
Special Issue - Antiphospholipid Syndrome
Austin J Clin Immunol. 2024; 10(1): 1061.
Pregnancy Complications and Therapy in Women with Antiphospholipid Syndrome (APS)
Ilias Pessach¹*; Emmanouil Kalampokas²; Theodoros Kalampokas³
¹Hematology Dept, Athens Medical Center, Athens, Greece
²Unit of Gynecologic Oncology, Second Department of Obstetrics and Gynecology, Aretaieion Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
³Unit of Obstetrics and Gynecology, Second Department of Obstetrics and Gynecology, Aretaieion Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
*Corresponding author: Ilias Pessach Hematology Dept, Athens Medical Center, Athens, Greece. Email: [email protected]
Received: December 01, 2023 Accepted: January 08, 2024 Published: January 15, 2024
Abstract
Women with antiphospholipid antibodies are at risk of unsuccessful pregnancy outcome, which may include many features such as preeclampsia, premature delivery, intrauterine growth restriction. The risk of thrombosis is higher during the third semester. The standard care in obstetric antiphospholipid syndrome includes aspirin and heparin, which have improved live births significantly, however one small percentage continue to have pregnancy complications. There are many new treatment approaches, but more randomized clinical trials are needed to assess the efficacy and safety. This review aims to highlight the risk factors associating the anti-antiphospholipid antibodies in pregnancy, as well the most severe form which is the catastrophic anti-phospholipid syndrome, and therapies currently available, but future directions through personalized new treatments for different aPL profiles, especially high-risk women with comorbidities. Novel promising drugs, with no complications, according to few case reports, are being identified, but we need time to evaluate the results.
Keywords: Antiphospholipid antibodies (aPL); Antiphospholipid syndrome (APS); Catastrophic anti-phospholipid syndrome (CAPS); Autoimmune disease; Pregnancy morbidity
Introduction
Antiphospholipid Syndrome (APS) is an autoimmune disorder characterized by the presence of heterogeneous antiphospholipid antibodies [aPL: Lupus Anticoagulants (LA), Anticardiolipin antibodies (aCL), antibodies against β2-glycoprotein-I (anti-β2GPI]. APS may be a contributory factor in 6.1% of cases of pregnancy morbidity [1]. Anti-β2GPIs are often referred as an important cause of APS. The phospholipid-binding β2-glycoprotein I (β2GPI) is the main autoantigen in APS. In fact, IgG antibodies targeting β2GPI (ab2GPI) is directly involved in thrombosis and pregnancy morbidity in several mouse models [2]. aPL can create a thrombogenic environment through several mechanisms involving various cell lines and clotting factors. One mechanism can be enhancing the production of Tissue Factor (TF) by activated monocytes and endothelial cells. Interference with natural regulators such as protein C, antithrombin, and tissue plasminogen activator, increases the production of von Willebrand factor by endothelial cells, and promotes platelet activation. Platelets have dynamic role in arterial thrombosis, and in APS specifically (Figure 1) [3]. Antibodies reacting with β2 glycoprotein I (β2GPI) bind to the surface of stimulated platelets triggering platelet activation [4], which can lead to the endothelium activation, thus following the fibrin generation, making platelet activation a primary mechanism of aPL-mediated arterial thrombosis [5]. Clinical manifestations of APS include thrombosis, obstetrical complications, but there are also the “noncriteria” manifestations [6], which includes thrombocytopenia and many other features, making the APS pathology understanding and therapy investigation very challenging, especially in pregnancy.
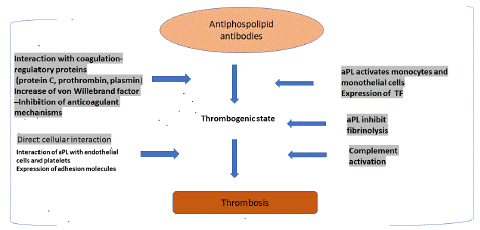
Figure 1: Antiphospholipid antibodies (aPL) prothrombotic activity. aPLs can have a direct effect on endothelial cells and platelets, interact with different coagulation cascade proteins, reduce fibrinolytic activity, and activate complement . These mechanisms contribute to the thrombotic events investigated in APS. aPLs:antiphospholipid antibodies; TF: tissue factor.
Pregnancy Complications and Treatment
APS may potentially cause adverse pregnancy outcomes. Morbidity in pregnancy associated with APS, includes the unexplained death of one or more morphologically normal foetuses at/or beyond the 10th week of gestation, the premature birth of one or more morphologically normal neonates before the 34th week of gestation due to eclampsia or severe preeclampsia, and/or three or more unexplained, consecutive spontaneous abortions before the 10th week of gestation [7]. Placental insufficiency has some features, one of which is Fetal Growth Restriction (FGR). FGR is the result of a complex of conditions such as maternal, fetal and placental ones, which results in severe perinatal mortality and morbidity [8]. A study estimated that one- fourth of pregnancies reaching 10 weeks of gestation in persistently aPL positive patients may result in pregnancy morbidity, independent of aPL-related history or treatment strategy [9]. A meta-analysis which included 22 studies (participation of 11745 pregnant women) showed that aPL positivity increased the risk of FGR and the risk of FGR varies with the aPL types [10]. ACA and β2GP1 are strongly associated with FGR. Therefore, it is necessary to measure aPL when FGR occurs. Women who have experienced adverse pregnancy outcomes, particularly severe FGR, should be screened for the presence of aPL.
Risk factors for poor pregnancy outcome can be divided into ‘’early risk factors’’ (presence of SLE / autoimmune diseases, history of pregnancy loss, thrombosis, triple aPL-positivity, double aPL-positivity) and ‘’late risk factors’’ (factors detected during pregnancy) [11]. The Obstetric monitoring during pregnancy is important to establish viability of the embryo. Human chorionic gonadotropin (hCG) titters during the first semester, which is anticipated to be increased regularly define a successful pregnancy [12]. The risk of thrombosis is higher during the third semester, compared to the first trimester and even higher during the early postpartum period [13]. Some major risk factors include previous history of thrombosis, systemic lupus erythematosus, thrombophilia, APS, heart disease, obesity, diabetes, smoking, immobility during the antepartum period, pre-eclampsia with foetal growth restriction, blood transfusion, postpartum haemorrhage ≥1,000mL with surgery and postpartum infection [14,15]. The presence of underlying autoimmune disease in general, is the additional risk, which demonstrates a significant association with APS pregnancy morbidity [16]. aPL positivity increases the risk of FGR, in fact, the risk of FGR varies with the aPL antibody type [10]. Maternal clinical manifestations vary from abdominal pain, altered mental status, seizure, general malaise, chest pain, hypertension, proteinuria, dyspnoea and others [17]. Diagnosis and treatment may be delayed as well, due to the acute fatty liver of pregnancy (AFLP) or Thrombotic Thrombocytopenic Purpura (TTP) [18,19].
Laboratory risk factors such that should be investigated, may be: persistent LA positivity alone or any combination of LA with IgG/IgM aCL antibodies or IgG/IgM anti-β2 glycoprotein I antibodies and triple aPL positivity (LA + IgG/IgM aCL antibodies + IgG/IgM anti-β2GPI antibodies) [20,21]. In the published research works so far, we could find how refractory and/or high-risk pregnancies may be treated with additional treatment protocol in association with the standard of care or observational or interventional studies with treatment of refractory and/or high-risk pregnancies treated with additional treatment protocols. It is of major importance to identify risk factors and start additional treatment protocols from the very beginning of the pregnancy. In 2007, El Hayeg et al., first describe the additional treatment combined with standard of care, used to prevent pregnancy failure in refractory APS pregnancies [22]. Eighteen pregnancies were treated, from 7.08±0.6 SD weeks of gestation, with prednisone 10 mg/daily + PEX for three sessions/week until the LAC activity was suppressed and IgG aCL lowered. 100% live birth was reporter, and these pregnancy complications were observed: mild pre-eclampsia in 5.5%, IUGR in 11.1%, preterm deliveries in 22.2%, and oligoidramnios and fetal distress in 16.6% Hydroxychloroquine (HCQ) is a promising drug in APS pregnancy yet results from ongoing studies need to be published [23]. In vitro studies show that HCQ reverses the aPL inhibition of trophoblast interleukin-6 secretion, increasing aPL inhibition of cell migration, and promotes trophoblast differentiation affected by anti-β2GP1 antibodies. These mechanisms are involved in the inhibition of syncytialization, an important process for syncytiotrophoblast renewal, negatively affecting the invasion of the maternal spiral arteries, a reason for early miscarriages [24]. HCQ is beneficial in women with aPL, as the treatment with HCQ is associated with a higher rate of live births (67 vs. 57%, p ¼ 0.05) and a lower prevalence of aPL-related pregnancy morbidity (47 vs. 63%, p ¼ 0.004) [23]. Interestingly, the HCQ at a dose of 400 mg is more efficacious than a lower dose of 200 mg [25], furthermore, when initiated during conception is associated with an increase in the live birth rate than when started during pregnancy and it is less effective in patients with thrombosis than in those without, for pregnancy loss prevention [26]. Hydroxychloroquine alone or in association with LDS has been used as an additional treatment to prevent pregnancy losses in refractory APS pregnancies. Ruffatti et al., showed a live birth of 95% in pregnancies treated with HCQ 200–400 mg alone and 55.5% in those treated with HCQ 200–400 mg + LDS in addition to the standard of care. Severe pregnancy complications were registered in 5 and 55.5% of those treated with HCQ 200–400 mg alone and HCQ 200–400 mg + LDS in addition to the standard of care [26]. The use of IVIG ≤2 gr/kg/ monthly alone or in addition to HCQ and LDS, although in a low number of patients treated, has a 100% of live birth rate, but high number of severe pregnancy complications, with none a live birth in women treated with standard of care, even when associated with HCQ [26,27].
A damaged trophoblast invasion with aPL-altering expression of adhesion molecules (placental growth factor or vascular endothelial growth factor), followed by a coagulation cascade activation, are responsible for late pregnancy loss and preeclampsia [28,29].
Another monoclonal antibody, Eculizumab, can prevent the rapid deterioration of clinical conditions in CAPS during pregnancy [30]. Other new treatments, such as 1N11, a fully human antibody that disrupts aPL recognition of b2-GPI, may be an anti-b2-GPI monoclonal antibody as a therapy [31]. In general, it is not recommended primary thrombo-prophylaxis for patients positive for aPL and no history of thrombosis. There is no investigation on the risk of the first thromboembolism and the protective role from LDA or anticoagulants in this patient group. Studies on asymptomatic aPL-positive patients has showed that LDA did not prevent thrombosis and do not reduce the risk a of thromboembolism [20,32]. Low Molecular Weight Heparin (LMWH) should be considered for thrombosis prevention in these patients during high-risk situations, such as the postpartum period [33]. Treatment options can include no treatment, LDA alone, or LDA (Low Dose Aspirin) and a prophylactic dose of LMWH [34]. aPL is highly predictive of pregnancy loss in animal models [35], so it is recommended that pregnant women who are positive for aPL to be treated with LDA [36] and LDA is generally recommended during pregnancy or at the beginning of attempting pregnancy, especially in women at high risk of pre-eclampsia [37]. Women with aPL may benefit from treatment with HCQ during pregnancy to improve pregnancy outcome. Through a retrospective study, one-hundred and seventy pregnancies in 96 women with persistent aPL were investigated, it was found that HCQ-treatment was associated with a higher rate of live births and a lower prevalence of aPL-related pregnancy morbidity [23]. The addition of first-trimester low-dose prednisolone to conventional treatment improved the rate of live births in refractory aPL-related first trimester pregnancy loss(es). After treatment with low-dose prednisolone, nearly two thirds (61%) of pregnancies resulted in live births, of which 8 (57%) were uncomplicated term pregnancies. However, the frequency of some complications remained elevated, mainly pre-term delivery (21%) [38]. Recent animal models and in vitro studies have suggested that the aetiology of adverse pregnancy events in aPL may be caused by inflammatory processes, including increased cytokine production, complement deposition, and immune cell activation [39]. Data from a European multi-center retrospective study including 30 patients with APS with 35 pregnancies showed a better outcome of pregnancies treated by the addition of HCQ when compared to previous pregnancies under the conventional treatment [25]. In 582 women with foetal loss at or after 20 weeks of gestation, Silver et al. reported 9.6% positivity of one or more antibodies including IgG/IgM aCL and anti-β2GPI. In a multicentric study, 3 out of 7 women with stillbirth were positive for at least one aPL. The prevalence of aPL positivity in women with late-onset pregnancy complications associated with placental insufficiency is reported to be around 31%, compared to 10% in those without placental insufficiency [40]. Low molecular weight heparin use has a good safety profile. In fact, low-dose heparin is given if patient had prior thrombosis and anticoagulant dose heparin if patient had prior thrombosis [41].The most recommended dose of aspirin is 50– 325 mg/day. A seven-year survey results from the European Registry of OAPS (Obstetric Antiphospholipid Syndrome) was reported, [42] with the participation of thirty hospitals throughout Europe Cases, with patients tested positive for aPL at least twice, prospectively and retrospectively with 1000 women and 3553 episodes (2553 were historical and 1000 were latest episodes). All cases fulfilled the Sydney classification criteria. According to the laboratory categories, 292 (29.2%) were in category I, 357 (35.7%) in IIa, 224 (22.4%) in IIb and 127 (12.7%) in IIc. Miscarriages were the most prevalent clinical manifestation in 386 cases (38.6%). The presence of early Preeclampsia (PE) and early Foetal Growth Rrestriction (FGR) appeared in 181 (18.1%) and 161 (16.1%) patients respectively. In this series, 448 (44.8%) women received the recommended OAPS treatment. Patients with recommended treatment had a good live birth rate (85%), but worse results (72.4%) were obtained in patients with any treatment (Low-Dose Aspirin (LDA) or Low-Molecular-weight Heparin (LMWH) not on recommended schedule, while patients with no treatment showed a poor birth rate (49.6%). A prospective observational study reported live births in 71% of pregnancies treated with aspirin in combination with either heparin or LMWH [43]. Randomized studies comparing aspirin have shown an increased rate of live births with aspirin and unfractionated heparin compared with aspirin alone, through different doses of heparin were employed [41,44]. The latter could not be replicated by prospective studies, mainly due to the better results in the aspirin group alone [45,46]. Moreover, a recent Cochrane review concluded that aspirin alone has not been demonstrated to improve pregnancy outcomes in APS and meta-analysis study concluded that the combination of aspirin and unfractionated heparin improves outcomes in obstetric APS while the efficacy of aspirin with LMWH is not proven [47]. Treatment with aspirin and low dose heparin leads to a significantly higher rate of live births than that when used aspirin alone in pregnant women with a history of recurrent miscarriage associated with phospholipid antibodies [41]. Low dose aspirin may improve pregnancy outcome in women with phospholipid antibodies by inevitaby blocking the action of cyclo-oxygenase in platelets, inhibiting platelet thromboxane synthesis and preventing thrombosis of the placental vasculature [48]. Heparin may reduce fetal loss by binding to phospholipid antibodies, protecting the trophoblast phospholipids from attack for successful implantation in early pregnancy, apart from its anticoagulant action [49].
A recent systematic review of 5 studies involving 154 pregnancies also concluded that primary prophylaxis with aspirin does not improve obstetric outcomes in otherwise healthy women who are asymptomatic APLA carriers [50]. In women with APS and prior thrombosis, aspirin and therapeutic dose LMWH should be recommended. Due to association of Warfarin with foetal malformations, it is employed that women on VKA (vitamin K antagonists) should be switched to LMWH prior to/or as soon become pregnant. There are conflicting results and limited evidence reported within observational studies on how to manage women who meet serological criteria for aPL but do not meet clinical criteria for obstetric APS, or alternatively those with three or more miscarriages and low titter aPL not meeting serologic criteria for aPL [51,52]. For women with a high-risk, concomitant SLE, or pre-eclampsia on individual basis, therapy with aspirin and LMWH could be considered. Further clinical studies are needed to determine the optimal management of aPL-positive women. A 17-years observational study of 1,592 non-thrombotic women with three consecutive spontaneous abortions before the 10th week of gestation or one foetal death at/or beyond the 10th week of gestation has been performed where the investigators compared the incidence of cancer diagnosis during follow-up among the cohort of women positive for antiphospholipid antibodies (n=517), the cohort of women carrying the F5 rs6025 or F2 rs1799963 polymorphism (n=279) and a cohort of women with negative thrombophilia screening results (n=796). The annualized rate of cancer was 0.300% (0.20%-0.44%) for women with obstetric APS and their cancer risk was substantially higher than that of women with negative thrombophilia screening [adjusted hazard ratio (aHR) 2.483; 95% Confidence Interval (CI) 1.27-4.85]. The computed standardized incidence ratio for women with obstetric APS was 2.89; 95% CI: 1.89-4.23. Among antiphospholipid antibodies, lupus anticoagulant was associated with cancer incidents (aHR 2.608; 95% CI: 1.091-6.236). This cohort shows that the risk of cancer is substantially higher in women with a history of obstetric APS than in the general population, and in women with a similar initial clinical history but negative for antiphospholipid antibodies [53]. The management of recurrent pregnancy failure in APS is based on prophylactic heparin, often in combination with aspirin. In this setting, data suggest that heparin may act via inhibition of complement activation rather than through an antithrombotic mechanism [54] which supports the above use of heparin. There are quite few randomized controlled clinical trials participating women with APS, which makes their management still to have barriers and uncertainty. Furthermore, clinical manifestations of APS may differ, with the complications to depend on clinical subtypes. A retrospective observational study demonstrated that that the rate of pre-eclampsia was higher in women with a history of thrombotic events than in women with a history of only obstetric complications [55].
A small number of patients (<1%) may develop the ‘’Catastrophic Anti-Phospholipid Syndrome’’ (CAPS), which is defined as small vessel thrombosis in three or more organs in less than one week in the presence of aPL, with histopathologic confirmation of small vessel thrombosis in the absence of inflammation [7,56,57]. Asherson defines the ‘Catastrophic’ Antiphospholipid Syndrome (CAPS) as a potential life-threatening variant of the APS, that can lead to multiorgan failure [58]. This is an extremely rare presentation, and due to the rarity of the syndrome, an international registry of CAPS patients was created in 2000 by the European Forum on Antiphospholipid Antibodies (aPL). The preliminary criteria for the classification of catastrophic APS were established in 2022. CAPS in pregnancy or puerperium is associated with earlier age of onset and fewer previous clinical manifestations, compared to the general population with CAPS. Because the patients with APS have increased risk of obstetric complications, it is important to diagnose APS as a possible and treat CAPS [57]. Treatment should be switched from anticoagulation therapy at onset to doses of enoxaparin throughout pregnancy and back again to an oral anticoagulant post-partum [58]. In the severe disease state, addition of high dose corticosteroids and/or IVIG is highly recommended [57]. Usually therapy consists of combined therapy (triple therapy) with anticoagulants, corticosteroids, plasmapheresis or intravenous immunoglobulins [30,60,61]. The plasmapheresis aim is to remove the pathologic antibodies and other proinflammatory and prothrombotic mediators. Usually 2–3 Lt of plasma is removed daily for 3–5 days. In terms of optimal therapy, there are few things to be considered: For the refractory CAPS, usually hydroxychloroquine is chosen due to its immunosuppressive properties.
Future Therapies-Directions
Further prospective studies need to investigate personalized new treatments for different aPL profiles, especially high-risk women with comorbidities. Prevention of pregnancy complication in women with APS begin with early detection. So far, randomized clinical trials have failed to prove if LMWH is beneficial for APS women with late pregnancy complications. The recommendation though to use LMWH for prophylaxis of recurrent complications is part of standard therapy. We need more studies to determine the diagnosis and treatment of APS in general, to understand the management of patients with extra-criteria clinical manifestations or non-criteria [62]. There are studies reporting the lower risk of VTE in population like Asian patients, something that makes necessary the search for individualized anticoagulation therapies based on population characteristics. Clinicians from the other hand should inform APS patients who are treated with oral anticoagulant drugs about potential teratogenic effects. Oral anticoagulation should be switched to Low-Molecular Weight Heparin (LMWH) once the pregnancy is confirmed. First-line APS treatments during pregnancy may vary but the combination of Low-Dose Aspirin (LDA) and LMWH administration is usually chosen [63]. Second-line therapies with steroids, Hydroxychloroquine (HCQ), intravenous immunoglobulin or plasmaphereses remain main option treatments, with HCQ to be the safest drug for use in pregnancy [64]. Although anticoagulants remain the basic treatment of APS, new biological designed drugs have caught the attention, basically for the treatment of refractory APS or CAPS [65].
In parallel, reports of their use in pregnancy are being captured, although few data are available, mainly from case reports. Novel drugs, such as eculizumab, are currently investigated. No complications are reported during pregnancy administration in few case reports available. Furthermore, fatal APS-related complications are prevented at the end of pregnancy, and there is no thrombosis or infectious complications identified during the postpartum period [66]. TNF-a blockers are also well tolerated without adverse effects [67]. Adalimumab, a TNF-a blocker, inhibits anti-b2GPI-induced TF expression in monocytes elevated levels of TNF-a levels in placental tissues , thus, by blocking TNF-a, it can improve endothelial dysfunction and prevent pregnancy loss [68]. Although these are promising drugs, still it is early to evaluate the outcome and there is need for more clinical trials.
Author Statements
Statement and Declaration
The authors declare no conflicts of interest to disclose.
Acknowledgements
The first author (I.P) was responsible for the main writing of the study, and all authors revised the paper.
Funding Information
Authors state no funding involved
Disclosure Statements
The authors declare no conflicts of interest to disclose.
References
- Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken). 2013; 65: 1869-73.
- McDonnell T, Wincup C, Buchholz I, Pericleous C, Giles I, Ripoll V, et al. The role of beta-2-glycoprotein I in health and disease associating structure with function: more than just APS. Blood Rev. 2020; 39: 100610.
- Huang S, Ninivaggi M, Chayoua W, de Laat B. VWF, platelets and the antiphospholipid syndrome. Int J Mol Sci. 2021; 22: 4200.
- Knight JS, Kanthi Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin Immunopathol. 2022; 44: 347-62.
- Proulle V, Furie RA, Merrill-Skoloff G, Furie BC, Furie B. Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood. 2014; 124: 611-22.
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018; 378: 2010-21.
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006; 4: 295-306.
- Copel JA, Bahtiyar MO. A practical approach to fetal growth restriction. Obstet Gynecol. 2014; 123: 1057-69.
- Erton ZB, Sevim E, de Jesús GR, Cervera R, Ji L, Pengo V, et al. Pregnancy outcomes in antiphospholipid antibody positive patients: prospective results from the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (”Registry”). Lupus Sci Med. 2022; 9: e000633.
- Xu J, Chen D, Tian Y, Wang X, Peng B. Antiphospholipid antibodies increase the risk of fetal growth restriction: A systematic meta-analysis. Int J Clin Pract. 2022; 2022: 4308470.
- De Carolis S, Tabacco S, Rizzo F, Giannini A, Botta A, Salvi S, et al. Antiphospholipid syndrome: an update on risk factors for pregnancy outcome. Autoimmun Rev. 2018; 17: 956-66.
- Chighizola CB, Andreoli L, Gerosa M, Tincani A, Ruffatti A, Meroni PL. The treatment of anti-phospholipid syndrome: A comprehensive clinical approach. J Autoimmun. 2018; 90: 1-27.
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ, 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005; 143: 697-706.
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006; 194: 1311-5.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 196. ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstet Gynecol. 2018; 132: e1-e17.
- Li C, Zuo Y, Zhang S, Makris UE, Karp DR, Li Z. Additional risk factors associated with thrombosis and pregnancy morbidity in a unique cohort of antiphospholipid antibody-positive patients. Chin Med J (Engl). 2022; 135: 658-64.
- Hoayek JG, Moussa HN, Rehman HA, Nasab SH, Blackwell SC, Sibai BM. Catastrophic antiphospholipid syndrome in pregnancy, a diagnosis that should not be missed. J Matern Fetal Neonatal Med. 2016; 29: 3950-5.
- Hakman E, Mikhael S. Clinical report of probable catastrophic antiphospholipid syndrome in pregnancy. Case Rep Obstet Gynecol. 2018; 2018: 4176456.
- Sadick V, Lane S, Fischer E, Seppelt I, Shetty A, McLean A. Post-partum catastrophic antiphospholipid syndrome presenting with shock and digital ischaemia - A diagnostic and management challenge. J Intensive Care Soc. 2018; 19: 357-64.
- Ruffatti A, Del Ross T, Ciprian M, Bertero MT, Sciascia S, Scarpato S, et al. Risk factors for a first thrombotic event in antiphospholipid antibody carriers: a prospective multicentre follow-up study. Ann Rheum Dis. 2011; 70: 1083-6.
- Walter IJ, Klein Haneveld MJ, Lely AT, Bloemenkamp KWM, Limper M, Kooiman J. Pregnancy outcome predictors in antiphospholipid syndrome: A systematic review and meta-analysis. Autoimmun Rev. 2021; 20: 102901.
- El-Haieg DO, Zanati MF, El-Foual FM. Plasmapheresis and pregnancy outcome in patients with antiphospholipid syndrome. Int J Gynaecol Obstet. 2007; 99: 236-41.
- Sciascia S, Hunt BJ, Talavera-Garcia E, Lliso G, Khamashta MA, Cuadrado MJ. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol. 2016; 214: 273.e1-8.
- Albert CR, Schlesinger WJ, Viall CA, Mulla MJ, Brosens JJ, Chamley LW, et al. Effect of hydroxychloroquine on antiphospholipid antibody-induced changes in first trimester trophoblast function. Am J Reprod Immunol. 2014; 71: 154-64.
- Mekinian A, Lazzaroni MG, Kuzenko A, Alijotas-Reig J, Ruffatti A, Levy P, et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: data from a European multicenter retrospective study. Autoimmun Rev. 2015; 14: 498-502.
- Ruffatti A, Tonello M, Hoxha A, Sciascia S, Cuadrado MJ, Latino JO, et al. Effect of additional treatments combined with conventional therapies in pregnant patients with high-risk antiphospholipid syndrome: A multicentre study. Thromb Haemost. 2018; 118: 639-46.
- Urban ML, Bettiol A, Serena C, Comito C, Turrini I, Fruttuoso S, et al. Intravenous immunoglobulin for the secondary prevention of stillbirth in obstetric antiphospholipid syndrome: A case series and systematic review of literature. Autoimmun Rev. 2020; 19: 102620.
- Marchetti T, Cohen M, de Moerloose P. Obstetrical antiphospholipid syndrome: from the pathogenesis to the clinical and therapeutic implications. Clin Dev Immunol. 2013; 2013: 159124.
- Tong M, Viall CA, Chamley LW. Antiphospholipid antibodies and the placenta: a systematic review of their in vitro effects and modulation by treatment. Hum Reprod Update. 2015; 21: 97-118.
- Rovere-Querini P, Canti V, Erra R, Bianchi E, Slaviero G, D’Angelo A, et al. Eculizumab in a pregnant patient with laboratory onset of catastrophic antiphospholipid syndrome: A case report. Med (Baltim). 2018; 97: e12584.
- Mineo C, Lanier L, Jung E, Sengupta S, Ulrich V, Sacharidou A, et al. Identification of a monoclonal antibody that attenuates antiphospholipid syndrome-related pregnancy complications and thrombosis. PLOS ONE. 2016; 11: e0158757.
- Erkan D, Derksen R, Levy R, Machin S, Ortel T, Pierangeli S, et al. Antiphospholipid Syndrome Clinical Research Task Force report. Lupus. 2011; 20: 219-24.
- Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, Brey R, Crowther M, Derksen R, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus. 2011; 20: 206-18.
- Derksen RH, Khamashta MA, Branch DW. Management of the obstetric antiphospholipid syndrome. Arthritis Rheum. 2004; 50: 1028-39.
- Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of anti-phospholipid syndrome in naive mice with mouse lupus monoclonal and human polyclonal anti-cardiolipin antibodies. Proc Natl Acad Sci USA. 1991; 88: 3069-73.
- Tincani A, Branch W, Levy RA, Piette JC, Carp H, Rai RS, et al. Treatment of pregnant patients with antiphospholipid syndrome. Lupus. 2003; 12: 524-9.
- Gibbins KJ, Ware Branch D. Pre-eclampsia as a manifestation of antiphospholipid syndrome: assessing the current status. Lupus. 2014; 23: 1229-31.
- Bramham K, Thomas M, Nelson-Piercy C, Khamashta M, Hunt BJ. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood. 2011; 117: 6948-51.
- Mulla MJ, Brosens JJ, Chamley LW, Giles I, Pericleous C, Rahman A, et al. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol. 2009; 62: 96-111.
- Foddai SG, Radin M, Cecchi I, Gaito S, Orpheu G, Rubini E, et al. The prevalence of antiphospholipid antibodies in women with late pregnancy complications and low-risk for chromosomal abnormalities. J Thromb Haemost. 2020; 18: 2921-8.
- Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ. 1997; 314: 253-7.
- Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Sáez-Comet L, Lefkou E, Mekinian A et al. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): a survey of 1000 consecutive cases. Autoimmun Rev. 2019; 18: 406-14.
- Backos M, Rai R, Baxter N, Chilcott IT, Cohen H, Regan L. Pregnancy complications in women with recurrent miscarriage associated with antiphospholipid antibodies treated with low dose aspirin and heparin. Br J Obstet Gynaecol. 1999; 106: 102-7.
- Kutteh WH, Franklin RD. Assessing the variation in antiphospholipid antibody (APA) assays: comparison of results from 10 centers. Am J Obstet Gynecol. 2004; 191: 440-8.
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002; 100: 408-13.
- Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker GA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. J Rheumatol. 2009; 36: 279-87.
- Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005; 2005: CD002859.
- Peaceman AM, Rehnberg KA. The effect of aspirin and indomethacin on prostacyclin and thromboxane production by placental tissue incubated with immunoglobulin G fractions from patients with lupus anticoagulant. Am J Obstet Gynecol. 1995; 173: 1391-6.
- McIntyre JA, Taylor CG, Torry DS, Wagenknecht DR, Wilson J, Faulk WP. Heparin and pregnancy in women with a history of repeated miscarriages. Haemostasis. 1993; 23: 202-11.
- Amengual O, Fujita D, Ota E, Carmona L, Oku K, Sugiura-Ogasawara M, et al. Primary prophylaxis to prevent obstetric complications in asymptomatic women with antiphospholipid antibodies: a systematic review. Lupus. 2015; 24: 1135-42.
- Arachchillage DR, Machin SJ, Mackie IJ, Cohen H. Diagnosis and management of non-criteria obstetric antiphospholipid syndrome. Thromb Haemost. 2015; 113: 13-9.
- Del Ross T, Ruffatti A, Visentin MS, Tonello M, Calligaro A, Favaro M, et al. Treatment of 139 pregnancies in antiphospholipid-positive women not fulfilling criteria for antiphospholipid syndrome: a retrospective study. J Rheumatol. 2013; 40: 425-9.
- Gris JC, Mousty É, Bouvier S, Ripart S, Cochery-Nouvellon É, Fabbro-Peray P, et al. Increased incidence of cancer in the follow-up of obstetric antiphospholipid syndrome within the NOH-APS cohort. Haematologica. 2020; 105: 490-7.
- Stephenson MD. Heparin prevents antiphospholipid antibody–induced fetal loss by inhibiting complement activation. Yearbook of obstetrics, gynecology and women’s health. 2006; 2006: 188-90.
- Bramham K, Hunt BJ, Germain S, Calatayud I, Khamashta M, Bewley S, et al. Pregnancy outcome in different clinical phenotypes of antiphospholipid syndrome. Lupus. 2010; 19: 58-64.
- Cervera R, Espinosa G. Update on the catastrophic antiphospholipid syndrome and the ’CAPS Registry’. Semin Thromb Hemost. 2012; 38: 333-8.
- Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003; 12: 530-4.
- Asherson RA. The catastrophic antiphospholipid syndrome. J Rheumatol. 1992; 19: 508-12.
- Carmi O, Berla M, Shoenfeld Y, Levy Y. Diagnosis and management of catastrophic antiphospholipid syndrome. Expert Rev Hematol. 2017; 10: 365-74.
- Makatsariya AD, Khizroeva J, Bitsadze VO. Catastrophic antiphospholipid syndrome (Ronald Asherson syndrome) and obstetric pathology. J Perinat Med. 2018; 46: 387-400.
- Fuentes Carrasco M, Mayoral Triana A, Cristóbal García IC, Pérez Pérez N, Izquierdo Méndez N, Soler Ruiz P, et al. Catastrophic antiphospholipid syndrome during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2021; 264: 21-4.
- Uthman I, Noureldine MHA, Ruiz-Irastorza G, Khamashta M. Management of antiphospholipid syndrome. Ann Rheum Dis. 2019; 78: 155-61.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 9th ed. 2012; 141: e691S-736S.
- Sperber K, Hom C, Chao CP, Shapiro D, Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009; 7: 9.
- Cohen H, Cuadrado MJ, Erkan D, Duarte-Garcia A, Isenberg DA, Knight JS, et al. 16th International Congress on Antiphospholipid Antibodies Task Force Report on antiphospholipid syndrome treatment trends. Lupus 16th International Congress on Antiphospholipid Antibodies Task Force. 2020; 29: 1571-93.
- Gustavsen A, Skattum L, Bergseth G, Lorentzen B, Floisand Y, Bosnes V, et al. Effect on mother and child of eculizumab given before caesarean section in a patient with severe antiphospholipid syndrome: A case report. Med (Baltim). 2017; 96: e6338.
- Alijotas-Reig J, Esteve-Valverde E, Llurba E, Gris JM. Treatment of refractory poor aPL-related obstetric outcomes with TNF-alpha blockers: maternal-fetal outcomes in a series of 18 cases. Semin Arthritis Rheum. 2019; 49: 314-8.
- Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol. 2005; 174: 485-90.