benefits. In therapeutic domains where advancements are sluggish, the implications can be dire. Even in the absence of disease-modifying therapies that directly address the underlying causes of Alzheimer’s, the progression of drug development must persist. This underscores the critical necessity to investigate and enhance innovative trial designs. Such methodologies can establish new operational guidelines for patient selection and testing criteria, thereby increasing the efficiency of trials. Adaptive clinical trial designs, which permit researchers to adjust various elements of the study without jeopardizing its scientific validity, represent another essential strategy. By adopting these novel approaches, research can continue to advance, bringing us closer to more effective therapeutic options.
Future Prospects in Alzheimer’s Disease Treatment
Despite the extensive number of clinical trials conducted, only five drugs are currently available for the treatment of Alzheimer's disease. Nevertheless, numerous potential therapies are under development. Among those that reach phase 3 clinical trials, the predominant mechanism of action is amylase inhibition, followed closely by acetylcholinesterase inhibition. However, due to the high attrition rate of drugs failing to progress beyond phase 2 trials, typically only three or fewer agents successfully transition from phase 2 to the final trial phase. While there is optimism regarding future treatments, significant challenges persist, including the need to accurately identify suitable candidates for medication and the necessity of establishing reliable predictive markers. To enhance the likelihood of successfully completing phase 3 clinical trials, it is imperative to consistently conduct long-term, effective phase 2 studies in conjunction with standard phase 3 trials. These studies must also navigate a complex bureaucratic landscape, which involves coordination with regulatory bodies and organizations, underscoring the importance of early preparation. For these trials to advance efficiently, collaboration between researchers and participants is essential, leveraging innovative programs that facilitate multicenter and large-scale studies to be completed more rapidly. This strategy could garner interest from various companies with aligned research objectives, thereby assisting in covering costs associated with organizational and study support.
Gene Therapy Approaches
The primary genetic risk factor associated with sporadic Alzheimer’s disease is the APOE4 allele, succeeded by more recently discovered genes such as ABCA7, BIN1, CD2AP, CD33, CR1, EPHA1, MS4A, and PICALM. This condition predominantly impacts the neocortex as individuals age, resulting in significant neuronal dysfunction in the hippocampus and other brain regions. Regrettably, there is currently no established method for regenerating neurons in the affected areas. Existing treatments have primarily focused on symptom management through the use of acetylcholinesterase inhibitors and glutamatergic receptor modulators, achieving only limited efficacy. In recent years, adeno-associated viruses (AAVs) have gained prominence in gene therapy investigations. These viruses possess the capability to transport genes, small promoters, receptors, or RNA sequences. Researchers have engineered various AAV vectors with distinct serotypes and capsids, enabling targeted delivery to cortical cells, the hippocampus, or other compromised brain areas. By employing a mini-promoter with an excision sequence flanked by loxP sites, these vectors can effectively introduce genes that code for specific proteins. To verify the efficacy of the therapy, scientists have utilized family members of the lacZ gene as reporter genes. Additionally, fluorescent genes have been incorporated into AAVs, facilitating the monitoring of protein expression via fluorescent microscopy. Looking forward, these AAV vectors hold promise for mitigating the impacts of Alzheimer’s disease by promoting neuronal regeneration or facilitating gene replacement therapies, such as substituting apoE4 with apoE3 in individuals who possess the apoE4 variant.
Immunotherapy (Vaccines and Antibody-Based Treatments)
The removal of Aβ deposits has been demonstrated to alleviate the pathological manifestations of Alzheimer’s disease (AD) in preclinical animal research. Currently, numerous clinical trials are being conducted to evaluate both active and passive immunotherapies aimed at targeting Aβ. In animal models of AD, both forms of immunotherapy have proven effective in diminishing amyloid accumulation. Specifically, they have been shown to significantly reduce the load of Aβ neuritic plaques and soluble aggregated Aβ. Notably, these reductions have been achieved without inducing severe inflammation in the central nervous system (CNS), without markedly decreasing the levels of full-length amyloid precursor protein (APP) or its C-terminal fragments, and without resulting in detrimental cognitive effects. Passive anti-Aβ immunotherapy has been particularly effective in rapidly clearing amyloid and neuritic plaques, both in transgenic APP-expressing mice and in TgCRND8 mice following stereotaxic injection. These encouraging findings from animal studies have significantly propelled the pursuit of immunotherapy as a potential strategy for the prevention or even treatment of AD.
Multiple research teams have evaluated the safety and efficacy of anti-human Aβ immunization in human subjects. Initial studies focused on the passive immunization of Alzheimer’s disease (AD) sera in vitro against postmortem brain tissue affected by the condition. Active vaccination functions by activating the recipient's immune system to generate a response against the designated antigen.
Conversely, passive immunization entails the direct administration of antibodies sourced externally, which attach to a specific antigen or epitope associated with the disease. Unlike active vaccination, passive immunization lacks antigen specificity and does not initiate primary T- or B-cell immune responses. Research conducted in mouse models of AD, along with preliminary clinical trial findings, indicates that vaccine therapy may effectively and swiftly eliminate amyloid plaques from the brain.
Concerns regarding cerebral microhaemorrhages in humans, previously associated with the use of full-length amino acid antigens, have been alleviated through the application of shorter amino acid antigens. Currently, researchers are diligently investigating whether this promising strategy can genuinely provide advantages for AD patients.
Personalized Medicine and Precision Health
The advancement of medicine is increasingly centered on tailored therapies that consider individual variances, alongside precision health initiatives aimed at forecasting and averting diseases. Alzheimer's disease exemplifies a multifaceted disorder shaped by an interplay of genetic predispositions, environmental influences, personality traits, and lifestyle choices. Approximately 25% of patients exhibit mixed pathology, complicating diagnosis, restricting treatment to only those causes that are curable, and hindering the approval of novel therapies under existing regulations. To tackle these issues, it is essential to design combinational trials specifically for older adults who, due to natural, sporadic, or experimental factors, demonstrate resistance to certain inhibitors and antibodies. The inclusion of orphan or less-researched populations in these trials may introduce additional complexities, further complicating the process. This paper aims to outline the primary scientific obstacles at the convergence of contemporary and innovative research with its clinical applications. We concentrate on the genetic, proteomic, neuroanatomical, and other markers associated with the preclinical phase of Alzheimer's disease, as examined within these research domains. Furthermore, we highlight the advantages of integrating and enhancing communication across these fields of study, which encompasses achieving more precise and timely diagnoses, informing the design of innovative biomarker-driven trials, refining the assessment of treatment outcomes, and ultimately fostering the development of multidisciplinary, personalized, and preventive strategies for Alzheimer's disease in the future.
Regenerative Medicine and Stem Cell Therapy
Regenerative medicine, particularly through stem cell therapy, presents promising opportunities for the comprehension and potential treatment of Alzheimer's disease. This intricate condition, marked by the buildup of amyloid-beta plaques and tau tangles, currently lacks effective therapies capable of modifying its progression. Researchers are investigating regenerative strategies to stimulate neurogenesis, the process of generating new neurons, which may aid in the restoration of impaired neural circuits. Stem cell therapies are designed to encourage the development of new neurons, potentially reversing cognitive impairments linked to Alzheimer's. Furthermore, research is examining the application of young plasma from donors devoid of genetic predispositions to Alzheimer's, which might exert rejuvenating effects on the aging brain. Although these approaches remain in preliminary phases, they signify groundbreaking methods to address a disease that impacts millions globally.
Challenges and Barriers in Alzheimer’s Research
The primary obstacle in the advancement of therapies for Alzheimer’s disease lies in the insufficient comprehension of the detrimental cycle that perpetuates the condition. To advance, it is imperative to create and implement an ultrasensitive diagnostic framework. Early detection of disease-related alterations with quantitative accuracy is crucial. Although clinical dementia manifests only after considerable neuronal damage has occurred, the degeneration of neurons and synapses is generally confirmed only postmortem. A diagnostic system that integrates and evaluates symptoms, cognitive function, and social behavior following the onset of the disease is also necessary. The development of instruments to address these deficiencies remains a significant challenge for the future. Surmounting these hurdles necessitates collaboration among academic institutions, industry stakeholders, and governmental bodies. Currently, the industry, particularly in collaboration with academia, is engaged in numerous preclinical trials utilizing disease models, alongside clinical investigations into potential therapies. However, much of the knowledge we acquire originates from direct observations made in clinical environments and with patients. Fostering collaboration between academia and industry is anticipated to facilitate substantial advancements. Concurrently, both fundamental and clinical research initiatives have yet to achieve the requisite scale. A deficiency in effective regional and international partnerships has impeded the progress of collaborative research endeavors. Enhancing these collaborations will expedite research advancements and yield significant breakthroughs in the treatment of Alzheimer’s disease.
Complexity of Alzheimer’s Pathology
Alzheimer’s disease (AD) is the most common cause of dementia and a leading contributor to mortality and morbidity [265]. It is marked by the buildup of amyloid-beta plaques and neurofibrillary tangles made of hyperphosphorylated tau in the brain [266]. Over time, these changes lead to the gradual degeneration of cholinergic neurons, which play a key role in memory and learning [267]. As these neurons deteriorate, there is a decline in the strength and duration of extracellular acetylcholine spikes, which are essential for transmitting signals between synapses [268]. This happens despite the ongoing loss of cholinergic neurons and the drop in extracellular acetylcholine levels [269]. As a result, gamma oscillations, which are important for cognitive function, weaken, giving way to slower brain wave activity [270].
Regulatory and Ethical Considerations
Rapid technological advancements in every field have accelerated scientific development at an astonishing pace [271]. In medical science, particularly in biotechnology, progress has pushed into areas that raise complex ethical and moral questions [272]. As new possibilities emerge, there is a growing need to balance what is scientifically feasible with what should be pursued in line with humanitarian concerns [273]. To address potential bioethical challenges, various organizations have established guidelines with general principles and recommendations that healthcare researchers must consider [274]. Scientific research and technological development also require proper and timely regulation [275]. However, with biotechnology evolving so quickly, the industry’s infrastructure has become too complex for easy oversight [276]. The commercialization of new products is advancing just as fast as the technology itself [277]. Regulatory agencies and collaborative data-sharing efforts have helped streamline some processes, such as the approval of new vaccines and ensuring the ethical supply of drugs [278]. Still, there is more work to be done [279]. Addressing ethical and regulatory issues in personalized medicine is crucial for shaping the future of biotechnology [280]. A strong ethical framework will guide future developments, improve regulatory protections, and promote collaboration to ensure access to better treatments, improve human health, and prevent diseases [281]. Additionally, planning and implementing prevention programs, along with educating future professionals, will play an essential role in this evolving landscape [282].
Financial and Societal Impacts on Research
The global cost of Alzheimer’s disease was estimated at $604 billion in 2010 [283]. In some countries, this expense exceeds 1% of their gross domestic product (GDP) [284]. Japan, for example, already spends 1% of its GDP on Alzheimer’s care, with 4 million people over the age of 65 affected [285]. In the next decade, this number is expected to rise to 7.3 million [286]. Both developed and developing countries are expected to see a significant increase in Alzheimer’s cases in the future [287]. Advancements in medical therapy will help reduce the social, healthcare, and economic burden of the disease [288]. As the population continues to age, these advancements will also have economic benefits [289]. Since 2013, GDP has been used to measure the purchasing power of an economy, reflecting a country’s economic activity [290]. Because healthcare is a part of GDP, the development of a drug that improves dementia outcomes would also benefit the healthcare industry as a whole [291].
While advancements in Alzheimer’s treatment can drive economic growth, they also require significant research investment [292]. If the condition continues to be classified somewhere between severe mental disorders and aging-related diseases, policy measures may fall short, reducing their effectiveness and leading to social instability and gaps in healthcare intervention [293]. However, the successful implementation of new therapies can positively impact policy and healthcare responses [294]. Developing effective treatments creates a win-win situation [295]. When medical advancements lead to cost reductions in welfare policies, governments benefit, for example, through lower tax burdens [296]. In Japan, where demand for such treatments is high, similar economic benefits extend to interestbearing defined-benefit pensions [297]. To maintain efficient pension fund management, a growing number of contributing individuals is essential [298]. The development and approval of dementia drugs play a crucial role in extending the healthy years of these individuals, ultimately supporting the sustainability of pension systems [299].
Conclusion
Alzheimer's disease presents a significant challenge, affecting not only individuals but also families and communities globally. Although existing treatments provide some alleviation of symptoms, they do not prevent the progression of the disease. Our comprehension of Alzheimer's is continually advancing, shifting from a narrow focus on amyloid plaques to a more comprehensive perspective that encompasses inflammation, synaptic dysfunction, and various other molecular factors. This enhanced understanding is vital for the development of effective therapies. The quest for a cure is ongoing, with researchers investigating numerous pathways, including the targeting of beta-amyloid and tau proteins, addressing neuroinflammation, and restoring synaptic function. Recent studies also emphasize the promise of novel molecular targets and innovative drug delivery systems, such as nasal insulin sprays. Despite many potential treatments encountering obstacles in clinical trials, these challenges stimulate further research and refinement of therapeutic strategies. Ultimately, addressing Alzheimer's necessitates a multifaceted strategy: early detection, holistic patient care, and the continuous advancement of new treatments. By integrating our expanding knowledge of the disease with innovative research and a compassionate approach to patient care, we can aspire to a future where Alzheimer's exerts a diminished impact.
References
- Mullard, A., Alzheimers Drug Approval Could Affect Other Diseases. Nature, 2021. 595: p. 162-163.
- Srivastava, S., R. Ahmad, and S.K. Khare, Alzheimer’s disease and its treatment by different approaches: A review. European Journal of Medicinal Chemistry, 2021. 216: p. 113320.
- Pardridge, W.M., Treatment of Alzheimer’s disease and blood–brain barrier drug delivery. Pharmaceuticals, 2020. 13(11): p. 394.
- Vaz, M. and S. Silvestre, Alzheimer’s disease: Recent treatment strategies. European journal of pharmacology, 2020. 887: p. 173554.
- Reardon, S., Alzheimer’s drug donanemab: what promising trial means for treatments. 2023, Nature Publishing Group.
- Beshir, S.A., et al., Aducanumab therapy to treat Alzheimer’s disease: a narrative review. International Journal of Alzheimer’s Disease, 2022. 2022(1): p. 9343514.
- Cummings, J.L., et al., The costs of developing treatments for Alzheimer’s disease: A retrospective exploration. Alzheimer’s & Dementia, 2022. 18(3): p. 469-477.
- Guo, T., et al., Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Molecular neurodegeneration, 2020. 15: p. 1-37.
- Pardo-Moreno, T., et al., Therapeutic approach to Alzheimer’s disease: current treatments and new perspectives. Pharmaceutics, 2022. 14(6): p. 1117.
- Peng, Y., et al., Current and future therapeutic strategies for Alzheimer’s disease: An overview of drug development bottlenecks. Frontiers in aging neuroscience, 2023. 15: p. 1206572.
- Löscher, W., et al., Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacological reviews, 2020. 72(3): p. 606-638.
- Gauthier, S., et al., Therapeutic targets for alzheimer’s disease: Amyloid vs. Non-amyloid. Where does consensus lie today? An ctad task force report. The journal of prevention of Alzheimer’s disease, 2022. 9(2): p. 231-235.
- Bekdash, R.A., The cholinergic system, the adrenergic system and the neuropathology of Alzheimer’s disease. International Journal of Molecular Sciences, 2021. 22(3): p. 1273.
- Jellinger, K.A., Neuropathological assessment of the Alzheimer spectrum. Journal of Neural Transmission, 2020. 127(9): p. 1229-1256.
- Ju, Y. and K.Y. Tam, Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural regeneration research, 2022. 17(3): p. 543-549.
- Montalbano, M., et al., Pathological tau signatures and nuclear alterations in neurons, astrocytes and microglia in Alzheimer’s disease, progressive supranuclear palsy, and dementia with Lewy bodies. Brain Pathology, 2023. 33(1): p. e13112.
- Rezaee, D., et al., The role of microRNAs in the pathophysiology of human central nervous system: a focus on neurodegenerative diseases. Ageing research reviews, 2023. 92: p. 102090.
- Islam, R., et al., An overview on microglial origin, distribution, and phenotype in Alzheimer’s disease. Journal of cellular physiology, 2024. 239(6): p. e30829.
- Xie, J., L. Van Hoecke, and R.E. Vandenbroucke, The impact of systemic inflammation on Alzheimer’s disease pathology. Frontiers in immunology, 2022. 12: p. 796867.
- Decourt, B., et al., The cause of Alzheimer’s disease: the theory of multipathology convergence to chronic neuronal stress. Aging and disease, 2022. 13(1): p. 37.
- Tahami Monfared, A.A., et al., Alzheimer’s disease: epidemiology and clinical progression. Neurology and therapy, 2022. 11(2): p. 553-569.
- Liang, C.-S., et al., Mortality rates in Alzheimer’s disease and non-Alzheimer’s dementias: a systematic review and meta-analysis. The Lancet Healthy Longevity, 2021. 2(8): p. e479-e488.
- Li, X., et al., Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Frontiers in Aging Neuroscience, 2022. 14: p. 937486.
- Zhang, X.-X., et al., The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. The journal of prevention of Alzheimer’s disease, 2021. 8: p. 313-321.
- Wu, T., et al., Amyloid cascade hypothesis for the treatment of alzheimer’s disease: progress and challenges. Aging and Disease, 2022. 13(6): p. 1745.
- Kepp, K.P., et al., The amyloid cascade hypothesis: an updated critical review. Brain, 2023. 146(10): p. 3969-3990.
- Caselli, R.J., D.S. Knopman, and G. Bu, An agnostic reevaluation of the amyloid cascade hypothesis of Alzheimer’s disease pathogenesis: The role of APP homeostasis. Alzheimer’s & Dementia, 2020. 16(11): p. 1582-1590.
- Alawode, D.O., et al., Alzheimer’s disease biomarkers revisited from the amyloid cascade hypothesis standpoint. Frontiers in Neuroscience, 2022. 16: p. 837390.
- Behl, C., In 2024, the amyloid-cascade-hypothesis still remains a working hypothesis, no less but certainly no more. Frontiers in Aging Neuroscience, 2024. 16: p. 1459224.
- Ardanaz, C.G., M.J. Ramírez, and M. Solas, Brain metabolic alterations in Alzheimer’s disease. International journal of molecular sciences, 2022. 23(7): p. 3785.
- Yan, X., et al., Metabolic dysregulation contributes to the progression of Alzheimer’s disease. Frontiers in neuroscience, 2020. 14: p. 530219.
- Błaszczyk, J.W., Energy metabolism decline in the aging brain—pathogenesis of neurodegenerative disorders. Metabolites, 2020. 10(11): p. 450.
- Aquilani, R., et al., Is the brain undernourished in Alzheimer’s disease? Nutrients, 2022. 14(9): p. 1872.
- Szablewski, L., Brain glucose transporters: role in pathogenesis and potential targets for the treatment of Alzheimer’s disease. International Journal of Molecular Sciences, 2021. 22(15): p. 8142.
- Kim, C.K., et al., Alzheimer’s disease: key insights from two decades of clinical trial failures. Journal of Alzheimer’s Disease, 2022. 87(1): p. 83-100.
- Chopade, P., et al., Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioengineering & Translational Medicine, 2023. 8(1): p. e10367.
- Athar, T., K. Al Balushi, and S.A. Khan, Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Molecular biology reports, 2021. 48(7): p. 5629-5645.
- Kabir, M.T., et al., Combination drug therapy for the management of Alzheimer’s disease. International Journal of Molecular Sciences, 2020. 21(9): p. 3272.
- Sanders, O. and L. Rajagopal, Phosphodiesterase inhibitors for Alzheimer’s disease: a systematic review of clinical trials and epidemiology with a mechanistic rationale. Journal of Alzheimer’s Disease Reports, 2020. 4(1): p. 185-215.
- Tolar, M., S. Abushakra, and M. Sabbagh, The path forward in Alzheimer’s disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimer’s & Dementia, 2020. 16(11): p. 1553-1560.
- Fedele, E., Anti-amyloid therapies for Alzheimer’s disease and the amyloid cascade hypothesis. International journal of molecular sciences, 2023. 24(19): p. 14499.
- Daly, T., et al., Amyloid-β in Alzheimer’s disease: A study of citation practices of the amyloid cascade hypothesis between 1992 and 2019. Journal of Alzheimer’s Disease, 2020. 74(4): p. 1309-1317.
- Yadang, F.S.A., et al., Scopolamine‐Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. International Journal of Alzheimer’s Disease, 2020. 2020(1): p. 6372059.
- Huang, Z., et al., Blood-brain barrier integrity in the pathogenesis of Alzheimer’s disease. Frontiers in neuroendocrinology, 2020. 59: p. 100857.
- Tatulian, S.A., Challenges and hopes for Alzheimer’s disease. Drug discovery today, 2022. 27(4): p. 1027-1043.
- Mullane, K. and M. Williams, Alzheimer’s disease (AD) therapeutics–1: Repeated clinical failures continue to question the amyloid hypothesis of AD and the current understanding of AD causality. Biochemical Pharmacology, 2018. 158: p. 359-375.
- Cao, J. and Y.-W. Zhang, Targeting synaptic pathology in Alzheimer’s disease. Zoological Research, 2024. 45(4): p. 875.
- Ong, C.-T., Enrichment of novel Tau isoform with altered biochemical properties in Alzheimer’s disease. Neural Regeneration Research, 2023. 18(11): p. 2397-2398.
- Abbas, M., Potential role of nanoparticles in treating the accumulation of amyloid-beta peptide in Alzheimer’s patients. Polymers, 2021. 13(7): p. 1051.
- Bellesia, R. and G. Manca, The potential for Artificial Intelligence in the diagnosis of Alzheimer’s Disease: A Systematic Literature Review. 2024.
- Sehar, U., J. Kopel, and P.H. Reddy, Alzheimer’s disease and its related dementias in US Native Americans: A major public health concern. Ageing research reviews, 2023. 90: p. 102027.
- Thomas, L., T. Florio, and C. Perez-Castro, Extracellular vesicles loaded miRNAs as potential modulators shared between glioblastoma, and Parkinson’s and Alzheimer’s diseases. Frontiers in Cellular Neuroscience, 2020. 14: p. 590034.
- Čarna, M., et al., Failure of the choroid plexus in Alzheimer’s disease. medRxiv, 2021: p. 2021.07. 29.21260696.
- Thakur, V., et al., Neurodegenerative disease: Alzheimer’s disease. 2023.
- Dhapola, R., et al., Environmental toxins and Alzheimer’s disease: a comprehensive analysis of pathogenic mechanisms and therapeutic modulation. Molecular Neurobiology, 2024. 61(6): p. 3657-3677.
- Uddin, M.S. and L.W. Lim, Glial cells in Alzheimer’s disease: From neuropathological changes to therapeutic implications. Ageing research reviews, 2022. 78: p. 101622.
- Mangalmurti, A. and J.R. Lukens, How neurons die in Alzheimer’s disease: implications for neuroinflammation. Current opinion in neurobiology, 2022. 75: p. 102575.
- Liu, S. and D. Geng, Key developments and hotspots of exosomes in Alzheimer’s disease: a bibliometric study spanning 2003 to 2023. Frontiers in Aging Neuroscience, 2024. 16: p. 1377672.
- Leng, F., et al., Neuroinflammation, amyloid, NFT markers and initial cognitive status predict cognitive decline in MCI patients. Alzheimer’s & Dementia, 2021. 17: p. e055983.
- Uddin, M.S., et al., Estrogen signaling in Alzheimer’s disease: molecular insights and therapeutic targets for Alzheimer’s dementia. Molecular Neurobiology, 2020. 57: p. 2654-2670.
- Weber, C., A. Dilthey, and P. Finzer, The role of microbiome-host interactions in the development of Alzheimer´ s disease. Frontiers in cellular and infection microbiology, 2023. 13: p. 1151021.
- Li, D., et al., Association between behavioural risks and Alzheimer’s disease: Elucidated with an integrated analysis of gene expression patterns and molecular mechanisms. Neuroscience & Biobehavioral Reviews, 2023. 150: p. 105207.
- Cai, H., et al., Plasma biomarkers predict Alzheimer’s disease before clinical onset in Chinese cohorts. Nature communications, 2023. 14(1): p. 6747.
- Wang, F., et al., Iron dyshomeostasis and ferroptosis: a new Alzheimer’s disease hypothesis? Frontiers in aging neuroscience, 2022. 14: p. 830569.
- Zhang, X., et al., Effect of physical activity on risk of Alzheimer’s disease: a systematic review and meta-analysis of twenty-nine prospective cohort studies. Ageing Research Reviews, 2023. 92: p. 102127.
- Volicer, L., Physiological and pathological functions of beta-amyloid in the brain and Alzheimer’s disease: a review. Journal of Physiological Investigation, 2020. 63(3): p. 95-100.
- Loeffler, D.A., Antibody-mediated clearance of brain amyloid-β: mechanisms of action, effects of natural and monoclonal anti-Aβ antibodies, and downstream effects. Journal of Alzheimer’s disease reports, 2023. 7(1): p. 873-899.
- Bruno, F., et al., Antimicrobial peptides (AMPs) in the pathogenesis of Alzheimer’s disease: implications for diagnosis and treatment. Antibiotics, 2022. 11(6): p. 726.
- Schreiner, T.G., et al., The roles of the amyloid beta monomers in physiological and pathological conditions. Biomedicines, 2023. 11(5): p. 1411.
- Sridhar, G.R., Acetylcholinesterase inhibitors (galantamine, rivastigmine, and donepezil). NeuroPsychopharmacotherapy, 2021: p. 1-13.
- VARIA, A. and R. SONI, 13. Multiple Roles of Fungal Organic Molecules (Nutraceuticals) in Different Fields. 2022.
- Li, W., et al., Alzheimer’s disease and COVID-19: Interactions, intrinsic linkages, and the role of immunoinflammatory responses in this process. Frontiers in immunology, 2023. 14: p. 1120495.
- Safdar, A., et al., Advancement in the Treatment of ALZHEIMER’S Disease, 2023. Amyloid, 2024. 5: p. 6.
- Kabir, M.T., et al., Molecular mechanisms of metal toxicity in the pathogenesis of Alzheimer’s disease. Molecular neurobiology, 2021. 58: p. 1-20.
- Uddin, M.S., et al., Molecular insight into the therapeutic promise of flavonoids against Alzheimer’s disease. Molecules, 2020. 25(6): p. 1267.
- Mieling, M., H. Meier, and N. Bunzeck, Structural degeneration of the nucleus basalis of Meynert in mild cognitive impairment and Alzheimer’s disease– Evidence from an MRI-based meta-analysis. Neuroscience & Biobehavioral Reviews, 2023. 154: p. 105393.
- Pashova-Dimova, S., et al., Changes in the Public IgM Repertoire and its Idiotypic Connectivity in Alzheimer’s Disease and Frontotemporal Dementia. bioRxiv, 2024: p. 2024.07.15.603559.
- Kanel, P., et al., Challenges and innovations in brain PET analysis of neurodegenerative disorders: a mini-review on partial volume effects, small brain region studies, and reference region selection. Frontiers in Neuroscience, 2023. 17.
- Qiu, T., et al., Degeneration of cholinergic white matter pathways and nucleus basalis of Meynert in individuals with objective subtle cognitive impairment. Neurobiology of Aging, 2023. 132: p. 198-208.
- Huang, L.-T., et al., Association of Peripheral Blood Cell Profile With Alzheimer’s Disease: A Meta-Analysis. Frontiers in Aging Neuroscience, 2022. 14.
- Gong, C.-X., et al., Multi-Targets: An Unconventional Drug Development Strategy for Alzheimer’s Disease. Frontiers in Aging Neuroscience, 2022. 14.
- Fronza, M.G., et al., The neurobiology and therapeutic potential of multitargeting β-secretase, glycogen synthase kinase 3β and acetylcholinesterase in Alzheimer’s disease. Ageing Research Reviews, 2023. 90: p. 102033.
- Pirola, J.P., et al., Astrocytic Signatures in Neuronal Activity: A Machine Learning-Based Identification Approach. bioRxiv, 2024: p. 2024.12.16.628802.
- Cantón-Habas, V., et al., Depression as a risk factor for dementia and Alzheimer’s disease. Biomedicines, 2020. 8(11): p. 457.
- Bai, R., et al., Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Research Reviews, 2022. 77: p. 101619.
- Morsy, A. and P.C. Trippier, Current and emerging pharmacological targets for the treatment of Alzheimer’s disease. Journal of Alzheimer’s disease, 2019. 72(s1): p. S145-S176.
- Bagga, S. and M. Kumar, Current status of Alzheimer’s disease and pathological mechanisms investigating the therapeutic molecular targets. Current Molecular Medicine, 2023. 23(6): p. 492-508.
- Raj, S., A. Vishnoi, and A. Srivastava, Classify Alzheimer genes association using Naïve Bayes algorithm. Human Gene, 2024. 41: p. 201309.
- Jain, U., S. Johari, and P. Srivastava, Current insights of Nanocarriermediated gene therapeutics to treat potential impairment of amyloid Beta protein and tau protein in Alzheimer’s disease. Molecular Neurobiology, 2024. 61(4): p. 1969-1989.
- Wightman, D.P., et al., The genetic overlap between Alzheimer’s disease, amyotrophic lateral sclerosis, Lewy body dementia, and Parkinson’s disease. Neurobiology of Aging, 2023. 127: p. 99-112.
- Sethi, P., et al., Exploring advancements in early detection of Alzheimer’s disease with molecular assays and animal models. Ageing Research Reviews, 2024: p. 102411.
- Sharma, M., P. Pal, and S.K. Gupta, Advances in Alzheimer’s disease: A multifaceted review of potential therapies and diagnostic techniques for early detection. Neurochemistry international, 2024: p. 105761.
- Koszła, O. and P. Sołek, Misfolding and aggregation in neurodegenerative diseases: Protein quality control machinery as potential therapeutic clearance pathways. Cell Communication and Signaling, 2024. 22(1): p. 421.
- Sidoryk-Węgrzynowicz, M., K. Adamiak, and L. Strużyńska, Targeting Protein Misfolding and Aggregation as a Therapeutic Perspective in Neurodegenerative Disorders. International Journal of Molecular Sciences, 2024. 25(22): p. 12448.
- Shastri, D., V. Raj, and S. Lee, Revolutionizing Alzheimer’s treatment: harnessing human serum albumin for targeted drug delivery and therapy advancements. Ageing Research Reviews, 2024: p. 102379.
- Chopra, H., et al., Nanomedicines in the management of Alzheimer’s disease: current view and future prospects. Frontiers in Aging Neuroscience, 2022. 14: p. 879114.
- Noor, A., et al., Molecular profiles of amyloid-β proteoforms in typical and rapidly progressive Alzheimer’s disease. Molecular neurobiology, 2022: p. 1-18.
- Verde, F., Tau proteins in blood as biomarkers of Alzheimer’s disease and other proteinopathies. Journal of Neural Transmission, 2022. 129(2): p. 239- 259.
- Karanth, S.D., et al., Cancer history associates with a lower burden of dementia and Alzheimer’s‐type neuropathology in autopsied research volunteers. Alzheimer’s & Dementia, 2021. 17: p. e054076.
- Uchida, K., Waste clearance in the brain and neuroinflammation: a novel perspective on biomarker and drug target discovery in Alzheimer’s disease. Cells, 2022. 11(5): p. 919.
- Singh, R., et al., Protein misfolding, ER stress and chaperones: An approach to develop chaperone-based therapeutics for Alzheimer’s disease. International Journal of Neuroscience, 2023. 133(7): p. 714-734.
- Nagar, P., et al., Endoplasmic reticulum stress in Alzheimer’s disease: Molecular mechanisms and therapeutic prospects. Life Sciences, 2023. 330: p. 121983.
- Gonçalves, P.B., A.C.R. Sodero, and Y. Cordeiro, Natural products targeting amyloid-β oligomer neurotoxicity in Alzheimer’s disease. European Journal of Medicinal Chemistry, 2024: p. 116684.
- Miku, E., Recent advancements in electrochemical biosensors for Alzheimer’s disease biomarkers detection. Current Medicinal Chemistry, 2021. 28(20): p. 4049-4073.
- Klyucherev, T.O., et al., Advances in the development of new biomarkers for Alzheimer’s disease. Translational neurodegeneration, 2022. 11(1): p. 25.
- Rani, S., et al., Advanced overview of biomarkers and techniques for early diagnosis of alzheimer’s disease. Cellular and Molecular Neurobiology, 2023. 43(6): p. 2491-2523.
- Espay, A.J., et al., The proteinopenia hypothesis: Loss of Aβ42 and the onset of Alzheimer’s Disease. Ageing Research Reviews, 2023. 92: p. 102112.
- Nordvall, G., J. Lundkvist, and J. Sandin, Gamma-secretase modulators: a promising route for the treatment of Alzheimer’s disease. Frontiers in Molecular Neuroscience, 2023. 16: p. 1279740.
- Bader, J.M., et al., Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer’s disease. Molecular systems biology, 2020. 16(6): p. e9356.
- Jellinger, K.A., Recent update on the heterogeneity of the Alzheimer’s disease spectrum. Journal of Neural Transmission, 2022. 129(1): p. 1-24.
- Kang, S., et al., Disentangling brain atrophy heterogeneity in Alzheimer’s disease: A deep self-supervised approach with interpretable latent space. NeuroImage, 2024. 297: p. 120737.
- Delaby, C., et al., Blood amyloid and tau biomarkers as predictors of cerebrospinal fluid profiles. Journal of Neural Transmission, 2022. 129(2): p. 231-237.
- Kang, S., et al., Dopamine transporter positron emission tomography in patients with Alzheimer’s disease with Lewy body disease features. Neurobiology of Aging, 2024. 134: p. 57-65.
- Zhang, Y., et al., Advances in retina imaging as potential biomarkers for early diagnosis of Alzheimer’s disease. Translational neurodegeneration, 2021. 10: p. 1-9.
- Vrahatis, A.G., et al., Revolutionizing the early detection of Alzheimer’s disease through non-invasive biomarkers: the role of artificial intelligence and deep learning. Sensors, 2023. 23(9): p. 4184.
- Veitch, D.P., et al., Using the Alzheimer’s Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimer’s & Dementia, 2022. 18(4): p. 824-857.
- Hachinski, V. and A. Avan, From dementia to eumentia: a new approach to dementia prevention. Neuroepidemiology, 2022. 56(3): p. 151-156.
- Avan, A. and V. Hachinski, Stroke and dementia, leading causes of neurological disability and death, potential for prevention. Alzheimer’s & Dementia, 2021. 17(6): p. 1072-1076.
- Hu, S., C. Yang, and H. Luo, Current trends in blood biomarker detection and imaging for Alzheimer’s disease. Biosensors and Bioelectronics, 2022. 210: p. 114278.
- Kaur, I. and R. Sachdeva, Prediction Models for Early Detection of Alzheimer: Recent Trends and Future Prospects. Archives of Computational Methods in Engineering, 2025: p. 1-28.
- Palmqvist, S., et al., Blood biomarkers to detect Alzheimer disease in primary care and secondary care. JAMA, 2024. 332(15): p. 1245-1257.
- Ali, H., AI in neurodegenerative disease research: Early detection, cognitive decline prediction, and brain imaging biomarker identification. Int J Eng Technol Res Manag, 2022. 6(10): p. 71.
- Dubois, B., et al., Biomarkers in Alzheimer’s disease: role in early and differential diagnosis and recognition of atypical variants. Alzheimer’s Research & Therapy, 2023. 15(1): p. 175.
- Rahman, M.M. and C. Lendel, Extracellular protein components of amyloid plaques and their roles in Alzheimer’s disease pathology. Molecular Neurodegeneration, 2021. 16(1): p. 59.
- Dwivedi, A.R. and P. Raghuveer, Future Barriers and Possible Solutions with Recent Advances in Alzheimer’s Disease. Multi-Factorial Approach as a Therapeutic Strategy for the Management of Alzheimer’s Disease, 2025: p. 413-430.
- Liao, W., et al., The Current Status and Challenges of Olfactory Dysfunction Study in Alzheimer’s Disease. Ageing Research Reviews, 2024: p. 102453.
- Weinstock, M., Therapeutic agents for Alzheimer’s disease: a critical appraisal. Frontiers in Aging Neuroscience, 2024. 16: p. 1484615.
- Moloney, C.M., V.J. Lowe, and M.E. Murray, Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: A clinicopathologic perspective for biomarker research. Alzheimer’s & dementia, 2021. 17(9): p. 1554-1574.
- Murray, M.E., et al., Global neuropathologic severity of Alzheimer’s disease and locus coeruleus vulnerability influences plasma phosphorylated tau levels. Molecular neurodegeneration, 2022. 17(1): p. 85.
- Tsering, W. and S. Prokop, Neuritic plaques—gateways to understanding Alzheimer’s disease. Molecular Neurobiology, 2024. 61(5): p. 2808-2821.
- Xu, C., et al., Association of APOE gene with longitudinal changes of CSF amyloid beta and tau levels in Alzheimer’s disease: racial differences. Neurological Sciences, 2024. 45(3): p. 1041-1050.
- Asveda, T., P. Talwar, and P. Ravanan, Exploring microglia and their phenomenal concatenation of stress responses in neurodegenerative disorders. Life Sciences, 2023. 328: p. 121920.
- Hirschfeld, L.R., White Matter Microstructural Changes in the Cingulum Bundle of the Hippocampus in Alzheimer’s Disease. 2023: Indiana University-Purdue University Indianapolis.
- Nelson, M.R., et al., Exploring white matter microstructural alterations in mild cognitive impairment: a multimodal diffusion MRI investigation utilizing diffusion kurtosis and free-water imaging. Frontiers in Neuroscience, 2024. 18: p. 1440653.
- Sacchi, L., et al., A “glympse” into neurodegeneration: Diffusion MRI and cerebrospinal fluid aquaporin‐4 for the assessment of glymphatic system in Alzheimer’s disease and other dementias. Human Brain Mapping, 2024. 45(12): p. e26805.
- Gong, C.-X., et al., Multi-targets: An unconventional drug development strategy for Alzheimer’s disease. Frontiers in aging neuroscience, 2022. 14: p. 837649.
- Hampel, H., et al., Omics sciences for systems biology in Alzheimer’s disease: State-of-the-art of the evidence. Ageing Research Reviews, 2021. 69: p. 101346.
- Contador, I., et al., Charting Alzheimer’s disease and dementia: epidemiological insights, risk factors and prevention pathways. Journal of Clinical Medicine, 2024. 13(14): p. 4100.
- Gardener, S.L., et al., Validation of the Alzheimer’s disease-Commonwealth Scientific and Industrial Research Organisation food frequency questionnaire using weighed food records and biomarkers: the method of triads model. International Journal of Food Science and Technology, 2025: p. vvae073.
- Sutin, A.R., et al., Loneliness and risk of all-cause, Alzheimer’s, vascular, and frontotemporal dementia: a prospective study of 492,322 individuals over 15 years. International Psychogeriatrics, 2023. 35(6): p. 283-292.
- Liu, X., et al., Self-calibrating surface-enhanced Raman scattering-lateral flow immunoassay for determination of amyloid-β biomarker of Alzheimer’s disease. Biosensors and Bioelectronics, 2024. 245: p. 115840.
- Arvidsson, I., et al., Comparing a pre-defined versus deep learning approach for extracting brain atrophy patterns to predict cognitive decline due to Alzheimer’s disease in patients with mild cognitive symptoms. Alzheimer’s Research & Therapy, 2024. 16(1): p. 61.
- Macías, M., et al., Liquid Biopsy in Alzheimer’s Disease Patients Reveals Epigenetic Changes in the PRLHR Gene. Cells, 2023. 12(23): p. 2679.
- Hampel, H., et al., Future avenues for Alzheimer’s disease detection and therapy: liquid biopsy, intracellular signaling modulation, systems pharmacology drug discovery. Neuropharmacology, 2021. 185: p. 108081.
- Gaitsch, H., R.J. Franklin, and D.S. Reich, Cell-free DNA-based liquid biopsies in neurology. Brain, 2023. 146(5): p. 1758-1774.
- Macías, M., et al., Towards a Personalized Medicine through Liquid Biopsy in Alzheimer’s disease: Epigenome of cell-free DNA reveals methylation differences linked to APOE status. 2024.
- Carballo, Á., et al., Association of periodontitis with cognitive decline and its progression: contribution of blood‐based biomarkers of Alzheimer’s disease to this relationship. Journal of clinical periodontology, 2023. 50(11): p. 1444-1454.
- Martínez-Gallego, I., H. Coatl-Cuaya, and A. Rodriguez-Moreno, Astrocytes mediate two forms of spike timing-dependent depression at entorhinal cortex-hippocampal synapses. Elife, 2024. 13: p. RP98031.
- Gaeta, A.M., et al., Predicting Alzheimer’s disease CSF core biomarkers: a multimodal Machine Learning approach. Frontiers in Aging Neuroscience, 2024. 16: p. 1369545.
- Armijo-Weingart, L., et al., Loss of glycine receptors in the nucleus accumbens and ethanol reward in an Alzheimer s Disease mouse model. Progress in Neurobiology, 2024. 237: p. 102616.
- Zhang, J., et al., Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal transduction and targeted therapy, 2024. 9(1): p. 211.
- Cummings, J., et al., Alzheimer’s disease drug development pipeline: 2024. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 2024. 10(2): p. e12465.
- Cummings, J.L., et al., Alzheimer’s disease: combination therapies and clinical trials for combination therapy development. CNS drugs, 2024. 38(8): p. 613-624.
- Behl, R., I. Kashyap, and B. Dwivedi, Real-World Applications and Case Studies of Deep Generative Models in Alzheimer’s Disease Research, in Deep Generative Models for Integrative Analysis of Alzheimer’s Biomarkers. 2025, IGI Global. p. 381-398.
- Tondo, G., et al., Novel therapeutic strategies in Alzheimer’s disease: Pitfalls and challenges of anti-amyloid therapies and beyond. Journal of Clinical Medicine, 2024. 13(11): p. 3098.
- Prabha, S., et al., Recent advancement in understanding of Alzheimer’s disease: Risk factors, subtypes, and drug targets and potential therapeutics. Ageing research reviews, 2024: p. 102476.
- Xie, S., et al., Repurposing Anidulafungin for Alzheimer’s Disease via Fragment-Based Drug Discovery. ACS Chemical Neuroscience, 2024. 15(16): p. 2995-3008.
- Mishra, A.S., et al., Transforming Alzheimer’s Treatment: Unveiling New Potential with Drug Repurposing Strategies. Current Medicinal Chemistry, 2024.
- Feldman, H.H., et al., A framework for translating tauopathy therapeutics: Drug discovery to clinical trials. Alzheimer’s & Dementia, 2024. 20(11): p. 8129-8152.
- Shukla, D., et al., Recent Advances in the Treatment and Management of Alzheimer’s Disease: A Precision Medicine Perspective. Current Topics in Medicinal Chemistry, 2024. 24(19): p. 1699-1737.
- Majolo, F., et al., Recent Drugs Tested in Clinical Trials for Alzheimer´ s and Parkinson´ s Diseases Treatment: Current Approaches in Tracking New Drugs. Frontiers in Clinical Drug Research-CNS and Neurological Disorders: Volume 12, 2024: p. 1-58.
- Li, V.O., et al., DeepDrug as an expert guided and AI driven drug repurposing methodology for selecting the lead combination of drugs for Alzheimer’s disease. Scientific Reports, 2025. 15(1): p. 2093.
- Patwekar, M., et al., Navigating the Alzheimer’s Treatment Landscape: Unraveling Amyloid-beta Complexities and Pioneering Precision Medicine Approaches. Current Topics in Medicinal Chemistry, 2024. 24(19): p. 1665- 1682.
- Thawabteh, A.M., et al., Recent Advances in Therapeutics for the Treatment of Alzheimer’s Disease. Molecules, 2024. 29(21): p. 5131.
- Hake, G., A. Mhaske, and R. Shukla, Drug Delivery Strategies in Alzheimer’s Disease, in Drug Delivery Strategies in Neurological Disorders: Challenges and Opportunities. 2024, Springer. p. 271-303.
- Daly, T., Improving clinical trials of antioxidants in Alzheimer’s disease. Journal of Alzheimer’s Disease, 2024. 99(s1): p. S171-S181.
- Gbayisomore, T.J., et al., Innovative approaches and challenges in antibody-based therapeutics for Alzheimer’s disease. INNOSC Theranostics and Pharmacological Sciences, 2024. 7(3): p. 2953.
- Ortiz-Islas, E., et al., Evolution of Alzheimer’s Disease Therapeutics: From Conventional Drugs to Medicinal Plants, Immunotherapy, Microbiotherapy and Nanotherapy. Pharmaceutics, 2025. 17(1): p. 128.
- Maniam, S. and S. Maniam, Screening techniques for drug discovery in alzheimer’s disease. ACS omega, 2024. 9(6): p. 6059-6073.
- Angelucci, F., et al., Integrating AI in fighting advancing Alzheimer: Diagnosis, prevention, treatment, monitoring, mechanisms, and clinical trials. Current Opinion in Structural Biology, 2024. 87: p. 102857.
- Umar, M., et al., Innovative Approaches to Alzheimer’s Therapy: Harnessing the Power of Heterocycles, Oxidative Stress Management, and Nanomaterial Drug Delivery System. Ageing Research Reviews, 2024: p. 102298.
- Liu, E., Y. Zhang, and J.-Z. Wang, Updates in Alzheimer’s disease: from basic research to diagnosis and therapies. Translational Neurodegeneration, 2024. 13(1): p. 45.
- Kulkarni, A., et al., Novel Drug Delivery Systems for the Treatment of Chronic Alzheimer’s, in Novel Drug Delivery Systems in the Management of Chronic Diseases. 2025, Apple Academic Press. p. 113-134.
- Silvestro, S., et al., Innovative insights into traumatic brain injuries: biomarkers and new pharmacological targets. International Journal of Molecular Sciences, 2024. 25(4): p. 2372.
- Nystuen, K.L., et al., Alzheimer’s disease: Models and molecular mechanisms informing disease and treatments. Bioengineering, 2024. 11(1): p. 45.
- De Ninno, G., et al., Current perspectives on Alzheimer’s disease fluid biomarkers and future challenges: A narrative review. Journal of Laboratory and Precision Medicine, 2024. 9.
- Halder, A. and E. Drummond, Strategies for translating proteomics discoveries into drug discovery for dementia. Neural Regeneration Research, 2024. 19(1): p. 132-139.
- Sun, X. and W. Zhang, Alzheimer’s Disease from Modeling to Mechanism Research. Systems Neuroscience, 2024: p. 153-170.
- Khare, N., P. Khare, and S. Priyadarshi, Investigating Neurodegenerative Diseases From Alzheimer’s to Parkinson’s, in Advancing Medical Research Through Neuroscience. 2025, IGI Global Scientific Publishing. p. 399-436.
- Peretti, D.E., et al., Association of glial fibrillary acid protein, Alzheimer’s disease pathology and cognitive decline. Brain, 2024. 147(12): p. 4094- 4104.
- Imbimbo, B.P., et al., Initial failures of anti-tau antibodies in Alzheimer’s disease are reminiscent of the amyloid-β story. Neural regeneration research, 2023. 18(1): p. 117-118.
- Lista, S., et al., A critical appraisal of blood-based biomarkers for Alzheimer’s disease. Ageing Research Reviews, 2024: p. 102290.
- Wang, C., et al., The potential of targeting microRNA-23b-3p signaling in Alzheimer’s disease: opinions on recent findings. Frontiers in pharmacology, 2023. 14: p. 1245352.
- Subramanian, K., et al. Exploring intervention techniques for Alzheimer’s disease: Conventional methods and the role of AI in advancing care. in Artificial Intelligence and Applications. 2024.
- Shan, G., et al., Application of adaptive designs in clinical research, in Modern Inference Based on Health-Related Markers. 2024, Elsevier. p. 229-243.
- Ballard, C., et al., Challenges and proposed solutions to conducting Alzheimer’s disease psychosis trials. Frontiers in Psychiatry, 2024. 15: p. 1384176.
- Dhaenens, B.A., et al., Platform trial design for neurofibromatosis type 1, NF2-related schwannomatosis and non-NF2-related schwannomatosis: A potential model for rare diseases. Neuro-Oncology Practice, 2024. 11(4): p. 395-403.
- Cummings, J., et al., Key considerations for combination therapy in Alzheimer’s clinical trials: Perspectives from an expert advisory board convened by the Alzheimer’s drug discovery foundation. The Journal of Prevention of Alzheimer’s Disease, 2025. 12(1): p. 100001.
- Baligács, N., et al., Homeostatic microglia initially seed and activated microglia later reshape amyloid plaques in Alzheimer’s Disease. Nature Communications, 2024. 15(1): p. 1-14.
- Rathee, S., et al., Advances in Understanding and Managing Alzheimer’s Disease: From Pathophysiology to Innovative Therapeutic Strategies. Current Drug Targets, 2024. 25(11): p. 752-774.
- Vashisth, K., et al., Immunotherapy in Alzheimer’s Disease: Current Status and Future Directions. Journal of Alzheimer’s Disease, 2024. 101(s1): p. S23-S39.
- Chanu, M.T., Alzheimer’s Disease and Related Dementia Drug Trials, Failures and Progress: Data Update 2024. 2024.
- Collij, L.E., et al., Quantification supports amyloid PET visual assessment of challenging cases: results from the AMYPAD diagnostic and patient management study. Journal of Nuclear Medicine, 2025. 66(1): p. 110-116.
- Mestre, T.A., et al., Patient-centered development of clinical outcome assessments in early Parkinson disease: key priorities and advances. npj Parkinson’s Disease, 2024. 10(1): p. 101.
- Wang, C., et al., A multimodal deep learning approach for the prediction of cognitive decline and its effectiveness in clinical trials for Alzheimer’s disease. Translational psychiatry, 2024. 14(1): p. 105.
- Yao, M., et al., Deciphering proteins in Alzheimer’s disease: A new Mendelian randomization method integrated with AlphaFold3 for 3D structure prediction. Cell Genomics, 2024. 4(12).
- Cummings, J., et al., Anti-amyloid monoclonal antibodies for the treatment of Alzheimer’s disease. BioDrugs, 2024. 38(1): p. 5-22.
- Habibi, F., et al., Harnessing artificial intelligence in Alzheimer’s disease management: navigating ethical challenges in AI. AI and Ethics, 2025: p. 1-17.
- Aljassabi, A., et al., Alzheimer’s disease immunotherapy: current strategies and future prospects. Journal of Alzheimer’s Disease, 2024. 98(3): p. 755- 772.
- Ohno, M., A strategy for allowing earlier diagnosis and rigorous evaluation of BACE1 inhibitors in preclinical Alzheimer’s disease. Journal of Alzheimer’s Disease, 2024. 99(2): p. 431-445.
- Balkhi, S., et al., Immune Modulation in Alzheimer’s Disease: From Pathogenesis to Immunotherapy. Cells, 2025. 14(4): p. 264.
- Jun, H., Z. Shi, and S. Mattke, Projected savings to Canadian provincial budgets from reduced long-term care home utilization due to a diseasemodifying Alzheimer’s treatment. The Journal of Prevention of Alzheimer’s Disease, 2024. 11(1): p. 179-184.
- Liu, J.L., et al., Modeling Early Detection and Geographic Variation in Health System Capacity for Alzheimer’s Disease-Modifying Therapies. 2024: RAND.
- Lozupone, M., et al., The impact of apolipoprotein E (APOE) epigenetics on aging and sporadic Alzheimer’s disease. Biology, 2023. 12(12): p. 1529.
- Ekundayo, B.E., et al., Donepezil-based combination therapy for Alzheimer’s disease and related neuropathies. Comparative Clinical Pathology, 2023. 32(4): p. 699-708.
- Li, J., et al., The efficacy and safety of anti-Aβ agents for delaying cognitive decline in Alzheimer’s disease: A meta-analysis. Frontiers in aging neuroscience, 2023. 15: p. 1257973.
- Chunhui, G., et al., Exosomes and non-coding RNAs: bridging the gap in Alzheimer’s pathogenesis and therapeutics. Metabolic Brain Disease, 2025. 40(1): p. 84.
- Gao, S., et al., LncRNA ENST00000440246. 1 Promotes Alzheimer’s Disease Progression by Targeting PP2A. Biochemical Genetics, 2024. 62(3): p. 2100-2116.
- Dimkovski, A., et al., Design and synthetic approach of novel hybrid molecules for treatment of Alzheimer’s disease.
- Carmona-Iragui, M., et al., Clinical and research application of fluid biomarkers in autosomal dominant Alzheimer’s disease and Down syndrome. EBioMedicine, 2024. 108.
- Serrano-Pozo, A., et al., Astrocyte transcriptomic changes along the spatiotemporal progression of Alzheimer’s disease. Nature neuroscience, 2024: p. 1-17.
- Jackson, R.J., B.T. Hyman, and A. Serrano-Pozo, Multifaceted roles of APOE in Alzheimer disease. Nature Reviews Neurology, 2024. 20(8): p. 457-474.
- Jaisa-Aad, M., et al., Characterization of monoamine oxidase-B (MAO-B) as a biomarker of reactive astrogliosis in Alzheimer’s disease and related dementias. Acta Neuropathologica, 2024. 147(1): p. 66.
- Liu, H., et al., Astrocytic proteins involved in regulation of the extracellular environment are increased in the Alzheimer’s disease middle temporal gyrus. Neurobiology of Disease, 2025. 204: p. 106749.
- Groenewald, C.A., et al., Exploring The Efficacy Of Dietary Interventions In Neurological Disorders: A Systematic Analysis. Journal of Advanced Zoology, 2024. 45.
- Liu, J.-Y., et al., Effect of berberine on cognitive function and β-amyloid precursor protein in Alzheimer’s disease models: a systematic review and meta-analysis. Frontiers in Pharmacology, 2024. 14: p. 1301102.
- Geng, J., et al., Associations between Alzheimer’s disease biomarkers and postoperative delirium or cognitive dysfunction: A meta-analysis and trial sequential analysis of prospective clinical trials. European Journal of Anaesthesiology| EJA, 2024. 41(3): p. 234-244.
- Tang, X., et al., A multimodal meta-analytical evidence of functional and structural brain abnormalities across Alzheimer’s disease spectrum. Ageing Research Reviews, 2024: p. 102240.
- Zhang, Z., et al., Correlation between serum biomarkers, brain volume, and retinal neuronal loss in early-onset Alzheimer’s disease. Neurological Sciences, 2024. 45(6): p. 2615-2623.
- Lozupone, M. and F. Panza, Impact of apolipoprotein E isoforms on sporadic Alzheimer’s disease: beyond the role of amyloid beta. Neural Regeneration Research, 2024. 19(1): p. 80-83.
- Herrejon, I.A., et al., Functional Connectivity Differences in Distinct Dentato-Cortical Networks in Alzheimer’s Disease and Mild Cognitive Impairment. bioRxiv, 2024.
- McAlary, L., et al., Amyloidogenic regions in beta-strands II and III modulate the aggregation and toxicity of SOD1 in living cells. Open Biology, 2024. 14(6): p. 230418.
- Vander Zanden, C.M. and E.Y. Chi, Passive immunotherapies targeting amyloid beta and tau oligomers in Alzheimer’s disease. Journal of pharmaceutical sciences, 2020. 109(1): p. 68-73.
- Yi, F., et al., Identifying underlying patterns in Alzheimer’s disease trajectory: a deep learning approach and Mendelian randomization analysis. Eclinicalmedicine, 2023. 64.
- Zhang, Y., et al., Large-scale proteomic analyses of incident Alzheimer’s disease reveal new pathophysiological insights and potential therapeutic targets. Molecular Psychiatry, 2024: p. 1-15.
- Wang, Y., et al. Learning the Irreversible Progression Trajectory of Alzheimer’s Disease. in 2024 IEEE International Symposium on Biomedical Imaging (ISBI). 2024. IEEE.
- Alshamrani, M., Recent trends in active and passive immunotherapies of Alzheimer’s disease. Antibodies, 2023. 12(2): p. 41.
- Stazi, M., et al., A combination of caffeine supplementation and enriched environment in an Alzheimer’s disease mouse model. International Journal of Molecular Sciences, 2023. 24(3): p. 2155.
- Bhadane, P., et al., Immunotherapeutic approaches for Alzheimer’s disease: Exploring active and passive vaccine progress. Brain Research, 2024: p. 149018.
- Wang, Q., et al., The protective role of carnosine against type 2 diabetesinduced cognitive impairment. Food Science & Nutrition, 2024. 12(6): p. 3819-3833.
- Shajahan, S.R., S. Kumar, and M.D.C. Ramli, Unravelling the connection between COVID-19 and Alzheimer’s disease: a comprehensive review. Frontiers in Aging Neuroscience, 2024. 15: p. 1274452.
- Yu, S.P., et al., Acute and chronic excitotoxicity in ischemic stroke and lateonset Alzheimer’s disease. Neural Regeneration Research, 2025. 20(7): p. 1981-1988.
- Ferdowsi, M., et al., Automated Detection of Alzheimer’s Disease Using EEG Signal Processing and Machine Learning, in Artificial Intelligence Enabled Signal Processing based Models for Neural Information Processing. 2024, CRC Press. p. 118-135.
- Guo, Y., et al., Multiplex cerebrospinal fluid proteomics identifies biomarkers for diagnosis and prediction of Alzheimer’s disease. Nature human behaviour, 2024. 8(10): p. 2047-2066.
- Al-Rawashdeh, H.S., et al., Hybrid feature extraction technique-based Alzheimer’s disease detection model using MRI images. Journal of Disability Research, 2024. 3(6): p. 20240073.
- Veronika, P., et al., Distinctive whole-brain cell types predict tissue damage patterns in thirteen neurodegenerative conditions. eLife, 2024. 12.
- Samanta, S., et al., New cyclophilin D inhibitor rescues mitochondrial and cognitive function in Alzheimer’s disease. Brain, 2024. 147(5): p. 1710- 1725.
- Samanta, S., S. Chakraborty, and D. Bagchi, Pathogenesis of neurodegenerative diseases and the protective role of natural bioactive components. Journal of the american nutrition association, 2024. 43(1): p. 20-32.
- Coppedè, F., Mitochondrial DNA methylation and mitochondria-related epigenetics in neurodegeneration. Neural Regeneration Research, 2024. 19(2): p. 405-406.
- Ramani, S., et al., Unveiling Alzheimer’s Early Signs: AI-Driven Insights Through Neuroimaging and Biomarkers. AI-Driven Alzheimer’s Disease Detection and Prediction, 2024: p. 223-236.
- Suresh, S. and C. Vellapandian, Cyanidin improves spatial memory and cognition in bisphenol A-induced rat model of Alzheimer’s-like neuropathology by restoring canonical Wnt signaling. Toxicology and Applied Pharmacology, 2024. 487: p. 116953.
- Dhanush, L., et al., Navigating the Administrative Landscape of AI in Alzheimer’s Disease Detection: A Comprehensive Management Studies Perspective, in AI-Driven Alzheimer’s Disease Detection and Prediction. 2024, IGI Global. p. 264-275.
- Bajaj, S. and R. Mahesh, Converged avenues: depression and Alzheimer’s disease–shared pathophysiology and novel therapeutics. Molecular Biology Reports, 2024. 51(1): p. 225.
- Ikanga, J., et al., Preliminary reference values for Alzheimer’s disease plasma biomarkers in Congolese individuals with and without dementia. Frontiers in Aging Neuroscience, 2024. 16: p. 1477047.
- Bougea, A. and P. Gourzis, Biomarker-Based Precision Therapy for Alzheimer’s Disease: Multidimensional Evidence Leading a New Breakthrough in Personalized Medicine. Journal of Clinical Medicine, 2024. 13(16): p. 4661.
- Bhatt, A., H. Bhardwaj, and P. Shrivastava, Mesenchymal stem cell therapy for Alzheimer’s disease: A novel therapeutic approach for neurodegenerative diseases. Neuroscience, 2024.
- Pandey, K., et al., Stem Cells as a Novel Source for Regenerative Medicinal Applications in Alzheimer’s Disease: An Update. Current Molecular Medicine, 2025. 25(2): p. 146-166.
- Bankar, A.A. and S.M. Nair, Stem Cell Therapy for Alzheimer’s Disease: A Promising Avenue for Neuroregeneration. Journal of Asian Medical Students’ Association, 2024.
- Segal, J., Investigating Stem Cell Therapy for Alzheimer’s Disease: A Promising Approach. Berkeley Pharma Tech Journal of Medicine, 2024. 4(1): p. 1-13.
- Wang, J., et al., Enhancing regenerative medicine: the crucial role of stem cell therapy. Frontiers in Neuroscience, 2024. 18: p. 1269577.
- Shah, A.J., et al., Neural Stem Cell Therapy for Alzheimer’s Disease: A-State-of-the-Art Review. Journal of Dementia and Alzheimer’s Disease, 2024. 1(2): p. 109-125.
- Iqbal, I., et al., Alzheimer’s disease and drug delivery across the blood– brain barrier: approaches and challenges. European journal of medical research, 2024. 29(1): p. 313.
- Chauhan, B., et al., Drug delivery for Alzheimer’s disease using nanotechnology: Challenges and advancements, in Alzheimer’s Disease and Advanced Drug Delivery Strategies. 2024, Elsevier. p. 361-371.
- Nagori, K., et al., Innovative strategies for overcoming blood-brain barrier challenges in Alzheimer’s disease: A focus on green-synthesized metallic nanoparticles. Inorganic Chemistry Communications, 2024: p. 113604.
- Lee, A.Y., et al., Barriers to Alzheimer disease clinical trial participation in a minority population. Cognitive and Behavioral Neurology, 2024. 37(1): p. 40-47.
- Pappolla, M.A., et al., Oxidative Stress in Alzheimer’s Disease: The Shortcomings of Antioxidant Therapies. Journal of Alzheimer’s Disease, 2024. 101(s1): p. S155-S178.
- Zeynalzadeh, E., et al., Navigating the neurological frontier: Macromolecular marvels in overcoming blood-brain barrier challenges for advanced drug delivery. Heliyon, 2024. 10(15).
- Zivari-Ghader, T., et al., Recent progresses in natural based therapeutic materials for Alzheimer’s disease. Heliyon, 2024. 10(4).
- Daraban, B.S., A.S. Popa, and M.S. Stan, Latest Perspectives on Alzheimer’s Disease Treatment: The Role of Blood-Brain Barrier and Antioxidant-Based Drug Delivery Systems. Molecules, 2024. 29(17): p. 4056.
- Vishwas, S., et al., Neuroprotective Role of Phytoconstituents-based Nanoemulsion for the Treatment of Alzheimer’s Disease. Current Topics in Medicinal Chemistry, 2024. 24(19): p. 1683-1698.
- Ma, Y.-n., et al., Exploring the multiple therapeutic mechanisms and challenges of mesenchymal stem cell-derived exosomes in Alzheimer’s disease. BioScience Trends, 2024. 18(5): p. 413-430.
- Wong, C.Y.J., et al., Insulin Delivery to the Brain via the Nasal Route: Unraveling the Potential for Alzheimer’s Disease Therapy. Drug Delivery and Translational Research, 2024. 14(7): p. 1776-1793.
- Zhao, J., et al., GSK3: A potential target and pending issues for treatment of Alzheimer’s disease. CNS Neuroscience & Therapeutics, 2024. 30(7): p. e14818.
- Dong, N., et al., Nanomedicine in the treatment of Alzheimer’s disease: bypassing the blood-brain barrier with cutting-edge nanotechnology. Neurological Sciences, 2024: p. 1-19.
- Sun, M. and Z. Chen, Unveiling the Complex Role of Exosomes in Alzheimer’s Disease. Journal of Inflammation Research, 2024: p. 3921- 3948.
- Turgutalp, B. and C. Kizil, Multi-target drugs for Alzheimer’s disease. Trends in Pharmacological Sciences, 2024.
- Fu, H., et al., Aβ-Aggregation-Generated Blue Autofluorescence Illuminates Senile Plaques as well as Complex Blood and Vascular Pathologies in Alzheimer’s Disease. Neuroscience Bulletin, 2024. 40(8): p. 1115-1126.
- Mazur, T., M. Malik, and D.C. Bieńko, The impact of chelating compounds on Cu2+, Fe2+/3+, and Zn2+ ions in Alzheimer’s disease treatment. Journal of inorganic biochemistry, 2024: p. 112601.
- Gorgoni, M., et al., The role of the sleep K‐complex on the conversion from mild cognitive impairment to Alzheimer’s disease. Journal of Sleep Research, 2024. 33(1): p. e14046.
- Palihati, N., et al., Clusterin is a potential therapeutic target in Alzheimer’s disease. Molecular Neurobiology, 2024. 61(7): p. 3836-3850.
- Schicktanz, S., et al., Moving Towards Ethical-Practical Recommendations for Alzheimer’s Disease Prediction: Addressing Interindividual, Interprofessional, and Societal Aspects. Journal of Alzheimer’s Disease, 2024. 101(4): p. 1063-1081.
- Erickson, C.M., A. Wexler, and E.A. Largent, Alzheimer’s in the modern age: Ethical challenges in the use of digital monitoring to identify cognitive changes. Informatics for Health and Social Care, 2024. 49(1): p. 1-13.
- Gheorghe-Moisii, M., C.-G. Gheorghe, and S. Soviany, Ethical considerations on the use of Al technology in eHealth applications for neurodegenerative diseases. Romanian Journal of Information Technology and Automatic Control, 2024. 34(1): p. 97-108.
- Mehta, N., et al., Regulatory and ethical concerns in the use of nanomaterials, in Alzheimer’s Disease and Advanced Drug Delivery Strategies. 2024, Elsevier. p. 197-212.
- Ayyalasomayajula, M.M.T., Ethical Considerations in AI Implementation for Neurodegenerative Disease Diagnosis and Treatment, in Integrating Neuroimaging, Computational Neuroscience, and Artificial Intelligence. 2025, CRC Press. p. 105-127.
- Mundhra, S., S.K. Kadiri, and P. Tiwari, Quality Control in Precision Medicine for Alzheimer’s Disease. Journal of Pharmaceutical Quality Assurance and Quality Control, 2024: p. 30-39.
- Mani, K., K.K. Singh, and R. Litoriya. Transformative Potential IoT Sensor Frameworks for Real-Time Alzheimer’s Detection and Monitoring in Elderly Populations. in 2024 International Conference on Advances in Computing Research on Science Engineering and Technology (ACROSET). 2024. IEEE.
- Dino, F.R., et al., Ethics in digital phenotyping: considerations regarding Alzheimer’s disease, speech and artificial intelligence. Journal of Medical Ethics, 2025.
- Jia, J., et al., Biomarker changes during 20 years preceding Alzheimer’s disease. New England Journal of Medicine, 2024. 390(8): p. 712-722.
- Huang, D., et al., Alzheimer’s Disease: Status of Low‐Dimensional Nanotherapeutic Materials. Advanced Functional Materials, 2024. 34(4): p. 2302015.
- Agrawal, M. and R. Dayaramani, Stimuli-Responsive Polymers for Brain Delivery, in Application of Nanocarriers in Brain Delivery of Therapeutics. 2024, Springer. p. 263-283.
- Rai, H., et al., Rhodanine composite fluorescence probes to detect pathological hallmarks in Alzheimer’s disease models. Sensors and Actuators B: Chemical, 2024. 407: p. 135364.
- Fox, J., et al., Indirect Costs of Alzheimer’s Disease: Unpaid Caregiver Burden and Patient Productivity Loss. Value in Health, 2024.
- Saha, S., et al. A Study on the Impact of Microstructures Inside the Human Brain in Understanding the Progression of Alzheimer’s Disease. in 2024 IEEE 16th International Conference on Computational Intelligence and Communication Networks (CICN). 2024. IEEE.
- Anderson, C., et al., The potential of a stratified approach to drug repurposing in Alzheimer’s disease. Biomolecules, 2023. 14(1): p. 11.
- Akbarzadehmoallemkoalei, M., et al., Understanding Alzheimer’s Disease: A Multidisciplinary Perspective, in PsychoNeuroImmunology: Volume 2: Interdisciplinary Approaches to Diseases. 2025, Springer. p. 357-415.
- Hashemi, S.A., et al., The effects of eight weeks of aerobic training with vitamin C on the expression pathway of antioxidants in the hippocampus tissue of TMT induced Alzheimer’s disease rats. Brain Research, 2024. 1822: p. 148645.
- Huang, L.-T., et al., Association of peripheral blood cell profile with Alzheimer’s disease: a meta-analysis. Frontiers in aging neuroscience, 2022. 14: p. 888946.
- Huang, Y., et al., Research progress on vestibular dysfunction and visual– spatial cognition in patients with Alzheimer’s disease. Frontiers in Aging Neuroscience, 2023. 15: p. 1153918.
- Neel, I.C., Alzheimer’s disease and other dementias, in Geriatric Medicine: A Person Centered Evidence Based Approach. 2024, Springer. p. 1027- 1046.
- Patil, V.S., et al., Computational Modelling of BACE-1, AChE, and Phosphodiesterase Inhibitors as Anti-Alzheimer’s Agents, in Computational and Experimental Studies in Alzheimer’s Disease. CRC Press. p. 122-137.
- Stouffer, K.M., et al., Amidst an amygdala renaissance in Alzheimer’s disease. Brain, 2024. 147(3): p. 816-829.
- Rubin, R., A Blood Test for Alzheimer Disease, Repurposing GLP-1s, and Wildfire Smoke—Highlights From the AAIC. JAMA, 2024. 332(10): p. 777- 779.
- Grødem, E.O., et al., A minimalistic approach to classifying Alzheimer’s disease using simple and extremely small convolutional neural networks. Journal of Neuroscience Methods, 2024. 411: p. 110253.
- Vejandla, B., et al., Alzheimer’s disease: the past, present, and future of a globally progressive disease. Cureus, 2024. 16(1).
- Kulkarni, R., et al., Association Between the Gut Microbiota and Alzheimer’s Disease: An Update on Signaling Pathways and Translational Therapeutics. Molecular Neurobiology, 2024: p. 1-21.
- Qureshi, T., et al., Hurdles and Strategies in Treating Alzheimer’s Disease, in Multi-Factorial Approach as a Therapeutic Strategy for the Management of Alzheimer’s Disease. 2025, Springer. p. 113-138.
- Gong, Y., et al., Healthy dietary patterns in relation to cognitive performance and Alzheimer’s disease mortality. The Journal of Prevention of Alzheimer’s Disease, 2025: p. 100100.
- Huang, L., et al., Neuropsychiatric symptoms in Alzheimer’s continuum and their association with plasma biomarkers. Journal of Affective Disorders, 2024. 348: p. 200-206.
- Harding, E., et al., Exploring experiential differences in everyday activities–A focused ethnographic study in the homes of people living with memory-led Alzheimer’s disease and posterior cortical atrophy. Journal of Aging Studies, 2024. 69: p. 101226.
- Dickson, J., et al., The Primary Care Memory Assessment Pathway (MAP): a Narrative Summary of Best Practice and Uncertainties. 2024.
- Belder, C.R., et al., Brain volume change following anti-amyloid β immunotherapy for Alzheimer’s disease: amyloid-removal-related pseudoatrophy. The Lancet Neurology, 2024. 23(10): p. 1025-1034.
- Levites, Y., et al., Integrative proteomics identifies a conserved Aβ amyloid responsome, novel plaque proteins, and pathology modifiers in Alzheimer’s disease. Cell Reports Medicine, 2024. 5(8).
- Kloske, C.M., et al., Advancements in APOE and dementia research: Highlights from the 2023 AAIC Advancements: APOE conference. Alzheimer’s & Dementia, 2024. 20(9): p. 6590-6605.
- Prajapati, S.K., A. Pathak, and P.K. Samaiya, Alzheimer’s disease: from early pathogenesis to novel therapeutic approaches. Metabolic Brain Disease, 2024. 39(6): p. 1231-1254.
- Meng, J. and P. Lei, Testing cognitive normal for Alzheimer’s disease prediction. Journal of Neurochemistry, 2025. 169(1): p. e16272.
- Ma, M., et al., Ferroptosis in Cognitive Impairment Associated with Diabetes and Alzheimer’s Disease: mechanistic insights and New Therapeutic opportunities. Molecular Neurobiology, 2025. 62(2): p. 2435-2449.
- Meng, X., et al., Development of asparagine endopeptidase inhibitors for treating neurodegenerative diseases. Trends in Molecular Medicine, 2025.