Review Article
Austin J Pharmacol Ther. 2014;2(1): 1006.
Brain-Derived Neurotrophic Factor for Depression Therapeutics.
Kazuko Sakata *
Department of Pharmacology, University of Tennessee Health Science Center, Memphis, TN, USA
*Corresponding author: : Kazuko Sakata, Department of Pharmacology/Psychiatry, College of Medicine,University of Tennessee Health Science Center, 874 Union Ave, Room 430, Memphis, TN 38163
Received: January 01, 2014; Accepted: January 22, 2014; Published: January 27, 2014;
Abstract
Increasing evidence over the last two decades has indicated that the pathophysiology of major depressive disorder (MDD) and the action of antidepressants both involve brain-derived neurotrophic factor (BDNF), a major neuronal growth factor in the brain. MDD is a complex disorder that results from genetic and environmental influences, singly or in combination. This article reviews the current knowledge of BDNF and depression therapeutics, focusing especially on the gene regulation of BDNF and other BDNF–related mechanisms for recent depression therapeutics, including glutamatergic antidepressants and brain stimulation. It is still unclear why some people are more susceptible to MDD and why many show individual differences in their treatment responses.This article also briefly reviews more recent findings on the epigenetic and genetic status of the BDNF gene in brain and blood, which may explain MDD susceptibility and predict response to depression treatment.
Introduction
Major depressive disorder (MDD) is the leading cause of disability in developed countries (˜350 million people are affected worldwide), with devastating symptoms including depressed mood, loss of interest or pleasure, executive dysfunctions, psychomotor retardation, suicide ideation, and eating and sleep disturbances [1]. However, the current treatment outcome is suboptimal—only one–third of patients show remission after a first–line treatment and only about a half of patients show complete remission following multiple treatments that take several months to years [2]. A more efficacious treatment and preventions are needed to combat MDD and to increase quality of life and reduce the disease burden. It is therefore imperative to understand the mechanisms of this disorder and its recovery.
BDNF and depression
A large body of evidence over the past decade has suggested that the pathophysiology of MDD and its recovery involve gene regulation of brain–derived neurotrophic factor (BDNF) [3–6]. BDNF is a major neuronal growth factor in the brain, which regulates neurogenesis, neuronal maturation and survival, and synaptic plasticity. Low levels of BDNF have been observed in the brain of suicide subjects and depressed patients [7–10], particularly in the regions (i.e., hippocampus, prefrontal cortex, and amygdala) that show atrophy in depressed patients and stressed animals [11–13]. Reduced BDNF levels have been also observed in blood of depressed patients, and these low levels can be reversed following depression treatment [14]. Causal relations between stress and BDNF have been clarified by using rodents; physical stress (acute and chronic immobilization) and corticosterone (a hormone induced by stress) have been shown to decrease BDNF levels in the hippocampus [15]. Negative environmental effects like psychological stress (re–exposure to cue associated with foot shocks [16] and chronic social defeat [17]) and chronic alcohol intake [18] also decrease BDNF levels in the hippocampus. On the other hand, different types of depressiontherapeutics (e.g., antidepressants and brain stimulations, see 3 and 4 below) increase BDNF levels and can reverse the stress–induced BDNF reduction [19]. Direct antidepressant effects of BDNF have been also reported; infusion of BDNF into the hippocampus produced sustained antidepressant–like effects in rodents [20–23]. These findings give hope that increasing the levels of BDNF in the related brain regions and targeting the involved pathways may become a new strategy for the prevention and treatment of MDD.
Gene regulation of BDNF
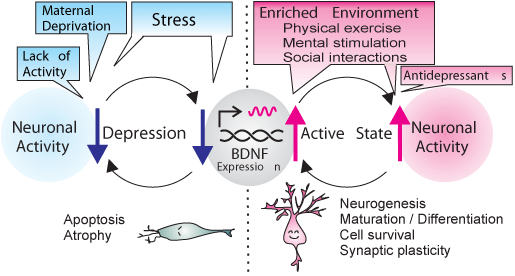
The expression of BDNF is tightly regulated by at least 9 promoters in both humans [24,25] and rodents [26,27]. Each promoter regulates BDNF expression differently in a region/cell–specific manner and has distinct function responding to stress, neuronal activity, and MDD treatments (see [6] for review). Stress reduces the activity of BDNF promoters IV and VI through epigenetic regulation processes that involve increases in histone H3 lysine 27 (H3K27) trimethylation [17,28] (see [29] for detailed epigenetic mechanisms of the BDNF gene). Recent studies have shown that the post–mortem brain of suicidal human subjects also display increased methylation at BDNF promoter/exon IV; this reduces transcription of the Bdnf gene [30]. Further, early–life maltreatment of infants has been reported to increase methylation of the promoter IV–controlled Bdnf DNA (exons IV and IX) and leads to persistent reduction in BDNF expression in the prefrontal cortex in adulthood [31]. Our group recently showed that a lack of promoter IV–driven BDNF [32] leads to depressionlike behavior in mice [33]. Promoter IV is the best–known activitydependent promoter among the known promoters; it responds to neuronal activity to increase BDNF levels [34–36], particularly in the cortex and hippocampus [37,38]. These findings suggest an intriguinghypothesis for critical roles of activity–dependent expression of BDNF in sustaining neuronal activity by a positive feedback mechanism [6]; namely, increased neuronal activity induces activity–dependent BDNF expression, which then induces neuronal activity to maintain active brain functions. Any disruption in the activity–dependentBDNF expression would therefore lead to a decrease in neuronal activity and function, which could in turn lead to depression [Figure 1].
It should be noted that BDNF increases activity/functions of both excitatory and inhibitory neurons. In particular, activity–driven BDNF expression is critical for increasing maturation and functions of the GABAergic inhibitory neurons [32,39–42]. Thus, the activated excitatory neurons likely receive tight inhibition by thenearby GABAergic neurons via the activity–driven BDNF expression. This enhancement of neuronal excitation and inhibition may increase synchronous neuronal activity in a neuronal circuit to control timingdependent signal processing [32]. The enhanced timing–dependentexcitation and inhibition may be critical for flexible learning (e.g., extinction of bad memories and fear) and recovery from MDD [43]. The neural functions of BDNF in the neuronal network including all kinds of neurons remain to be elucidated in the future.
Figure 1: Activity-dependent BDNF hypothesis of depression. Increased neuronal activity, induced by an enriched environment (e.g., physical exercise, mental stimulation with learning exercise and sensory input, and social interactions) and/or medication up-regulates BDNF expression in the hippocampus and cortex. This, in turn, increases neuronal activity and this positive feedback loop can maintain an active mind state. In contrast, any disruption in BDNF expression, caused by epigenetic regulation processes, stress, and/or reduced neuronal activity, would lead to decreases in neuronal activity and activity-driven BDNF expression. This vicious cycle of decreased neuronal activity and reduced BDNF expression may cause depression.Modified and used with permission from [6].
In contrast to stress⁄negative factors, healthy factors such as longterm (4–8 weeks) physical exercise [44–46], learning training [47,48], and being reared in an enriched environment [49,50], all induceexpression of BDNF in the hippocampus and cortex, controlled via its multiple promoters (see [6] for review). For example, physical exercise (4 weeks of running) induces relatively strong BDNF expression through promoters I, II, and III (but not through promoters IV and VI) [50–52], while novel objects (e.g. toys) induces moderate BDNF expression through promoter I, II, III, IV and VI in the hippocampus [52]. Stimuli that induce long–term potentiation (LTP), a form of synaptic plasticity, also induce BDNF mRNA expression in the hippocampus—the brain region important for memory formation, suggesting a role of BDNF in learning [53,54]. The currentlyprescribed monoaminergic antidepressants [e.g., selective serotonin reuptake inhibitors (SSRI), tricyclic antidepressants (TCA), tetracyclic antidepressants, and monoamine oxidase inhibitors treatments (MAOI)] increase monoamine levels in the brain, but direct application of monoamines (serotonin, norepinephrine, dopamine) to hippocampal neurons has failed to induce BDNF expression in an acute phase (3 hrs) [55]. However, chronic (<3 weeks) administration of different classes of monoaminergic antidepressants has been shown to increase Bdnf gene expression through different promoters in the hippocampus and cortex. For example, fluoxetine (SSRI) works on promoter II [56]; phenelzine (MAOI) works on promoters I and VI [56]; duloxetine (a serotonin–norepinephrine reuptake inhibitor, SNRI) works on promoters III and IXa [57]; and imipramine (TCA) works on promoters IV and VI [17]. However, the amount of increase is not robust compared to that seen with neuronal depolarization (see [6] for review).
The recent development of mutant mice that lack promoter IVdriven BDNF but retain intact other promoters and the BDNF coding region (KIV, [32]) has begun to answer some of the questions about the role of endogenous gene regulation of BDNF in antidepressant effects. In our investigations, we have not been able reproduce previous results showing that chronic (3 weeks) treatments with different kinds of antidepressants increased hippocampal BDNF levels in both normal and mutant mice [58]. The previously reported increases in BDNF levels in response to chronic monoaminergic antidepressant treatment may in fact have been a secondary effect due to increased behavioral activity over the time (>3 weeks) induced by the increased monoamine levels. The behavioral activity, and thus the behavioral activity–driven BDNF induction, may be compromised in a certain laboratory setting. In the depression mouse model that lacks promoter IV–driven BDNF, an enriched environment treatment showed a better effect in increasing BDNF levels through multiple promoters and neurogenesis and in reversing depression–like behavior than did treatments with different types of monoaminergic antidepressants [43,50]. These findings indicate that neuronal activity, rather than the monoamine modulation itself, may be a strong inducer of BDNF levels. It should be noted that the chronic treatment with the monoaminergic antidepressants and enriched environment produced antidepressanion–like behavioral effects tested in the tail suspension test in KIV mice [43,50]. This fact indicates that promoter IVdriven BDNF is not required for the preclinical antidepressant–like effects.
Bidirectional roles of BDNF depending on the brain regions: BDNF mRNA levels are abundant in the cortex and hippocampus but are much less in the ventral brain regions except some thalamic nucleus [59], where activity of promoters I, II and VI have been observed [38]. However, the ventral brain regions show moderate BDNF protein expression by anterograde transport of BDNF [60]. The BDNF regulation by stress and its effect in the ventral brain regions are opposite to that in the cortex and hippocampus: Stress increases BDNF protein levels in the nucleus accumbens and BDNF in this region causes depression–like behavior [61, 62]. It remains unclear which promoter is responsible for this stress–induced BDNF increase and how these changes affect the neural network functions in the connected brain regions.
New–lines of depression therapeutics
Antidepressants acting on glutamate receptors
Neuronal activity–driven BDNF induction via glutamate, the major excitatory neurotransmitter, and depolarization has been well studied over the last two decades [55,63,64]. Recently, glutamatergic drugs (including agonists and antagonists) have been shown to have acute antidepressant effects (see reviews [65,66]). Glutamate receptors are classified as ionotropic (i) and metabotropic (m) glutamate receptors (GluRs). The iGluRs include N–methyl–D–aspartate (NMDA) and a–amino–3–hydroxy–5–methyl–4–isoxazolepropionic acid (AMPA) receptors, while mGluRs, which are coupled with ion channels orG–proteins, include the group containing mGluR1 through mGluR8. Interestingly, almost all of these glutamatergic drugs that show antidepressant (–like) effects also increase BDNF levels and act on its signaling pathways [Table 1].
Target
Drug
Major mechanism of action
Antidepressant effects
in human
Antidepressant effects
in rodents
BDNF levels
in human
BDNF levels
in rodents
Reference
NMDAR
Ketamine
Non-competitive antagonist
Yes
Yes
↑
↑
[67-72]
NMDAR
MK-0657
Selective NR2B antagonist
Yes
↑
[73]
NMDAR
Acamprosate
NMDA and mGluR5 antagonist
Inconclusive
Yes
↑
[73-75]
NMDAR
Memantine
Non-competitive low-affinity antagonist
No
↑
[76-78]
AMPAR
Ampakines
Positive allosteric modulator
Yes
Yes
↑
[79-85]
mGluR2/3
LY379268
Agonist
Clinical trials undergoing
Yes ↑ (enhancement with antidepressants)
↑ (with antidepressants)
[86-90]
mGluR2/3
LY341495
Antagonist
Clinical trials undergoing
Yes
↑ (enhancement with DOI)
[91-92]
mGluR5
MPEP
Selective antagonist
Yes
↑ (hippocampus) ↓ (cortex)
[93-96]
Other
Riluzole
Reduces extra-synaptic glutamate by inhibiting presynaptic release and enhancing glial uptake
Yes (preliminary)
↑
[97-99]
Table 1: Glutamatergic drugs, antidepressant effects, and BDNF.
Ketamine, a NMDR receptor antagonist, produces an acute antidepressant effects that lasts for a week in patients with depression when provided at a low dose [67–69]. Ketamine is thought to block NMDA receptors on GABAergic neurons, thereby inhibiting GABA neurons (reducing GABAergic inhibition) and increasing the excitability of glutamatergic neurons [70]. Ketamine rapidly increases BDNF–protein levels without BDNF gene upregulation in the rodent brain, while protein synthesis is necessary for ketamine’s antidepressant–like effects [71]. This protein up–regulation, whilebypassing BDNF gene upregulation, may account for the fast action of ketamine. In a clinical study, ketamine has been also reported to increase plasma BDNF levels in depression patients at 4 hr postinfusion [72]. Interestingly, responders show a greater induction of BDNF than do non–responders [72].
MK–0657, a selective NR2B antagonist, also exerts a relatively acute antidepressant effect in treatment–resistant depression patients within 5 days, while increased plasma BDNF levels are observed after9 days of treatment [73].
Acamprosate, an NMDA and mGluR5 antagonist, has shown antidepressant effects in a preclinical study and promotes increasesin BDNF levels in the serum of human subjects [74]. However, the results of a clinical pilot study are still inconclusive regarding its efficacy as an antidepressant medication [75].
Memantine, another NMDA receptor antagonist, induces BDNF expression in rat brain [76,77]; however, a recent clinical study does not support its antidepressant efficacy [78].
Ampakines, which are drugs that potentiate AMPA receptors, also increase BDNF expression in the hippocampus and prefrontal cortex in rodents [79–83]. Ampakines show antidepressant effects in both rodents (LY392098 [84]) and in depressed patients (Org 26576, [85]).
The mGlu2⁄3 receptor ligands (both agonists and antagonists) involve BDNF and are being tested as adjunctive therapy in patients with major depression [90]. These compounds appear to shorten the latency of antidepressant medication. An mGluR2⁄3 agonist, LY379268, when administered to rodents, produces antidepressantlike behavioral effects when combined with 3 days of fluoxetine and chlorimipramine, although it produces only small antidepressant–like effects on its own [86]. LY379268 acutely increases the amount of Gadd45–β (growth arrest and DNA–damage–inducible beta) that binds to BDNF promoter IX [87]. Gadd45– β increases DNA demethylation. LY379268 also increases BDNF mRNA levels in the cerebral cortex and hippocampus with a peak at 3 h from treatment [88]. Chronic treatment (10 weeks) of LY379268 also increases cortical BDNF levels [89]. An mGluR2⁄3 antagonist, LY341495, also exerts antidepressantlike effects in 30 min and after 24 hr in rodents [91]. Interestingly, the sustained antidepressant–like effect (after 24 hr), but not theacute effect (in 30 min), depends on the activation of BDNF and its receptor TrkB signaling [91]. LY341495 has been also reported to enhance BDNF mRNA induction when combined with 5–dimethoxy– 4–iodoamphetamine (DOI), a serotonin receptor 2 agonist, but it does not induce BDNF levels on its own [92].
A selective mGluR5 antagonist, 2–methyl–6–(phenylethynyl) pyridine (MPEP), produces antidepressant–like effects in rodents ([93,94], see review [95]). Chronic MPEP treatment increases hippocampal but reduces cortical BDNF mRNA levels [96]. Repeated MPEP administration for 12 days reduces mRNA levels of the NMDA receptor NR1 subunit in the forebrain [100]; thus, a finalantidepressant effect of mGlu5 antagonism is proposed to be similar to that evoked by NMDA receptor antagonists [101], such as ketamine[67,68].
Riluzole, another type of glutamatergic modulator, reduces extrasynaptic glutamate by inhibiting presynaptic release and enhancing astroglial uptake. This compound has also shown antidepressant efficacy in clinical pilot studies: Open–label trials in treatmentresistant depression patients have yielded promising results [97,98]. Repeated, but not single, injections of riluzole have been reported to result in prolonged elevation of hippocampal BDNF [99]. The BDNF induction seems paradoxical since riluzole inhibits voltagedependent sodium channels and reduces extra–synaptic glutamate levels, whereas BDNF is induced by neuronal activity and glutamate. One explanation could be that the mechanisms other than neuronal activity⁄glutamate mediate the BDNF induction since riluzole has complex mechanisms of action (e.g., it dose and dependently affects various calcium⁄potassium channels and GABAA receptors [102]). Astudy has shown that the BDNF increase by riluzole requires activation of the p38 mitogen–activated protein kinase via N–type Ca2+ channels and adenosine A1 receptors [103]. It remains unknown whether thereduced neuronal activity and glutamate levels are actually involved in the BDNF induction by riluzole.
An agonist for the BDNF receptor, TrkB
Recently, 7,8–dihydroxyflavone (7,8–DHF) was identified as the first selective TrkB agonist [104]. Chronic oral administration of this compound has been reported to produce antidepressant–like effects in rodents [105], suggesting its future use in the treatment of various disorders, including MDD. A systemic administration of 7,8–DHF in rodents has been shown to enhance extinction of conditioned fear, and particularly strikingly in mice that had previously been stressed [106]. Fear extinction is a BDNF–dependent process, arising particularly within the pathways of hippocampus [107], prefrontal cortex [43,108], and amygdala [109]. Deficits in extinction of conditioned fear have been suggested to underlie aspects of stress disorders including posttraumatic stress disorder (PTSD) [110]. This TrkB agonist shows potential for the treatment of stress disorders including PTSD and MDD. However, some caution should be taken when considering TrkB activation. Previous studies have shown that the antidepressant effects of BDNF are bidirectional and dependent on dorsal or ventral brain regions: a BDNF increase in the dorsal brain regions (e.g., hippocampus and prefrontal cortex) produces antidepressant–like effects [20–23], while an increase in the ventral brain regions (e.g., nucleus accumbens and ventral tegmental area)causes depression [61,62]. Thus, 7,8–DHF may need to be directly targeted into specific brain regions (e.g., hippocampus and prefrontal cortex). The TrkB activation should also be within an endogenous range of TrkB activation in order to avoid desensitization of theendogenous BDNF system due to the exogenous force. Future studies need to investigate the safety (e.g., carcinogenic potential) and efficacy of this TrkB agonist for psychiatric disorders including MDD.
Brain stimulation
Electroconvulsive shock (ECS) therapy is most often used for patients with severe major depression who have not responded to other antidepressant treatments. Both acute (2 h) and chronic (10day) ECS has been shown to increase BDNF mRNA approximately 2– to 3–fold in the hippocampus [19]. Deep brain stimulation is also used for treatment resistant depression, and it also increases BDNF levels in the rat brain [111] and in human serum [112]. Vagal nerve stimulation (VNS) has been approved for treatment resistant depression by the Food and Drug Administration since 2005. VNS given for just three hours increases BDNF mRNA levels in rat hippocampus and cerebral cortex [113], while chronic VNS increases BDNF mRNA levels in the hippocampus [114].
Antidepressant effect acting on TrkB without gene induction of BDNF
Recent findings suggest that treatments for depression may acutely act on the BDNF pathway, but without BDNF gene induction, which takes hours. For example, the rapid antidepressant response seen following ketamine administration is mediated by BDNFprotein induction in the absence of BDNF gene up–regulation [71]. Antidepressant treatments (fluoxetine and imipramine) rapidly increase phosphorylation of the BDNF receptor, TrkB, in the PFC and hippocampus within 30–60 min of drug administration; these processes occur without BDNF induction [115,116]. Increased intracellular cAMP and membrane depolarization are known to rapidly increase the incorporation of TrkB into the neuronal plasmamembrane [117]. Moreover, both acute and chronic VNS also elevate phosphorylation of TrkB at tyrosines 515, 705, and 816 in the hippocampus, while traditional antidepressants (fluoxetine or desipramine) elevate phosphorylation of TrkB at tyrosines 705 and 816, but not at tyrosine 515 [118]. These TrkB phosphorylations induced by antidepressants and VNS activate phospholipase–Cγ signaling and lead to the phosphorylations of CREB [116,119] and Akt⁄ERK [118]. These phosphorylations can then induce transcription of genes involved in neurogenesis and neuronal growth, including induction of other growth factors as well as BDNF itself [120], thereby producing positive neurotrophic effects.
BDNF mRNA trafficking and local protein synthesis
The acute effects of antidepressants and VNS in increasing BDNF protein levels and TrkB phosphorylation may involve local protein synthesis and release of BDNF. The Bdnf gene has two polyadenylation sites that lead to either a short or long 3’ untranslated region (UTR). The long 3’–UTR, which is mainly localized in dendrites [121] and stabilized by neuronal activity [122], is involved in rapid activitydependent translation of the BDNF protein [123]. A single–nucleotide polymorphism (SNP) in the BDNF gene can create a difference in the local protein synthesis and release of BDNF. One of the best known of these SNPs is an amino acid substitution of valine (Val) to methionine (Met) at codon 66 (Val66Met) in the proBDNF protein. This BNDF Val66Met SNP influences the function of BDNF by reducing trafficking and activity–dependent secretion of BNDF protein in the brain [124–127]. These changes in BDNF mRNA trafficking and local protein synthesis may affect the activity–dependent remodeling of spine⁄dendrite structure and synaptic plasticity, and thus affect the response to depression treatments (see [4,11, 13,128–130] for review).
Individual differences in treatment responses due to epigenetic and genetic status
Recent studies have shown that different epigenetic and genetic statuses of the BDNF gene may account for variations in MDD treatment responses. Decreased methylation of H3K27 at BDNF promoter⁄exon IV (which increases expression of BDNF exon IV) in the postmortem prefrontal cortex has been observed in depressed subjects with a history of antidepressant treatment [131]. Only responders to chronic antidepressant treatment (8 weeks of citalopram), but not non–responders, showed decreased methylation of H3K27 at promoter⁄exon IV and increased BDNF exon IV mRNA levels in blood cells [132]. These findings suggest that the histone methylation at promoter⁄exon IV may be a biomarker of treatment response.
Another study by Perroud et al. recently reported that the presence of DNA methylation in CpG islands in promoter IV⁄exon IV in the blood cells can also predict the treatment response in borderline personality disorder [133]. They showed that 4 weeks of intensive dialectical behavior therapy decreased the BDNF methylation status (which increases BDNF transcription) in responders, but increased it in nonresponders [133]. They also found a relationship between child maltreatment and higher methylation of BDNF DNA, but found no orrelation between the BDNF DNA methylation levels and serum BDNF protein levels [133]. On the other hand, other studies have shown that a failure of BDNF to increase in serum [134] or plasma [135] during the first week of antidepressant treatment predicts the final non–response and non–remission with high sensitivity, uggesting that early changes in peripheral BDNF may constitute or reflect a necessary prerequisite for final treatment response.
Tadic et al. reported that major MDD patients showing hypomethylation of the BDNF promoter region (at CpG site –87 of exon IV) are unlikely to benefit from antidepressant pharmacotherapy, and that they show a decrease in plasma BDNF levels during the first week of treatment [136]. These studies suggest that measuring BDNF RNA levels and its methylation status in the blood cells may serve as a biomarker of depression recovery and response to antidepressants.
In addition to the epigenetic regulation of the BDNF promoters, studies have indicated that the differences in treatment effects for MDD can also depend on the polymorphisms of the BDNF gene. The well–studied Val66Met SNP (Met allele) causes reductions in dendritic trafficking and activity–dependent secretion of BDNF[125–127]. A recent study showed that the BDNF Met allele in mice caused a blockage of synaptogenic and antidepressant actions of ketamine, suggesting that the therapeutic response to this drug might be attenuated or blocked in depressed patients who carry the loss of function Met allele [137]. However, a meta–analysis did not reveal any BDNF Val66Met polymorphism associated with treatment response in patients with MDD [138]. Another study reported the opposite esult, where BDNF Met carriers showed a higher remission rate for geriatric depression than was seen for BDNF (Val⁄Val) homozygotes [139].
Studies on the MDD risk of the Val66Met SNP in human subjects have produced inconsistent results, which may reflect factors such as the size and ethnicity of the studied populations [140–142] (see review [6]). In addition to the Val66Met SNP, recent studies have revealed new SNPs in the Bdnf gene: Six SNPs and two haplotypes (one including Val66Met, another near exon VIIIh) are associated with MDD andeight SNPs are associated with response to antidepressant treatment [143]. The intronic variants 5’–upstream of the BDNF coding region located near exons VIIh and V show the most significant effects in MDD and antidepressant response, respectively [143]. Further, another SNP (rs12273363) in the upstream of the Bdnf gene has been associated with MDD susceptibility in patients with a history of childhood adversity [144], and that this SNP was recently found to reduce promoter IV activity [145]. In addition, novel SNPs in the DNF promoters I, –281A and G–712A, have been reported to protect gainst anxiety or are associated with substance abuse [146,147]. Promoter I is another activity–dependent promoter [37,148] and a recent study has also reported changes in its DNA methylation profiles in the blood cells of MDD patients [149]. Future studies remain to elucidate the effect of these SNPs on the Bdnf gene regulation in the brain and on MDD treatment responses
A combination of several independent risk alleles within the TrkB gene has also been associated with suicide attempts among patients with MDD [150]. These findings suggest that the individual differences in the BDNF–TrkB pathway due to epigenetic⁄genetic status may contribute to the risk of MDD and to the differences in treatment response.
Conclusions
Stress reduces BDNF levels via epigenetic regulations. On the other hand, most of the currently used therapeutics for MDD (e.g., reducing stress, antidepressant treatments, introducing exercise and enriched environments, and brain stimulation) increase BDNF levels. Drugs that can target the mechanisms that induce BDNF and activate BDNF–TrkB signaling (e.g., increasing BDNF promoter activity, transcription stability, trafficking, activity–driven release, TrkB agonists, drugs that increase TrkB phosphorylation and activate PI3⁄Akt⁄ERK, etc.) may therefore become potent antidepressant treatments. Further understanding of the BDNF mechanisms will provide information regarding the potential targets for novel drugs and other interventions for combating MDD. In particular, the mechanisms that explain how individual differences in epigenetic condition and SNPs in the Bdnf gene affect the BDNF mechanisms (transcription, translation, trafficking, secretion, receptor activation, etc.) may help to develop individualized treatment of MDD. We still do not understand why some people are more susceptible to MDD hile others better tolerate the same stress, and why so many patients fail to respond to current treatments. Specific epigenetic and genetic factors may act complementarily, and further clarification of this stress⁄treatment x genetic⁄epigenetic interaction may provide the required insight for prediction of MDD risks and MDD treatment responses, thereby leading to effective individualized prevention and treatment of MDD. Knowledge obtained from preclinical and clinicalstudies will be critical for further advancement of our understanding of the role of BDNF in MDD treatment. In addition, elucidating BDNF mechanisms in blood and monitoring blood BDNF levels may become useful for developing indicators⁄predictors of depression recovery.
References
- WHO. https://www.who.int/mental_health/management/depression/definition/en/. 2009
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006; 163: 1905-1917.
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006; 59: 1116-1127.
- Castren E. Is mood chemistry? Nat Rev Neurosci. 2005; 6: 241-246.
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of Depression. Neuron. 2002 2002/3/28; 34: 13-25.
- Sakata K. Brain Derived Neurotrophic Factor and Major Depression. Neurobiology of Depression, Editor Francisco López-Muñoz, Frontiers in Neuroscience. 2011; Chapter 19, 391-417.
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003; 60: 804-815.
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011; 36: 195-203.
- Dunham JS, Deakin JF, Miyajima F, Payton A, Toro CT. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. J Psychiatr Res. 2009; 43: 1175-1184.
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012; 17: 1130-1142.
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001; 16: S7-S19.
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011; 62: 431-445.
- Licznerski P, Duman RS. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013; 251: 33-50.
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008; 64: 527-532.
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. Journal of Neuroscience. 1995; 15: 1768-1777.
- Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002; 27: 133-142.
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006; 9: 519-525.
- MacLennan AJ, Lee N, Walker DW. Chronic ethanol administration decreases brain-derived neurotrophic factor gene expression in the rat hippocampus. Neurosci Lett. 1995; 197: 105-108.
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995; 15: 7539-7547.
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002; 22: 3251-3261.
- Deltheil T, Tanaka K, Reperant C, Hen R, David DJ, et al. Synergistic neurochemical and behavioural effects of acute intrahippocampal injection of brain-derived neurotrophic factor and antidepressants in adult mice. Int J Neuropsychopharmacol. 2009; 12: 905-915.
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005; 1037: 204-208.
- Naumenko VS, Kondaurova EM, Bazovkina DV, Tsybko AS, Tikhonova MA, et al. Effect of brain-derived neurotrophic factor on behavior and key members of the brain serotonin system in genetically predisposed to behavioral disorders mouse strains. Neuroscience. 2012; 214: 59-67.
- Liu QR, Walther D, Drgon T, Polesskaya O, Lesnick TG, et al. Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson's Disease. Am J Med Genet B Neuropsychiatr Genet. 2005; 134B: 93-103.
- Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007; 90: 397-406.
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, et al. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006; 1067: 1-12.
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007; 85: 525-535.
- Fuchikami M, Morinobu S, Kurata A, Yamamoto S, Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int J Neuropsychopharmacol. 2009; 12: 73-82.
- Karpova NN. Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology. 2014; 76 Pt C: 709-718.
- Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, et al. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry. 2010; 67: 258-267.
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009; 65: 760-769.
- Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, et al. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2009; 106: 5942-5947.
- Sakata K, Jin L, Jha S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 2010; 9: 712-721.
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993; 10: 475-489.
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998; 20: 709-726.
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998; 20: 727-740.
- Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci U S A. 1993; 90: 8802-8806.
- Malkovska I, Kernie SG, Parada LF. Differential expression of the four untranslated BDNF exons in the adult mouse brain. J Neurosci Res. 2006; 83: 211-221.
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994; 76: 989-999.
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, et al. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002; 22: 7580-7585.
- Kuczewski N, Langlois A, Fiorentino H, Bonnet S, Marissal T, et al. Spontaneous glutamatergic activity induces a BDNF-dependent potentiation of GABAergic synapses in the newborn rat hippocampus. J Physiol. 2008; 586: 5119-5128.
- Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, et al. A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc Natl Acad Sci U S A. 2011; 108: 12131-12136.
- Sakata K, Martinowich K, Woo NH, Schloesser RJ, Jimenez DV, et al. Role of activity-dependent BDNF expression in hippocampal-prefrontal cortical regulation of behavioral perseverance. Proc Natl Acad Sci U S A. 2013; 110: 15103-15108.
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995; 373: 109.
- Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, et al. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington's disease mice. Hippocampus. 2009; 20: 621-636.
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004; 363: 43-48.
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998; 112: 1012-1019.
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000; 3: 533-535.
- Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, et al. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci Lett. 1992; 138: 153-156.
- Jha S, Dong B, Sakata K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl Psychiatry. 2011; 1: e40.
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000; 101: 305-312.
- Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, et al. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington's disease mice. Hippocampus. 2009.
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992; 9: 1081-1088.
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996; 16: 1137-1145.
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990; 9: 3545-3550.
- Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006; 139: 1017-1029.
- Molteni R, Calabrese F, Cattaneo A, Mancini M, Gennarelli M, et al. Acute stress responsiveness of the neurotrophin BDNF in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology. 2009; 34: 1523-1532.
- Sakata K, Mastin JR, Duke SM, Vail MG, Overacre AE, et al. Effects of antidepressant treatment on mice lacking brain-derived neurotrophic factor expression through promoter IV. Eur J Neurosci. 2013; 37: 1863-1874.
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990; 9: 2459-2464.
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997; 17: 2295-2313.
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM E, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003; 54: 994-1005.
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006; 311: 864-868.
- Lindefors N, Ballarin M, Ernfors P, Falkenberg T, Persson H. Stimulation of glutamate receptors increases expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Ann N Y Acad Sci. 1992; 648: 296-299.
- Zafra F, Castren E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A. 1991; 88: 10037-10041.
- Hashimoto K. The role of glutamate on the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2011; 35: 1558-1568.
- Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013; 9: 1101-1112.
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000; 47: 351-354.
- Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006; 63: 856-864.
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009; 66: 522-526.
- Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol. 2008; 100: 959-965.
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011; 475: 91-95.
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 1-6.
- Ibrahim L, Diaz Granados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, et al. A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012; 32: 551-557.
- Erickson CA, Wink LK, Ray B, Early MC, Stiegelmeyer E, et al. Impact of acamprosate on behavior and brain-derived neurotrophic factor: an open-label study in youth with fragile X syndrome. Psychopharmacology (Berl). 2013; 228: 75-84.
- Witte J, Bentley K, Evins AE, Clain AJ, Baer L, et al. A randomized, controlled, pilot study of acamprosate added to escitalopram in adults with major depressive disorder and alcohol use disorder. J Clin Psychopharmacol. 2012; 32: 787-796.
- Marvanova M, Lakso M, Pirhonen J, Nawa H, Wong G, et al. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol Cell Neurosci. 2001; 18: 247-258.
- Reus GZ, Abelaira HM, Stringari RB, Fries GR, Kapczinski F, et al. Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats. Metab Brain Dis. 2012; 27: 175-182.
- Smith EG, Deligiannidis KM, Ulbricht CM, Landolin CS, Patel JK, et al. Antidepressant Augmentation Using the N-Methyl-d-Aspartate Antagonist Memantine: A Randomized, Double-Blind, Placebo-Controlled Trial. J Clin Psychiatry. 2013; 74: 966-973.
- Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999; 397: 72-76.
- Mackowiak M, O'Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology. 2002; 43: 1-10.
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000; 20: 8-21.
- Legutko B, Li X, Skolnick P. Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology. 2001; 40: 1019-1027.
- Fumagalli F, Calabrese F, Luoni A, Shahid M, Racagni G, et al. The AMPA receptor potentiator Org 26576 modulates stress-induced transcription of BDNF isoforms in rat hippocampus. Pharmacol Res. 2011; 65: 176-181
- Farley S, Apazoglou K, Witkin JM, Giros B, Tzavara ET. Antidepressant-like effects of an AMPA receptor potentiator under a chronic mild stress paradigm. Int J Neuropsychopharmacol. 2010; 13: 1207-1218
- Nations KR, Dogterom P, Bursi R, Schipper J, Greenwald S, et al. Examination of Org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: an exploratory, randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2012; 26: 1525-1539.
- Matrisciano F, Panaccione I, Zusso M, Giusti P, Tatarelli R, Iacovelli et al. Group-II metabotropic glutamate receptor ligands as adjunctive drugs in the treatment of depression: a new strategy to shorten the latency of antidepressant medication? Mol Psychiatry. 2007; 12: 704-706.
- Matrisciano F, Dong E, Gavin DP, Nicoletti F, Guidotti A. Activation of group II metabotropic glutamate receptors promotes DNA demethylation in the mouse brain. Mol Pharmacol. 2011; 80: 174-182.
- Di Liberto V, Bonomo A, Frinchi M, Belluardo N, Mudo G. Group II metabotropic glutamate receptor activation by agonist LY379268 treatment increases the expression of brain derived neurotrophic factor in the mouse brain. Neuroscience. 2010; 165: 863-873.
- Reiner A, Wang HB, Del Mar N, Sakata K, Yoo W, et al. BDNF may play a differential role in the protective effect of the mGluR2/3 agonist LY379268 on striatal projection neurons in R6/2 Huntington's disease mice. Brain Res. 2012; 1473: 161-172.
- https://www.clinicaltrials.gov/ct2/show/NCT01457677%3Fterm%3Dro4995819%26rank%3D2.
- Koike H, Fukumoto K, Iijima M, Chaki S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res. 2010; 238: 48-52.
- Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ. Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol Biochem Behav. 2002; 73: 317-326.
- Liu CY, Jiang XX, Zhu YH, Wei DN. Metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine produces antidepressant effects in rats: role of brain-derived neurotrophic factor. Neuroscience. 2012; 223: 219-224.
- Iijima M, Fukumoto K, Chaki S. Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behav Brain Res. 2012; 235: 287-292.
- Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007; 115: 116-147.
- Legutko B, Szewczyk B, Pomierny-Chamiolo L, Nowak G, Pilc A. Effect of MPEP treatment on brain-derived neurotrophic factor gene expression. Pharmacol Rep. 2006; 58: 427-430.
- Mathew SJ, Keegan K, Smith L. Glutamate modulators as novel interventions for mood disorders. Rev Bras Psiquiatr. 2005; 27: 243-248.
- Zarate CA, Manji HK. Riluzole in psychiatry: a systematic review of the literature. Expert Opin Drug Metab Toxicol. 2008; 4: 1223-1234.
- Katoh-Semba R, Asano T, Ueda H, Morishita R, Takeuchi IK, et al. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 2002; 16: 1328-1330.
- Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005; 315: 590-600.
- Skolnick P. Antidepressants for the new millennium. Eur J Pharmacol. 1999; 375: 31-40.
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther. 2010; 17: 4-31.
- Katoh-Semba R, Kaneko R, Kitajima S, Tsuzuki M, Ichisaka S, et al. Activation of p38 mitogen-activated protein kinase is required for in vivo brain-derived neurotrophic factor production in the rat hippocampus. Neuroscience. 2009; 163: 352-361.
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010; 107: 2687-2692.
- Liu X, Chan CB, Qi Q, Xiao G, Luo HR, et al. Optimization of a small tropomyosin-related kinase B (TrkB) agonist 7,8-dihydroxyflavone active in mouse models of depression. J Med Chem. 2012; 55: 8524-8537.
- Andero R, Heldt SA, Ye K, Liu X, Armario A, et al. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011; 168: 163-172.
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007; 12: 656-670.
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010; 328: 1288-1290.
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006; 9: 870-872.
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007; 164: 1684-1692.
- Friedman A, Frankel M, Flaumenhaft Y, Merenlender A, Pinhasov A, et al. Programmed acute electrical stimulation of ventral tegmental area alleviates depressive-like behavior. Neuropsychopharmacology. 2009; 34: 1057-1066.
- Hoyer C, Kranaster L, Sartorius A, Hellweg R, Gass P. Long-term course of brain-derived neurotrophic factor serum levels in a patient treated with deep brain stimulation of the lateral habenula. Neuropsychobiology. 65: 147-152.
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007; 1179: 28-34.
- Biggio F, Gorini G, Utzeri C, Olla P, Marrosu F, et al. Chronic vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. Int J Neuropsychopharmacol. 2009; 12: 1209-1221.
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003; 23: 349-357.
- Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007; 32: 2152-2162.
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998; 21: 681-693.
- Furmaga H, Carreno FR, Frazer A. Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PLoS One. 2012; 7: e34844.
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007; 7: 18-21.
- Yasuda M, Fukuchi M, Tabuchi A, Kawahara M, Tsuneki H, et al. Robust stimulation of TrkB induces delayed increases in BDNF and Arc mRNA expressions in cultured rat cortical neurons via distinct mechanisms. J Neurochem. 2007; 103: 626-636.
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, et al. Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008; 134: 175-187.
- Fukuchi M, Tsuda M. Involvement of the 3'-untranslated region of the brain-derived neurotrophic factor gene in activity-dependent mRNA stabilization. J Neurochem. 2010; 155: 1222-1233.
- Lau AG, Irier HA, Gu J, Tian D, Ku L, et al. Distinct 3'UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc Natl Acad Sci U S A. 2010; 107: 15945-15950.
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006; 314: 140-143.
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003; 112: 257-269.
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004; 24: 4401-4411.
- Chiaruttini C, Vicario A, Li Z, Baj G, Braiuca P, et al. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc Natl Acad Sci U S A. 2009; 106: 16481-16486.
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005; 76: 99-125.
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013; 14: 7-23.
- Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2013; 76 Pt C: 639-656.
- Chen ES, Ernst C, Turecki G. The epigenetic effects of antidepressant treatment on human prefrontal cortex BDNF expression. Int J Neuropsychopharmacol. 2011; 14: 427-429.
- 132. Lopez JP, Mamdani F, Labonte B, Beaulieu MM, Yang JP, et al. Epigenetic regulation of BDNF expression according to antidepressant response. Mol Psychiatry. 2013; 18: 398-399.
- Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli ME, et al. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl Psychiatry. 2013; 3: e207.
- Tadic A, Wagner S, Schlicht KF, Peetz D, Borysenko L, et al. The early non-increase of serum BDNF predicts failure of antidepressant treatment in patients with major depression: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2011; 35: 415-420.
- Dreimuller N, Schlicht KF, Wagner S, Peetz D, Borysenko L, et al. Early reactions of brain-derived neurotrophic factor in plasma (pBDNF) and outcome to acute antidepressant treatment in patients with Major Depression. Neuropharmacology. 2012; 62: 264-269.
- Tadic A, Muller-Engling L, Schlicht KF, Kotsiari A, Dreimuller N, et al. Methylation of the promoter of brain-derived neurotrophic factor exon IV and antidepressant response in major depression. Mol Psychiatry. 2013.
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, et al. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012; 71: 996-1005.
- Zou YF, Ye DQ, Feng XL, Su H, Pan FM, et al. Meta-analysis of BDNF Val66Met polymorphism association with treatment response in patients with major depressive disorder. Eur Neuropsychopharmacol. 2010; 20: 535-544.
- Alexopoulos GS, Glatt CE, Hoptman MJ, Kanellopoulos D, Murphy CF, et al. BDNF val66met polymorphism, white matter abnormalities and remission of geriatric depression. J Affect Disord. 2010; 125: 262-268.
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002; 7: 579-593.
- Tsai SJ, Cheng CY, Yu YW, Chen TJ, Hong CJ. Association study of a brain-derived neurotrophic-factor genetic polymorphism and major depressive disorders, symptomatology, and antidepressant response. Am J Med Genet B Neuropsychiatr Genet. 2003; 123B: 19-22.
- Iga J, Ueno S, Yamauchi K, Numata S, Tayoshi-Shibuya S, et al. The Val66Met polymorphism of the brain-derived neurotrophic factor gene is associated with psychotic feature and suicidal behavior in Japanese major depressive patients. Am J Med Genet B Neuropsychiatr Genet. 2007; 144B: 1003-1006.
- Licinio J, Dong C, Wong ML. Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. Arch Gen Psychiatry. 2009; 66: 488-497.
- Juhasz G, Dunham JS, McKie S, Thomas E, Downey D, et al. The CREB1-BDNF-NTRK2 pathway in depression: multiple gene-cognition-environment interactions. Biol Psychiatry. 2011; 69: 762-771.
- Hing B, Davidson S, Lear M, Breen G, Quinn J, et al. A polymorphism associated with depressive disorders differentially regulates brain derived neurotrophic factor promoter IV activity. Biol Psychiatry. 2012; 71: 618-626.
- Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, et al. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005; 30: 1353-1361.
- Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, et al. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer's disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006; 141B: 387-393.
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, et al. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem. 2000; 275: 17269-17275.
- Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, et al. DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One. 2011; 6: e23881.
- Kohli MA, Salyakina D, Pfennig A, Lucae S, Horstmann S, et al. Association of genetic variants in the neurotrophic receptor-encoding gene NTRK2 and a lifetime history of suicide attempts in depressed patients. Arch Gen Psychiatry. 2010; 67: 348-359.