
Research Article
Austin J Pharmacol Ther. 2020; 8(2).1121.
Effects of Constant Light on the Circadian System in Rats
Feng TR¹, Li Z² and Li SX³*
1Maryknoll Convent School (Secondary Section), China
2Shanghai University of Traditional Chinese Medicine, School of Pharmacy, China
3National Institute on Drug Dependence, Peking University, China
*Corresponding author: Su-Xia Li, National Institute on Drug Dependence, Peking University, 38 Xue Yuan Road, Haidian District, Beijing 100191, China
Received: May 07, 2020; Accepted: October 17, 2020; Published: October 24, 2020
Abstract
Light pollution is one of the most rapidly increasing types of environmental degradation, especially in the hospital ward and ICUs. Exposure to Constant Light (LL) condition disrupts circadian rhythms in behavioral, physiological and endocrinal processes. Whether melatonin can reverse those disruptions remains unclear. We used male Sprague - Dawley rats (7 weeks of age at the time of purchase) to explore 1) the effects of LL condition on social memory, anxietylike behavior and circadian rhythmic system, 2) whether exogenous melatonin can reverse the changes. Compared with LD+Veh (LD: standard 12h:12h lightdark cycles; Veh: receive vehicle intraperitoneal injection) group, rats exposed to LL for two weeks have less anxiety-like behavior, impaired the social memory, elongated the rhythm cycle of free running wheel and disruped secretion of Corticosterone (Cort) and Melatonin (Mel). Application of exogenous melatonin couldn't rescue the impairment of the social memory and the disrupted circadian system. These findings suggest that LL condition can disorganize the circadian system and exogenous melatonin cannot reverse these changes.
Keywords: Constant light; Free running wheel; Corticosterone; Melatonin; Diurnal rhythm
Introduction
The growing prevalence of exposure to artificial light at night is the most common type of change of our living environment at the turn of 20th century. Urban development has further exacerbated the issue of light at night as lighting from infrastructure strays into the atmosphere. We called such phenomenon as “light pollution”. Previous research indicated that more than 80% of the world and more than 99% of the US. and European populations live under lightpolluted skies and almost one-fifth of world terrain is under light polluted skies [1,2]. The artificial light of a typical shopping mall is up to 200 thousand times brighter than the illuminance experienced in the natural environment [3]. Additionally, Artificial lightings are rapidly increasing by around 6% per year (range from 0 to 20%) [4]. Now about 20% of the urban population work at night shifts and are chronically exposed to night time light [5]. Although artificial light has clearly improved the quality of our life, there is reliable evidence suggesting that artificial light at night has strong adverse effects on the health of animals and human [1-4]. Exposure to light at night disrupts circadian rhythm mediated by decreasing the secretion of pineal melatonin and some other mechanisms. Except for timing and exposure duration, the inhibition of secretion of melatonin is also related with light intensity and wavelength. Previous study found that even illuminance is as low as 1.5 lux also can affect circadian rhythm [6]. Some studies showed that typical bedroom light at home can reduce and delay the production of melatonin [7,8]. The pollution of air, noise, or water has been attached more importance for decades. However, light pollution has not been paid sufficient attention, especially in the hospital ward and the Intensive Care Unit (ICUs).
Currently, a growing number of people in the world are unintentionally or intentionally exposed to constant light (LL) during night, especially for patients in hospital. Lights are always present in the hospital ward, especially in the ICU for patients’ close observations and frequent patients care activities. It is reported that nocturnal illumination in ICUs varies from 5 to 1400 lux [9,10]. Previous study showed that patients who were hospitalized in the hospital ward or ICUs considered bright lights are noxious and disruptive [11]. Such as sleep-wake cycle and production of hormones were both disturbed with exposure to stimulation of ICUs light in healthy subjects [12]. A recent review showed that nocturnal light pollution, disrupts circadian rhythm in healthy participants; however, windowed rooms or real-time ambient lighting which is called physiologic light-dark patterns increases the speed of recovery for critical illness patients [13].
A cumulated evidences demonstrated that individuals exposed to light at night are at increasing risk of heart disease [14], cancer [15,16], sleep disturbances [17,18], circadian rhythm dysfunctions [19], disrupted rhythmicity of neuroendocrine function (such as corticotrophin releasing hormone, glucocorticoids, and prolactin) [20,21], mood disorders [22], and reproductive dysfunction [23]. A line of studies on laboratory animals indicate that maintaining animals in LL conditions is deleterious, but the mechanisms underlying these harmful effects remain unspecified [24]. Reports on the behavioral effects of LL condition in laboratory animals are inconsistent. Some study show that LL condition influences memory [25], but other study showed no any effects [26]. Additionally, although circadian disruption has been reported to lessen anxiety [27], the effects of LL condition on anxiety still have not been well established [25,26].
Melatonin was believed to be the major endocrine output signal of the endogenous time measuring and time keeping system [28]. Exogenous melatonin is used to treat desynchronization of circadian rhythms caused by jet lag or shiftwork [5,29-31]. Melatonin is also used to entrain the sleep–wake rhythm to a 24 h cycle for blind people [32] and to improve sleep, morning alertness and cognitive performance for aged individuals and patients with neurodegenerative disorders [33-35]. We make a hypothesis that melatonin can rescue the disruptions of circadian system induced by LL condition. We used two weeks’ LL condition to simulate the constant light in hospital ward and ICUs. Melatonin was administered for two weeks at the same time every day to mimic people taking melatonin. The aims of these studies were to investigate the circadian system and behavioral responses to LL condition exposure, and to find whether melatonin can reverse the changes induced by LL condition. Glucocorticoid is an important stress hormone in mammal. The production of melatonin is not only affected by the light, but also can feedback on the impact of the Suprachiasmatic Nuclei (SCN) [36,37] Previous studies have proved that melatonin can inhibit the secretion of glucocorticoid [21]. Melatonin and glucocorticoid hormons are known as biological markers of the circadian system [38,39].
Materials and Methods
Animals and housing
Forty male Sprague - Dawley rats were purchased from the Center of Laboratory Animal Science, Peking University Health Science Center. The rats were 7 weeks of age at the time of purchase. Rats were given sustaining food and water ad libitum and were allowed to adapt to the environment for 3 days before the commencement of the experiments. The average weight of rats was 260g (range 238-282g) at the beginning of the experiments. Each rat was isolated housed in cage equipped with running wheel. Five rats were placed in an isolated locker with controlled humidity (50%±5%) and temperature (23oC±1oC). Lighting conditions were controlled independently for each locker allowing in this way to have different schedules for different groups. The experimental procedures were abided by the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the University Animal Use Committee.
Rats (n=40) were randomly divided into four groups, including Melatonin (Mel) group and Vehicle (Veh) group under a 12h/12h light/dark cycle (7:00 light up; 19:00 light off, defined as LD+Mel and LD+Veh, respectively) and a constant light condition (defined as LL+Mel and LL+Veh,respectively) for two weeks.
Melatonin treatment
Melatonin (Sigma-Aldrich Trading Co.,Ltd, Shanghai China) was dissolved in 2% alcohol (concentration: 1.5mg/ml) and administered subcutaneously (sc) in an amount of 5mg/kg. Rats in Veh group were subcutaneous injected with the same volume of 2% alcohol. The administration of Mel or veh was at 18:00 daily (one hour before the light off). Two rats died during the melatonin treatment. One belongs to LD+Veh group, another belongs to LD+Mel group.
Social memory test (SMT)
SMT was performed in a new cage in the darkness with a red light at 21:00. We conducted SMT as previously described [40]. The procedure consisted of a 5 min learning trial and a 5 min retrieval trial, separated by an inter-trial interval of 5 min, using different groups of rats. During the learning trial, a juvenile rat A, 4 weeks of age, male SD rats, was introduced into the new cage with the experimental rat, and the time spent by the experimental rat investigating the juvenile rat (sniffing, licking and anogenital investigation) was measured as the social investigation time. During the inter-trial interval, the familiarized juvenile A (previously introduced during the learning trial) and the second juvenile B (the same age and gender as juvenile A, unknown to the experimental rat), were isolated in cages with clean sawdust. The retrieval trial was started by introducing both juveniles into the cage with the experimental rat, and social investigation times directed towards the known juvenile A and unknown juveniles B were measured separately. The juveniles were identified by using colored marks on the head with A or B. A discrimination index was calculated using the formula N=(b-a²)/(b+a²), where b=time with novel rat B, a²=time with familiar rat A. This discrimination index was used to measure short-term memory performance.
Light/Dark box Test (LDT)
The light/dark box test was frequently used for anxiety-like behavior on the basis of the rat's natural preference for darkness [41- 43]. This test was performed in a dark phase of the diurnal cycle. The dark/light box consisted of two different sized and different colored compartments (40cm×40cm×40cm, white and 20cm×20cm×40cm, black). The black compartment was dark and the white compartment was illuminated by a 40W bulb located 45cm above the box. The two compartments were connected by a 5cm×6cm tunnel. Rats were placed into the white compartment with access to the dark box. The time spent in the dark box, the total time spent in the light box and the number of visits to this anxiety - related compartment were scored by visual observation for 10 minutes.
Free running wheel activity
Voluntary wheel-running activity of rats was studied by use of a self-constructed running wheel system that consists of stainless steel running wheels (230mm diameter; 100mm width, 85mm distance from the wheel to the side wall of the cage) integrated in standard polycarbonate cages (400×270×170 mm; Suzhou feng's experimental animal equipment co. LTD, Suzhou, China) and a data acquisition unit (clocklab 2). The rotary movements of the running wheels create pulses using permanent magnets and reed contacts. A Clocklab 2 system (Actimetrics, Wilmette, IL, USA) acquires the rotary pulses and provides the data to the computer. A continuously operating computer equipped with Clocklab 2.0 software (Actimetrics, Wilmette, IL, USA) collects and saves the raw data in 5min bins for two weeks.
Tissue sample preparation, hormone and peptide measurement
The blood samples were collected through caudal vein at 8:00 (CT1) and 20:00 (CT13) on the same day. Each rat was placed into a special device for collecting caudal venous blood. The tail of rat was in the outside and the head and body was in a dark container with several holes for ventilation as our previously described [44]. Immediately after each blood sample was drawn, a 1ml sample was kept in a tube with the anticoagulant EDTA.
Blood samples were kept in a tube with EDTA at room temperature for about 30 minutes till to centrifugation. The supernatant was transferred from the samples immediately after 10 minutes of centrifugation at 2500rpm in room temperature. Plasma was stored in aliquots at -70oC until assays were performed. Plasma concentrations of corticosterone and melatonin were detected by ELISA kits (Rapidbio, California, USA). The sensitivities of the assay are about 0.7nmol/l for corticosterone and 2pg/ml for melatonin. The intra- and inter-assay coefficients of variation are less than 5% and 10% for corticosterone, and melatonin, respectively.
Statistical analysis
Data are presented as the mean±Standard Error of the Mean (SEM). The SMT and LDT data were analyzed using a between subjects 2×2 (photoperiod×treatment) ANOVA tested group differences for all behaviors in reference to each group’s light and treatment status. Corticosterone and melatonin data were analyzed using General Linear Models (GLMs). Two sampling points over 24 h (08:00, and 20:00) of the plasma concentration of corticosterone and melatonin were Analyzed Using Repeated- Measures Analysis Of Variance (ANOVA). Three - way repeated measures ANOVAs were conducted, with treatment (“photoperiod” LD and LL, “treatment” Veh and Mel ) as the between - subjects factor and sampling time as the within subjects factor to evaluate the levels of corticosterone and melatonin among groups. The circadian rhythms of these two dependent variables were calculated using paired comparisons after main effect analysis. Differences were considered to be significant when p values were less than 0.05. Four outliers, which belong to four groups respectively, were excluded from the statistics for corticosterone and melatonin.
Results
Effects of constant light on behaviors
Figure 1A shows that it is treatment factor, not the photoperiod factor significantly increased the time at black (1,19=1.253, p=0.281 for photoperiod factor; F1,19=16.603, p=0.001 for treatment factor), with a significant photoperiod×treatment interaction (F1,19=6.341, p=0.024). Paired comparisons showed that constant light condition significantly decreased rats stay the time at black (F1,15=6.249, p=0.025 for Veh group; F1,15=1.039, p=0.324 for Mel group), and adminstration of exogenous Mel significantly increased rats stay the time at black (F1,15=1.144, p=0.302 for LD condition; F1,15=23.092, p=0.000 for LL condition).

Figure 1: Effects of constant light exposure on behaviors in rats. (A&B):
light/dark box test; (C): Social memory test. Data represent mean±S.E.M.
(n=5 rats per group). Significance was determined as *p<0.05 compared with
the LD+Veh, #p<0.05 compared with the LL+Mel.
Figure 1B shows that it is the constant light, not melatonin significantly decreased the number of shuttles (F1,19=10.507, p=0.005 for photoperiod factor, F1,19=0.075, p=0.787 for treatment factor), without a significant photoperiod×treatment interaction (F1,19=0.000, p=0.986).
Figure 1C shows that it is the constant light, not melatonin significantly decreased the discrimination index (F1,31= 5.517, p=0.026 for photoperiod factor, F1,30=3.157, p=0.087 for treatment factor), without a significant photoperiod×treatment interaction (F1,31=0.775, p=0.387).
Effects of constant light and melatonin on running wheel activity
Rats with administration of Veh or Mel, showed primarily activity during the dark phase at LD condition (LD+Veh or Mel, Figure 2A&B). Constant light condition lengthened the period of wheel running activity (LL+Veh, Figure 2C). At the constant light condition, the exogenous melatonin can’t rescue the lengthened period of diurnal rhythmicity of free wheel - running pattern (LL+Mel, Figure 2D). The periods of each group rats were showed in Table 1.The tau values are presented in the Figure 2.
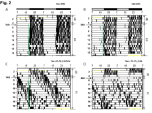
Figure 2: Free running wheel of rats during constant light exposure. (A):
LD+Veh group; (B): LD+Mel group; (C): LL+Veh group; (D): LL+Mel group.
LD: Light: Dark 12:12 h; LL: Constant light. Veh: Vehicle; Mel: Melatonin.
The green lines represent the time of Mel administration. The yellow lines
represent no wheel running.
group
LD+Veh
LD+Mel
LL+Veh
LL+Mel
period (h)
24.1±0.04
24.1±0.03
25.5±0.08
25.42±0.06
Table 1: The period of each group.
Effects of constant light on plasma Cort and melatonin
Figure 3A shows that it is the photoperiod factor, not the treatment factor significantly influence the plasma Cort concentration (F1,29=5.89, p=0.022 for photoperiod factor; F1,29=1.864, p=0.183 for treatment factor), without a significant photoperiod×melatonin interaction (F1,29=2.767, p=0.107). Paired comparisons show that the plasma Cort concentration in LD+Veh group has a significant daily rhythm with significant increase at CT 13. The ditrnal rhythmicity of the plasma Cort concentration in other three groups is disrupted.
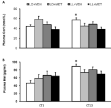
Figure 3: Effects of constant light exposure on hormone levels in
rats. Plasma levels of (A): Corticosterone and (B): Melatonin in rats after
constant light exposure. Data represent mean±SEM. (n=8-9 rats per group).
Significance was determined as *p<0.05 compared with the LD+Veh. Cort:
Corticosterone; Mel: Melatonin.
Figure 3B shows that neither the photoperiod factor, nor the treatment factor dose influence the plasma Mel concentration (F1,30=0.327, p=0.572 for photoperiod factor; F1, 30 = 0.349, p=0.559 for melatonin factor), without a significant photoperiod×melatonin interaction (F1, 30=0.116, p=0.736). A paired comparison shows that the plasma Mel concentration in LD+Veh group has a significant daily rhythm with significant increase at CT 13. The diurnal rhythmicity of the plasma Mel concentration in other three groups is disrupted.
Discussion
Our results showed that constant light impaired the social memory in rats, and melatonin could not rescue the decreased social memory. As for the light/dark test, constant light significantly decreased the time at black, which means constant light lessened anxiety-like behavior in rats. Exogenous melatonin reversed the decreased anxiety-like behavior in the light/dark box and decreased locomotor activity in LL rats but had no effect in LD rats. Moreover constant light prolonged the extent of tau values and disrupted circadian rhythms of plasma Cort and Mel, while melatonin did not rescue the disturbed circadian rhythms. Previous studies indicated that disruption of circadian rhythm can impair long-term passive avoidance memory in rats and mice, and dim nighttime light can impair learning and memory in the Barnes maze by reducing dendritic length in DG and basilar CA1 dendrites [45]. Moreover, the phase shift of 6h or 12 h had no effects on social memory, while these phase shifts did impair passive avoidance memory. The authors explained it that social memory might use brain systems were not susceptible to circadian rhythm disruption [46]. Our results show that constant light impaired social memory and exogenous melatonin had no effects on it. The difference between our study and previous study may be caused by the severity of the disruption of circadian rhythm. The constant light continued for two weeks in our study is more severe than the phase shifts used in the previous study. As for the effects of melatonin, it had been demonstrated that hippocampal slices still persist differences of photoperiod in the absence of circulating or exogenously administered melatonin [47]. Chaudhury also showed that the circadian rhythmicity of Long Term Potentiation (LTP) and neural excitability in CA1 exist independent of melatonin [48]. This previous results evidenced that melatonin does not affect the discrimination index, our results show the similar results in either LD condition or LL condition.
Rats exposed to a regular LD cycle remained rhythmicity, with predominant activity during the night. Rats in LL condition exhibited the spontaneous free-running patterns with a period in general activity longer than 24 h and thus, did not exhibit a loss of overt expression of circadian rhythmicity. Melatonin did not affect the spontaneous free-running patterns at LD cycle condition, but the while they are disrupted at the LL condition. Our results of two weeks’ constant light were not similar to previous reports, in which the constant light for 8 weeks resulted in progressive development of arrhythmic locomotor patterns [49,50] including ultradian bouts of activity with a period of approximately 4 h [51]. The main reason of such difference may due to the duration of rats’ exposure to constant light condition. Our two weeks’ constant light condition was too short to express the arrhythmic free running-wheel activity. But our result is similar as a previous study that exposure to constant light condition for 13 days, 66.7% (20/30) experimental rats lengthened the period of locomotor activity, 33.3% (10/30) lost behavioral rhythm [52]. Daily injections of melatonin in rats kept in constant light condition had been shown either to synchronize or partially synchronize disrupted circadian activity [53]. And some research suggests that daily injection of melatonin failed to synchronize disrupted locomotor activity in rats maintained at constant bright light, but could entrain intact freerunning rhythms at constant dim light [54,55]. These differences may due to the species of experimental animals and the time of administration of melatonin.
Previous studies on the effect of constant light condition on the glucocorticoid level are inconsistent. Some studies found that exposure to LL condition elevated corticosterone level [23,56], and another study showed that corticosterone level was reduced at LL condition for two weeks [1]. In our study, LL condition did not significantly influence the plasma corticosterone concentrations when comparing with LD condition. Previous study showed that constant light exposure is a strong stressful stimulus, leading to the disruption of circadian rhythms [57] and a subsequent alteration of melatonin and Cort secretion [58]. Another previous study indicated that exposure to a stressful environmental situation for several weeks, the mice may have down-regulated their stress response [1]. The exact mechanism underlying such phenomenon is still not clear.
Limitations
The alterations in behaviors and peripheral markers found in the present study are just with male rats. Female rats should be further studied in the future. An additional methodological consideration is that the nocturnal Sprague-Dawley rat was used to model a constant light - related influence of diurnal humans. As discussed in previous publications, the impact of light in nocturnal versus diurnal species is different and nocturnal animal models of constant light influence on the rhythmic system may lead to inconclusive results [59]. Therefore, the use of a diurnal species to model a constant light related influence should be further studied in the future.
Implication
Our findings indicate that exposure to constant light condition can disrupt the circadian rhythm of free running-wheel activity, the secretion of melatonin and corticosterone, and impair the social memory. Melatonin can’t rescue these processes. So the most important thing to control light pollution is reduction of the usage of artificial light, but for some special places, such as hospital ward, especially ICU, where the light for patients’ observations and patient care activities, eye masks for patients may be a relatively cheap and effective way [12].
Acknowledgment
This work was supported by the Natural Science Foundation of China (no 81171251) and the Natural Science Foundation of Beijing (no 7162101). The authors declare that they do not have any conflicts of interest related to the data presented in this manuscript.
References
- Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, et al. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009; 205: 349-354.
- Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, et al. The new world atlas of artificial night sky brightness. Sci Adv. 2016; 2: e1600377.
- Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. Limiting the impact of light pollution on human health, environment and stellar visibility. J Environ Manage. 2011; 92: 2714-2722.
- Holker F, Wolter C, Perkin EK, Tockner K. Light pollution as a biodiversity threat. Trends Ecol Evol. 2010; 25: 681-682.
- Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001; 358: 999- 1005.
- Wright KP, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near- 24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA. 2001; 98: 14027-14032.
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011; 96: E463-472.
- Burgess HJ, Molina TA. Home lighting before usual bedtime impacts circadian timing: a field study. Photochem Photobiol. 2014; 90: 723-726.
- Meyer TJ, Eveloff SE, Bauer MS, Schwartz WA, Hill NS, Millman RP. Adverse environmental conditions in the respiratory and medical ICU settings. Chest. 1994; 105: 1211-1216.
- Walder B, Francioli D, Meyer JJ, Lancon M, Romand JA. Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels. Crit Care Med; 2000; 28: 2242-2247.
- Ugras GA, Oztekin SD. Patient perception of environmental and nursing factors contributing to sleep disturbances in a neurosurgical intensive care unit. Tohoku J Exp Med. 2007; 212: 299-308.
- Hu RF, Jiang XY, Zeng YM, Chen XY, Zhang YH. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care, 2010; 14: R66.
- Oldham MA, Lee HB, Desan PH. Circadian Rhythm Disruption in the Critically Ill: An Opportunity for Improving Outcomes. Crit Care Med. 2016; 44: 207- 217.
- Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health. 2005; 47: 89-95.
- Davis S, Mirick DK. Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control. 2006; 17: 539-545.
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst. 2001; 93: 1563-1568.
- Deboer T, Detari L, Meijer JH. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep. 2007; 30: 257-262.
- Kohyama J. A newly proposed disease condition produced by light exposure during night: asynchronization. Brain Dev. 2009; 31: 255-273.
- Borugian MJ, Gallagher RP, Friesen MC, Switzer TF, Aronson KJ. Twentyfour- hour light exposure and melatonin levels among shift workers. J Occup Environ Med. 2005; 47: 1268-1275.
- Claustrat B, Valatx JL, Harthe C, Brun J. Effect of constant light on prolactin and corticosterone rhythms evaluated using a noninvasive urine sampling protocol in the rat. Horm Metab Res. 2008; 40: 398-403.
- Persengiev S, Kanchev L, Vezenkova G. Circadian patterns of melatonin, corticosterone, and progesterone in male rats subjected to chronic stress: effect of constant illumination. J Pineal Res. 1991; 11: 57-62.
- Dumont M, Beaulieu C. Light exposure in the natural environment: relevance to mood and sleep disorders. Sleep Med. 2007; 8: 557-565.
- Van der Meer E, Van Loo PL, Baumans V. Short-term effects of a disturbed light-dark cycle and environmental enrichment on aggression and stressrelated parameters in male mice. Lab Anim. 2004; 38: 376-383.
- Navara KJ, Nelson RJ. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res. 2007; 43: 215- 224.
- Ma WP, Cao J, Tian M, Cui MH, Han HL, Yang YX, et al. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci Res. 2007; 59: 224-230.
- Castro JP, Frussa-Filho R, Fukushiro DF, Chinen CC, Abilio VC, Silva RH. Effects of long-term continuous exposure to light on memory and anxiety in mice. Physiol Behav. 2005; 86: 218-223.
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci. USA. 2007; 104: 6406-6411.
- Pevet P. Melatonin. Dialogues Clin Neurosci. 2002; 4: 57-72.
- Arendt J, Skene DJ, Middleton B, Lockley SW, Deacon S. Efficacy of melatonin treatment in jet lag, shift work, and blindness. J Biol Rhythms. Dec. 1997; 12: 604-617.
- Arendt J. Managing jet lag: Some of the problems and possible new solutions. Sleep Med Rev. 2009; 13: 249-256.
- Arendt J. Shift work: coping with the biological clock. Occup Med (Lond). 2010; 60: 10-20.
- Skene DJ, Arendt J. Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med. 2007; 8: 651-655.
- Emens JS, Burgess HJ. Effect of Light and Melatonin and Other Melatonin Receptor Agonists on Human Circadian Physiology. Sleep Med Clin. 2015; 10: 435-453.
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008; 299: 2642-2655.
- Cardinali DP, Vigo DE, Olivar N, Vidal MF, Brusco LI. Tryptophan Metabolism: Implications for Biological Processes, Health and Disease. 197-238.
- Vriend J, Reiter RJ. Melatonin feedback on clock genes: a theory involving the proteasome. J Pineal Res 2015; 58: 1-11.
- Pfeffer M, Korf HW, Wicht H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen Comp Endocrinol. 2017; S0016-6480.
- Claustrat B, Valatx JL, Harthe C, Brun J. Effect of constant light on prolactin and corticosterone rhythms evaluated using a noninvasive urine sampling protocol in the rat. Horm Metab Res. 2008; 40: 398-403.
- Forsling ML, Stoughton RP, Zhou Y, Kelestimur H, Demaine C. The role of the pineal in the control of the daily patterns of neurohypophysial hormone secretion. J Pineal Res. 1993; 14: 45-51.
- Li SX, Fujita Y, Zhang JC, Ren Q, Ishima T, Wu J, et al. Role of the NMDA receptor in cognitive deficits, anxiety and depressive-like behavior in juvenile and adult mice after neonatal dexamethasone exposure. Neurobiol Dis. 2014; 62: 124-134.
- Onaivi ES, Martin BR. Neuropharmacological and physiological validation of a computer-controlled two-compartment black and white box for the assessment of anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 1989; 13: 963-976.
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003; 463: 55-65.
- Kulesskaya N, Voikar V. Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: role of equipment and procedure. Physiol Behav. 2014; 133: 30-38.
- Xu LZ, Liu LJ, Yuan M, Li SX, Yue XD, Lai JL. Short photoperiod condition increases susceptibility to stress in adolescent male rats. Behav Brain Res. 2016; 300: 38-44.
- Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms. 2012; 27: 319-327.
- Reijmers LG, Leus IE, Burbach JP, Spruijt BM, van Ree JM. Social memory in the rat: circadian variation and effect of circadian rhythm disruption. Physiol Behav. 2001; 72; 305-309.
- Walton JC, Chen Z, Weil ZM, Pyter LM, Travers JB, Nelson RJ. Photoperiodmediated impairment of long-term potention and learning and memory in male white-footed mice. Neuroscience. 2011; 175: 127-132.
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005; 20: 225-236.
- Tapia-Osorio A, Salgado-Delgado R, Angeles-Castellanos M, Escobar C. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav Brain Res. 2013; 252: 1-9.
- Tavernier RJ, Largen AL, Bult-Ito A. Circadian organization of a subarctic rodent, the northern red-backed vole (Clethrionomys rutilus). J Biol Rhythms. 2004; 19: 238-247.
- Chiesa JJ, Cambras T, Carpentieri AR, Diez-Noguera A. Arrhythmic rats after SCN lesions and constant light differ in short time scale regulation of locomotor activity. J Biol Rhythms. 2010; 25: 37-46.
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. 2004; 24: 615-619.
- Chesworth MJ, Cassone VM, Armstrong SM. Effects of daily melatonin injections on activity rhythms of rats in constant light. Am J Physiol. 1987; 253: R101-107.
- Marumoto N, Murakami N, Katayama T, Kuroda H, Murakami T. Effects of daily injections of melatonin on locomotor activity rhythms in rats maintained under constant bright or dim light. Physiol Behav. 1996; 60; 767-773.
- Murakami N, Marumoto N, Nakahara K, Murakami T. Daily injections of melatonin entrain the circadian activity rhythms of nocturnal rats but not diurnal chipmunks. Brain Res. 1997; 775: 240-243.
- Abilio VC, Freitas FM, Dolnikoff MS, Castrucci AM, Frussa-Filho R. Effects of continuous exposure to light on behavioral dopaminergic supersensitivity. Biol Psychiatry. 1999; 45: 1622-1629.
- Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014; 35: 648-670.
- Wideman CH, Murphy HM. Constant light induces alterations in melatonin levels, food intake, feed efficiency, visceral adiposity, and circadian rhythms in rats. Nutr Neurosci. 2009; 12: 233-240.
- Kronfeld-Schor N, Einat H. Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology. 2012; 62: 101- 114.