1Department of Medicinal Chemistry, Faculty of Pharmacy, University of Zagazig, Egypt
2Quality Control Department, EIPICO, Egypt
*Corresponding author: Hafez HM, Quality Control Department, EIPICO, Egypt.
Received: November 24, 2014; Accepted: December 04, 2014; Published: December 05, 2014
Citation: Hafez HM, Elshanawany AA, Abdelaziz LM, Mohram MS. Development of a Stability-Indicating HPLC Method for Simultaneous Determination of Amlodipine Besylate and Atorvastatin Calcium in Tablets. Austin J Anal Pharm Chem. 2014;1(6): 1028.
The fixed dose combination which contains the antihypertensive agent Amlodipine and the statin, Atorvastatin is the first combination of its kind designed to treat two risk factors for cardiovascular disease (CVD).
A Validated HPLC method was developed as a Stability-Indicating HPLC Method for both drugs and four of their impurities. The method was performed on Phenomenex kinetex C18 100A (250x4.6 mm, 2.6μ) and the mobile phase consisted of Potassium dihydrogen phosphate (pH 5.5, 0.03M) - Acetonitrile (65:35 V/V) which pumped at a flow rate 1.2 ml/min at 40°C. 20 μl of drugs sample solutions were monitored at wavelength 240 nm.
The proposed method was validated in terms of linearity ranged between [(5.18-15.54 and 5.26-15.78 μg/ml) corresponding levels of 50-150% w/w of the nominal analytical concentration] with linear regression equations were [{y = 44667X - 405.02 (r= 0.9999) and Y = 41443X - 2251.4 (r= 0.9999)], accuracy [99.73± 0.46 and 100.22±1.04 %], precision [100.34± 0.61 and 101.01± 1.72 %], limits of detection [0.16 and 0.17 μg/ml] and limits of quantitation [0.48 and 0.52 μg/ml] for Amlodipine besylate and Atorvastatin calcium respectively.
Amlodipine Besylate, Atorvastatin Calcium and Caduet tablets bulk powders were stressed under different conditions in forced degradation studies. Method validation was developed following the recommendations for analytical method validation of International Conference on Harmonization (ICH) and Food and Drug administration (FDA) organizations.
Keywords: Stability-Indicating; HPLC; Amlodipine Besylate; Atorvastatin Calcium; Phenomenex kinetex C18
Hypertension and dyslipidemia are the most commonly co-occurring cardiovascular risk factors. Coronary artery disease (CAD) is the leading cause of morbidity and mortality worldwide accounting for in excess of 930,000 deaths. It is a multi factorial disease, emphasis is to treat overall cardiovascular risk rather than single risk factors in isolation.
The third National Health and Nutrition Examination Survey (NHANES) estimated that more than 64% of patients with hypertension have dyslipidemia; conversely, approximately 47% of patients with dyslipidemia have hypertension. Antihypertensive and lipid lowering medications substantially reduce the risk of CAD, stroke and death in patients with cardiovascular risk factors.
The fixed dose combination which contains the antihypertensive agent Amlodipine and the statin, Atorvastatin, is the first combination of its kind designed to treat two risk factors for cardiovascular disease (CVD).
The pharmacokinetic and pharmacodynamic properties of Amlodipine and Atorvastatin make them well suited for combination in a single pill to manage cardiovascular risk. The half lives of both agents facilitate once daily dosing and both can be administered at any time of day with or without food. Each drug has no adverse effects on the other’s efficacy or tolerability [1].
Caduet® tablets combine the calcium channel blocker Amlodipine Besylate with the HMG CoA-reductase inhibitor Atorvastatin Calcium. Amlodipine Besylate is chemically described as 3-ethyl-5- methyl (4RS)-2-[(2-aminoethoxy) methyl]-4-(o-chlorophenyl)-6- methyl-1, 4-dihydro pyridine-3, 5-dicarboxylate benzene sulphonate, its empirical formula is C20H25ClN2O5 ·C6H6O3S. Atorvastatin calcium is chemically described as calcium [R-(3R, 5R)]-7-[2-(4- fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-(phenyl carbamoyl)- 1H-pyrrol-1-yl] -3, 5-dihydroxy heptanoate trihydrate. Its empirical formula is (C33H34 FN2O5)2Ca ·3H2O. The structural formulas for Amlodipine Besylate and Atorvastatin Calcium are shown in Figure 1.
Amlodipine Besylate is a white to off-white crystalline powder with a molecular weight of 567.1. It is slightly soluble in water and sparingly soluble in ethanol. Atorvastatin calcium is a white to off-white crystalline powder and it has a molecular weight of 1209.42. It is insoluble in aqueous solutions of pH 4 and below, it is very slightly soluble in distilled water, pH 7.4 phosphate buffer and acetonitrile. It is freely soluble in methanol [2].
Caduet® is indicated in patients for Hypertension, Coronary Artery Disease (CAD) like Angina, Prevention of Cardiovascular Disease and Hyperlipidemia [2].
The preparation of new combinations of drugs in pharmaceuticals encourages researchers to develop new and efficient methods for multi-quantification with separation procedures. HPLC is a dominant separation technique, especially in pharmaceutical analysis [3].
Literature survey indicated that several analytical methods have been described for the determination of Amlodipine Besylate, Atorvastatin Calcium alone [4-5] or in combination with other compounds [6-7]. Several method were reported for simultaneous determination of Amlodipine Besylate and Atorvastatin Calcium such as Spectrophotometry [8-10], HPTLC [11] Spectrofluorimetry, HPLC Coupled with Fluorescence Detection [12] Capillary Electrophoresis [3] HPLC Coupled with UV Detection [13-17], HPLC–MS–MS [18- 20] and RP-UPLC [21-22].
Stability testing and stress testing (forced degradation) studies are critical components of drug development strategy. The studies lead to understand the mechanism of a drug’s decomposition which further helps in obtaining information on physical and chemical factors that result in instability.
These factors are then controlled in order to stabilize the drug or drug formulation which leads to increase shelf-life or improve efficacy [23]. Stress testing is defined as the stability testing of drug substances and drug products under conditions exceeding those used for accelerated testing.
These studies are undertaken to elucidate the intrinsic stability of the drug substance. According to International Conference on Harmonization (ICH) guideline Q1A (R2), the stability testing of drug substances should be carried out under different stress conditions (hydrolysis, oxidation, photolysis, and thermal degradation) to validate the stability-indicating supremacy of analytical methods used for the analysis of stability samples [24].
The standard conditions for photo stability testing are described in ICH guideline Q1B [25]. These tests allow accurate and precise quantification of drugs and their degradation and interaction products.
Few analytical methods have been reported as a stability indicating method for simultaneous determination of Amlodipine and Atorvastatin in presence of their degradation products [26-29].
The focus of the present study was to develop a simple, rapid, precise, and accurate isocratic reversed-phase stability-indicating HPLC method for the simultaneous determination of Amlodipine and Atorvastatin and their impurities in tablet dosage form.
Analysis was performed on a chromatographic system of WATERS 2695 separation module connected to WATERS 2487 UV/ VIS detector. The system equipped by Empower PC program. The chromatographic separation was achieved on Phenomenex kinetex 2.6 μm C18 100A (100x 4.6 mm).
All reagents used were of analytical grade or HPLC grade. Potassium dihydrogen phosphate and ortho-phosphoric acid were supplied by (Merck, Darmstadt, Germany), Acetonitrile and Methanol HPLC grade were supplied by (Fischer scientific, U.K.) and Distilled water was obtained from Milli-RO and Milli-Q systems (Millipore, Bedford, MA).
Amlodipine Besylate and Atorvastatin Calcium working standard powders were kindly supplied by Egyptian international pharmaceutical industries company (EIPICO) (10th Ramadan, Egypt), and were used without further purification.
Caduet®, Pfizer (Egypt) contains (Amlodipine (as Besylate) 10 mg per tablet and Atorvastatin (as Calcium) 10 mg per tablet B.NO: 0795049.
20 μl of drugs sample solutions were monitored at fixed wavelength (lambda =240 nm for Amlodipine Besylate and Atorvastatin Calcium). Liquid chromatography was performed on Phenomenex kinetex 2.6 μm C18 100A (100x 4.6 mm) and the mobile phase consisted of Potassium dihydrogen phosphate (pH 5.5, 0.03M) - Acetonitrile (65:35 V/V) which pumped at a flow rate equals to 1.2 ml/min at 40 °C.
Potassium dihydrogen phosphate (pH 5.5, 0.03M) was prepared by dissolving 4.08 g of Potassium dihydrogen phosphate in approximately 950 ml distilled water. The pH was adjusted to 5.5 with sodium hydroxide then water was added to 1000 ml.
Mobile phase was filtered through a 0.45 μl Nylon membrane filter (Millipore, Milford, MA, USA) under vacuum and degassed by ultrasonication (Cole Palmer, Vernon Hills, USA) before usage. Mixture of acetonitrile and distilled water (50:50 V/V) was prepared to be used as diluents.
Stock standard solutions containing 1 mg/ml of Amlodipine (as Besylate) ( actual weight =103.6 mg ) and 1 mg/ml of Atorvastatin (as Calcium) ( actual weight =105.2 mg ) were prepared by dissolving 100 mg of each in 40 ml methanol in 100 ml volumetric flask respectively. It was sonicated for 5 minutes and the final volume of solutions was made up to 100 ml with diluent to get stock standard solutions.
To construct calibration plots, the stock standard solutions were diluted with diluent to prepare working solutions in the concentration ranges (5.18-15.54 and 5.26-15.78 μg/ml) for Amlodipine Besylate and Atorvastatin Calcium respectively.
Each solution (n=5) was injected in triplicate and chromatographed under the mentioned conditions above. Linear relationships were obtained when average drug standard peak area were plotted against the corresponding concentrations for each drug. Regression equation was computed.
A composite of ten Caduet® 10/10 tablets was prepared by grinding it to a fine, uniform size powder, triturated using mortar and pestle. After calculating the average tablet weight, amounts of powder equivalent to one tablet was accurately weighed and transferred to 100 ml volumetric flasks then completed with diluent up to 100 ml.
The solutions were solicited for 15 min and the solutions were then filtered through 0.45 μm Nylon membrane filters (Millipore, Milford, MA, USA). Aliquots of appropriate volume (10 ml) were transferred to 100 ml calibrated flasks and diluted to volume with diluent to obtain the mentioned concentration above (10 μg/ml). The diluted solutions were analyzed under optimized chromatographic conditions and chromatogram is showed in (Figure 2A).
Amlodipine Besylate, Atorvastatin Calcium and Caduet tablets bulk powders were stressed under different conditions in forced degradation studies. Stock solutions of Amlodipine Besylate, Atorvastatin Calcium and Caduet tablets bulk powders – used to forced degradation studies - were prepared by dissolving it in diluents [30].
Acidic degradation: Hydrochloric acid (HCl) (1 M, 10 ml) was added to 10 ml prepared stock solutions of Amlodipine Besylate, Atorvastatin Calcium and Caduet tablets respectively. These solutions were separately heated at 70C˚ for 6 hours in the dark (to exclude the possible degradative effect of light). 2 ml of previous solutions were then transferred to 25 ml volumetric flasks, neutralized by addition of 1ml of 1 M NaOH and diluted to final volume with diluents [30, 31].
Alkaline degradation: Sodium hydroxide (NaOH) (1 M, 10 ml) was added to 10 ml prepared stock solutions of Amlodipine Besylate, Atorvastatin Calcium and Caduet tablets respectively. These solutions were separately heated at 70C˚ for 2 hours in the dark (to exclude the possible degradative effect of light). 2 ml of previous solutions were then transferred to 25 ml volumetric flasks, then it was neutralized by addition of 1ml of 1 M HCl and diluted to final volume with diluents [30, 31].
Oxidation: Hydrogen peroxide (H2O2; 10%, v/v, 10 ml) was added to 10 ml prepared stock solutions of Amlodipine Besylate, Atorvastatin Calcium and Caduet tablets respectively. These solutions were separately heated at 70C˚ for 6 hours in the dark. 2 ml of previous solutions were then transferred to 25 ml volumetric flasks and diluted to final volume with diluent [30, 31].
Neutral degradation (Thermal degradation): Prepared stock solutions of Amlodipine Besylate, Atorvastatin Calcium and Caduet tablets respectively were heated at 70C˚ for 6 hours in the dark to study the effect of thermal stress. Also the experiment was performed on solid-state samples which could be stressed under previous condition and then diluted with a known amount of mobile phase. The experiment was performed in the dark to exclude the possible degradative effect of light. 1 ml of previous solutions was then transferred to 25 ml volumetric flasks and diluted to final volume with diluents [30, 31].
Photo stability: Prepared stock solutions of Amlodipine Besylate, Atorvastatin Calcium and Caduet tablets respectively (10 ml) were exposed to light providing an overall illumination of not less than 1.2 million lux hours and an integrated near ultraviolet energy of not less than 200 watt hours/square meter. The experiment was also performed on solid-state samples which could be stressed under previous condition and then diluted with a known amount of mobile phase. 1 ml of previous solutions was then transferred to 25 ml volumetric flasks and diluted to final volume with diluents [30, 31].
Specificity is the ability to assess unequivocally the analyte in the presence of components which may be expected to be present. Typically these might include impurities, degradiants and matrix [32].
A placebo of tablet was prepared by mixing the respective excipients. Solutions were prepared by following the procedure described in the section on sample preparation. The commonly used tablet excipients did not interfere with the method. The diluent chromatogram shows that the tablet diluent had negligible contribution after the void volume at the method detection wavelength of 240 nm.
The method were also evaluated by assessing whether impurities like (Amlodipine impurity A, Amlodipine impurity B, Atorvastatin impurity A and Atorvastatin impurity C ) and degradation products present in the pharmaceutical formulations - obtained from stress studies involving acid, base, peroxide, and heat stored under ICH stability conditions - interfered with the analysis of Amlodipine Besylate and Atorvastatin Calcium (Figure.2A).
Each Caduet film-coated tablet also contains calcium carbonate, croscarmellose sodium, microcrystalline cellulose, pregelatinized starch, polysorbate 80, hydroxypropyl cellulose, purified water, colloidal silicon dioxide (anhydrous), magnesium stearate and Opadry® II Blue 85F10919 (polyvinyl alcohol, titanium dioxide, PEG 3000, talc, and FD&C blue #2).
The linearity of an analytical procedure is its ability (within a given range) to obtain test results which are directly proportional to the concentration (amount) of analyte in the sample. For the establishment of linearity, a minimum of 5 concentrations are recommended [32].
Five Concentrations were chosen in the ranges (5.18-15.54 and 5.26-15.78 μg/ml) for corresponding levels of 50-150% w/w of the nominal analytical concentration of Amlodipine Besylate and Atorvastatin Calcium respectively. The linearity of peak area responses versus concentrations was demonstrated by linear least square regression analysis. The linear regression equations were {Y = 44667X - 405.02 (r= 0.9999) and Y = 41443X - 2251.4 (r= 0.9999)} for Amlodipine Besylate and Atorvastatin Calcium respectively. Where Y is the peak area of standard solution and X is the drug concentration.
For impurities of both drugs, 5 mg of each of Amlodipine impurity A, Amlodipine impurity B, Atorvastatin impurity A and Atorvastatin impurity C (50μg / ml) respectively was weighed in 100 ml volumetric flask then completed with diluents and sonicated.
Five Concentrations were chosen in the ranges (1- 3 μg/ml) for corresponding levels of 50-150% w/w of the nominal analytical concentration of all impurities respectively. The linear regression equations were {Y = 467.93X + 1187.1 (r= 0.9991), Y = 3055.2X – 3901.2 (r= 1.0), Y = 2355.2X + 1979.7 (r= 0.9999) and Y = 2278.7X – 1943.3 (r= 0.9998)} for Amlodipine impurity A, Amlodipine impurity B, Atorvastatin impurity A and Atorvastatin impurity C respectively. Where Y is the peak area of standard solution and X is the drug concentration.
The precision of the assay was investigated by measurement of both repeatability and Intermediate precision.
Repeatability: Repeatability was investigated by injecting a minimum of 9 determinations covering the specified range for the procedure (e.g., 3concentrations/3 replicates each) and percentage RSD were calculated in Table 1.
Intermediate precision: In the inter-day studies, standard and sample solutions prepared as described above, were analyzed in triplicate on three consecutive days at specified range for the procedure (e.g., 3 concentrations/3 replicates each) of the test concentration and percentage RSD were calculated in Table 1.
Accuracy was assessed using 9 determinations over 3 concentration levels covering the specified range (80,100 and 120%). Accuracy was reported in Table 1 as percent recovery by the assay of known added amount of analyte in the sample.
According to the ICH recommendations, determination of limits of detection and quantitation was based on the standard deviation of the y-intercepts of regression lines (n=3) and the slope of the calibration plots in Table 2 and Figure 2B [32].
System suitability tests were used to verify that the resolution and reproducibility were adequate for the performed analysis. The system suitability tests included number of theoretical plates, resolution, peak tailing, capacity factor and selectivity factor. Results are revealed in Table 3.
Robustness of an analytical procedure is a measure of its capacity to remain unaffected by small variations in method parameters and provides an indication of its reliability during normal usage [32]. Robustness was tested by studying the effect of changing mobile phase pH by ± 0.3, the percentage of organic solvent (Acetonitrile) in the mobile phase by ± 2 %, temperature ± 2C°, wavelengths ± 2 nm and flow rate ± 0.1 ml/min had no significant effect on the chromatographic resolution of the method in Figure 3. Changes in pH degree and Acetonitrile percent in mobile phase have a greater effect on resolution than other factors. (Table 4).
Stability of analytical solution: Also as part of evaluation of robustness, solution stability was evaluated by monitoring the peak area response. Standard stock solutions in methanol were analyzed right after its preparation 1, 2 and 3 days after at 5 C°. The change in standard solution peak area response over 3 days was (0.57 and 0.58 %) for Amlodipine Besylate, Atorvastatin Calcium respectively. Their solutions were found to be stable for 3 days at 5 C° at least.
The proposed methods were successfully used to determine Amlodipine Besylate, Atorvastatin Calcium respectively in Caduet 10/10 mg/tablet. Seven replicate determinations were performed. Satisfactory results were obtained for each compound in good agreement with label claims.
The results obtained were compared statistically with those from published method [26] by using Student’s t-test and the variance ratio F-test. The results showed that the t and F values were smaller than the critical values. So, there were no significant differences between the results obtained from this method and published methods (Table 5).
Several trials were carried out to obtain optimized chromatographic condition for simultaneous determination of Amlodipine Besylate and Atorvastatin Calcium in their pharmaceutical preparations.
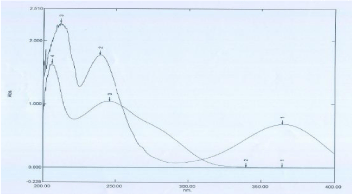
Firstly, maximum absorption wavelengths (240 nm) for Amlodipine Besylate and Atorvastatin Calcium were selected by scanning from 350-200 nm under UV (Figure 4).
Potassium Dihydrogen Phosphate buffer has no effect on absorption at wavelength more than 200 nm [33]. Concentration of buffer (0.03M) is adequate for most reversed phase applications. This concentration is also moderate enough to avoid problems with precipitation when significant amounts of organic modifiers are used in the mobile phase [34].
On the basis of pka of Amlodipine Besylate and Atorvastatin Calcium are 8.6 and 4.5 respectively which means that Amlodipine Besylate has more basic properties than Atorvastatin Calcium due to free and cyclic amino group and vice versa Atorvastatin Calcium has free carboxylic group.
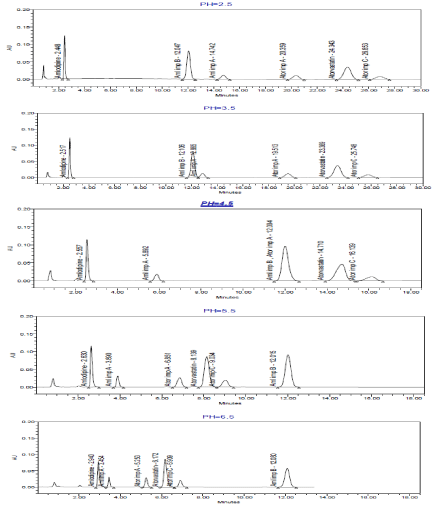
Several degrees of pH of phosphate buffer (2.5 -6.5) were examined (Figure.5). At lower pH (2.5-3.5) shows a fast eluting of Amlodipine (it becomes difficult to separate it from its predictable degradation products, lately one for Atorvastatin (long time about 25 min) and co-elution between Amlodipine imp A and Amlodipine imp B.
At pH 4.5, there is an interfering between Amlodipine imp B and Atorvastatin imp A.
A good separation was obtained at higher pH (5.5-6.5), pH 5.5 is more suitable than pH 6.5 because resolution between Amlodipine and its impurity A are greater than resolution between Atorvastatin and its impurity C at pH 5.5 and the former is more critical than the latter one as it is more affected by increasing in organic solvent percentage.
Several types of columns were tried like Agilent eclipse plus C18 (3.5 μm, 4.6 x 100 mm), Thermo BDS HYPERSIL C18 (5μm, 150 X 4.6 mm) and Thermo BDS HYPERSIL Cyano (5μm, 250 X 4.6 mm) in addition to Phenomenex kinetex 2.6 μm C18 100A (100x 4.6 mm). The first and second columns have bad peak shape and resolution (Figure 6). On cyano column, order of peaks was changed Atorvastatin early eluted than amlodipine which resulted in further examination at different PH and organic solvent percentages but finally we did not have good peak shape or resolution (Figure.7).
Ammonium acetate and sodium dihydrogen phosphate were also tried, no significant difference between sodium dihydrogen phosphate and potassium dihydrogen phosphate but Ammonium acetate give less resolution power (Figure.8).
Methanol exhibits poor separation and peak shape as an organic solvent (Figure 9).
After all of previous had been carried out, previous optimized chromatographic conditions were selected.
If the compound is poorly water-soluble, organic co-solvents may be used in combination with acid or base. Organic solvents that have been commonly used for stress-testing studies are DMSO, acetic acid acetonitrile and methanol. As usual, when conducting stress testing, the analyst should be wary of possible side reactions that may affect the drug, for example, methanol should be avoided for compounds containing –CO2H, –CO2R, amide groups. Acetonitrile is the co-solvent of choice for photochemical reaction [30].
Amlodipine Besylate, Atorvastatin calcium authentic standards and Caduet® tablets were subjected to various stress condition.
In acidic conditions, both drugs exhibited high percentage of degradation about 79% and 62% for Amlodipine Besylate and Atorvastatin calcium respectively (Figure.10 and Table 6). The pyridine analogue of Amlodipine (impurity D) and Atorvastatin lactone (impurity G) were the main degradation products of Amlodipine Besylate and Atorvastatin calcium respectively (Figure 11a-c).
In alkaline conditions, both drugs degraded about 95% and 14% for Amlodipine Besylate and Atorvastatin calcium respectively. The major degradation products of Amlodipine Besylate eluted early at 0.9 and 1.09 minute (Figure 12 and Table 6).
Amlodipine Besylate and Atorvastatin calcium are more stable under neutral degradation (Thermal degradation); only about 13% degradation of two drugs was observed (Figure 13 and Table 6).
In oxidative conditions, both drugs were found to be highly labile to oxidative hydrolysis in 10% H2O2 at 70% after 6 hours. Approximately 80%, 75% degradation was observed for Amlodipine Besylate and Atorvastatin calcium respectively due to its nitrogenous content.
The major degradation product of Amlodipine Besylate was its pyridine analogue (Figure 14 and Table 6). Both drugs were affected significantly by photolytic degradation (Figure 15 and Table 6).
New developed method has several merits than other published methods in literature, it didn´t use ion pair buffer in mobile phase like RJ Eranki et al [28] which shorten column life time and require long time for system stabilization and column washing. Run time is shorter (50% decrease) and sensitivity is more than ZM Turabi et al [29]. Specificity was proven clearly after impurities of both drugs had been separated in addition to degradation products which were identified and its pathways were mentioned, in contrary to BG Chaudhari et al [27]. A. Mohammadi et al [26] didn´t mention how to optimize and develop his method and little information about system suitability test.
A simple, accurate, precise, robust and reliable LC method has been established as stability indicating method for Amlodipine Besylate and Atorvastatin Calcium respectively in bulk and in their pharmaceutical dosage form.
Repeatability, Intermediate precision, Reproducibility and Accuracy of Amlodipine Besylate and Atorvastatin Calcium respectively.
Drug Name |
Conc. |
Amlodipine Besylate |
Atorvastatin Calcium |
|||
|
AV±SD mg/ml |
AV±RSD % |
AV±SD mg/ml |
AV±RSD % |
||
Repeat- ability |
80% |
8.36±0.10 |
100.92±1.14% |
8.46±0.13 |
100.55±1.55% |
|
100% |
10.36±0.03 |
100.00±0.26% |
10.54±0.07 |
100.21±0.68% |
||
120% |
12.40±0.03 |
99.77±0.24% |
12.58±0.06 |
99.63±0.51% |
||
Intermediate precision |
80% |
8.35±0.07 |
100.77±0.79% |
8.47±0.11 |
100.62±1.26% |
|
100% |
10.40±0.06 |
100.34±0.61% |
10.63±0.18 |
101.01±1.70% |
||
120% |
12.40±0.02 |
99.73±0.19% |
12.59±0.07 |
99.75±0.58% |
||
Accuracy |
80% |
8.22±0.05 |
99.17±0.65% |
8.39±0.15 |
99.72±1.76% |
|
100% |
10.33±0.05 |
99.73±0.46% |
10.54±0.11 |
100.22±1.04% |
||
120% |
12.32±0.02 |
99.1±0.14% |
12.54±0.07 |
99.37±0.59% |
||
N.B. (80%, 100% and 120%) Concentration of Amlodipine Besylate and Atorvastatin Calcium are [(8.29, 12.43, 15.54), (8.42, 10.52, 12.62)] respectively.
Calibration data was resulted from method validation of Amlodipine Besylate and Atorvastatin Calcium respectively.
Item |
Amlodipine Besylate |
Atorvastatin Calcium |
Linear range (µg/ml) |
5.18-15.54 |
5.26-15.78 |
Detection limit (µg/ml) |
0.16 |
0.17 |
Quantitation limit (µg/.ml) |
0.48 |
0.52 |
Regression data |
|
|
No. |
5 |
5 |
slope (b) |
44667 |
41443 |
Standard deviation of the slope |
115.01 |
157.99 |
intercept (a) |
-405.02 |
-2251.4 |
Standard deviation of the intercept |
2163.61 |
2152.91 |
correlation coefficient ® |
0.9999 |
0.9999 |
Standard error of regression |
2407.38 |
1819.8 |
(Y = a + bC, where C is the concentration of the compound (μg/ml) and Y is the drug peak area)
System suitability parameters of all drugs were obtained from Method Validation.
Drugs/Parameters |
Theoretical Plates (N) |
Resolution (R) |
Capacity Factor (K) |
Tailing Factor (T) |
Selectivity (α) |
Amlodipine Besylate |
4841.32 |
5.45 |
3.24 |
1.59 |
1.57 |
Amlodipine impurity A |
8140.99 |
4.96 |
4.38 |
1.13 |
1.36 |
Atorvastatin impurity A |
10890.33 |
13.56 |
8.33 |
1.09 |
1.9 |
Atorvastatin Calcium |
11591.13 |
4.61 |
10.07 |
1.18 |
1.21 |
Atorvastatin impurity C |
12602.44 |
2.87 |
11.27 |
1.05 |
1.12 |
Amlodipine impurity B |
16468.69 |
11.29 |
16.83 |
1.01 |
1.49 |
Effect of Changes of Some Parameters on Resolution during Method Robustness.
Parameters |
Flow Rate |
PH |
Acetonitrile % |
Wavelength |
||||
Value |
1.1 |
1.3 |
5.2 |
5.8 |
33 |
37 |
238 |
242 |
Amlodipine Besylate |
4.9 |
4.53 |
4.42 |
5.26 |
8.39 |
3.37 |
5.45 |
5.43 |
Amlodipine impurity A |
6.29 |
5.86 |
8.87 |
3.94 |
6.93 |
3.34 |
4.96 |
4.92 |
Atorvastatin impurity A |
13.99 |
13.35 |
16.7 |
11.07 |
16.12 |
11.03 |
13.56 |
13.51 |
Atorvastatin Calcium |
4.61 |
4.48 |
5.15 |
4.25 |
5.39 |
3.86 |
4.61 |
4.62 |
Atorvastatin impurity C |
2.77 |
2.69 |
2.92 |
2.82 |
3.41 |
2.33 |
2.87 |
2.87 |
Amlodipine impurity B |
8.97 |
8.6 |
14.83 |
14.37 |
9.66 |
12.22 |
11.29 |
11.25 |
Statistical comparison of the proposed and published methods for determination of Amlodipine Besylate and Atorvastatin Calcium respectively in their dosage forms by reported method (T- student test) and (F –test for variance).
Drug name |
Recovery ± SD |
Calculated t- values |
Calculated F- values |
|
Proposed methods |
Reference method |
|||
Amlodipine Besylate |
100 .78±1.79 |
100.39±2.56 |
0.36 |
0.49 |
Atorvastatin Calcium |
100.53±1.23 |
100.18±2.02 |
0.49 |
0.37 |
(Where the Tabulated t-values and F -ratios at p = 0.05 are 2.365 and 3.79).
Results of recovery% of degradations for Amlodipine Besylate and Atorvastatin Calcium respectively under different stress conditions.
Amlodipine |
Atorvastatin |
|||||||
Assay of Amlodipine |
Detected Degradation |
Assay of Atorvastatin |
Detected Degradation |
|||||
Peak Area |
Recovery % |
Peak Area |
Recovery % |
Peak Area |
Recovery % |
Peak Area |
Recovery % |
|
Standard |
446291 |
100.0 |
446291 |
100.0 |
413153.36 |
100.0 |
446291 |
100.0 |
Thermal degradation at 75 C°/ 6 hours |
408244 |
91.5 |
4285 |
1.0 |
384622 |
93.1 |
0 |
0.0 |
Acid degradation at (1M HCl) 75 C°/ 6 hours |
97027.5 |
21.7 |
65631 |
14.7 |
165496 |
40.1 |
3053 |
0.7 |
Alkaline degradation at (1M NaOH) 75 C°/ 6 hours |
7990 |
1.8 |
121085 |
27.1 |
373890 |
90.5 |
0 |
0.0 |
Oxidative degradation at (1M NaOH) 75 C°/ 6 hours |
101286 |
22.7 |
55000 |
12.3 |
109630 |
26.5 |
90520 |
21.9 |
Photolytic degradation at 1.2 million lux / hours |
415503 |
93.1 |
408244 |
91.5 |
362433 |
87.7 |
0 |
0.0 |
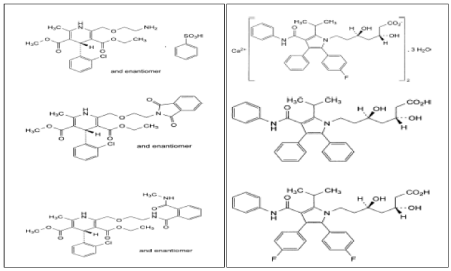
Structures of Amlodipine Besylate, Amlodipine impurity A, Amlodipine impurity B, Atorvastatin Calcium, Atorvastatin impurity A and Atorvastatin impurity C respectively.
Amlodipine impurity A is 3-ethyl 5-methyl (4RS)-4-(2-chlorophenyl)-2-[[2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethoxy]methyl]-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
Amlodipine impurity B is 3-ethyl 5-methyl (4RS)-4-(2-chlorophenyl)-6-methyl-2-[[2- [[2-(methylcarbamoyl) benzoyl] amino] ethoxy] methyl]-1, 4-dihydropyridine-3,5-dicarboxylate
Atorvastatin impurity A is (3R, 5R) - 3, 5-dihydroxy -7-[5-(1-methylethyl)-2,3-diphenyl-4-(phenyl carbamoyl)-1H-pyrrol-1-yl] - heptanoic acid (desfluoroatorvastatin).
Atorvastatin impurity C is (3R, 5R) -7-[2, 3-bis (4-fluorophenyl)-5-(1-methylethyl) -4-(phenyl carbamoyl)-1H-pyrrol-1-yl] - 3, 5-dihydroxy heptanoic acid (fluoroatorvastatin).
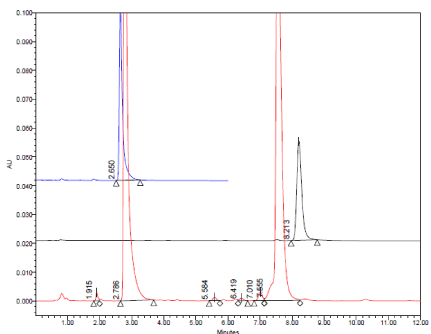
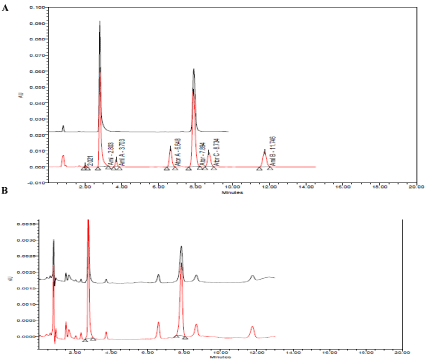
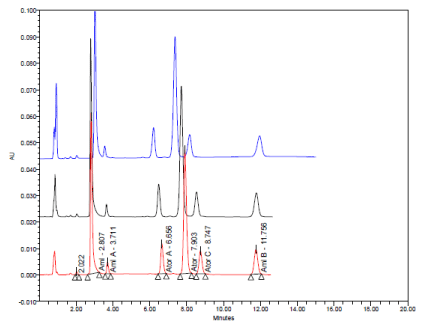
(A, B) Typical HPLC chromatograms obtained from 20 μl injections of Amlodipine Besylate (2.8 min.) Amlodipine impurity A (3.7 min.), Atorvastatin impurity A (6.6 min.), Atorvastatin Calcium (7.8 min.), Atorvastatin impurity C (8.7 min.) and Amlodipine impurity B (11.7 min.) respectively under optimized chromatographic conditions.
A. At 100% conc. Level of all compounds and tablet.
B. At DL and QL.
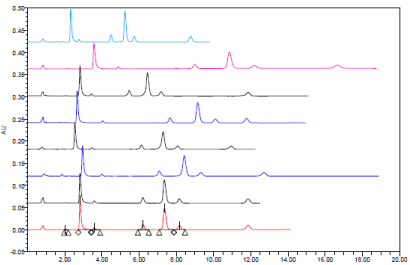
Typical HPLC chromatograms obtained from 20 μl injections of Amlodipine Besylate Amlodipine impurity A, Atorvastatin impurity A , Atorvastatin Calcium , Atorvastatin impurity C and Amlodipine impurity B respectively under optimized chromatographic conditions.
(Ascending order)
1, 2- At 238 and 242 nm
3, 4- at 1.1 and 1.3 ml/min
5, 6- at phosphate buffer pH = 5.2 and 5.8
7, 8- at 33% and 37% of acetonitrile.
Typical UV spectru of Amlodipine Besylate and Atorvastatin Calcium respectively.
Typical HPLC chromatograms obtained from 20 μl injections of Amlodipine Besylate Amlodipine impurity A, B, Atorvastatin Calcium, Atorvastatin impurity A and C under chromatographic conditions of mobile phase consisted of acetonitrile 35%: phosphate buffer 65% (different PH) was pumped on Phenomenex kinetex 2.6 u C18 100A columns at a flow rate 1.2 ml/min and detected at 240 nm at constant temperature 40 °C.
Typical HPLC chromatograms obtained from 20 μl injections of Amlodipine Besylate Amlodipine impurity A, B, Atorvastatin Calcium, Atorvastatin impurity A and C under chromatographic conditions of mobile phase consisted of acetonitrile: phosphate buffer (PH = 6.5) was pumped on columns at a flow rate 1.2 ml/min and detected at 240 nm at constant temperature 40 °C.
(Ascending order)
1- Agilent eclipse plus C18 (3.5 um, 4.6 x 100 mm) and acetonitrile 30%
2- Agilent eclipse plus C18 (3.5 um, 4.6 x 100 mm) and acetonitrile 35%
3- Thermo BDS HYPERSIL C18 (5um, 150 X 4.6 mm) and acetonitrile 30%
Thermo BDS HYPERSIL C18 (5um, 150 X 4.6 mm) and acetonitrile 35%
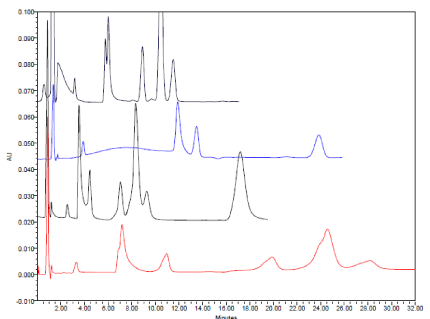
Typical HPLC chromatograms obtained from 20 μl injections of Amlodipine Besylate Amlodipine impurity A, B, Atorvastatin Calcium, Atorvastatin impurity A and C under chromatographic conditions of a mobile phase consisted of acetonitrile: phosphate buffer was pumped on Thermo BDS HYPERSIL Cyano (5um, 250 X 4.6 mm) column at a flow rate 1.2 ml/ min and detected at 240 nm at constant temperature 40 °C.
(Ascending order)
1- acetonitrile 35 %: phosphate buffer 65% PH = 6.5
2- acetonitrile 35 %: phosphate buffer 65% PH = 4.5
3- acetonitrile 30 %: phosphate buffer 70% PH = 4.5
4- acetonitrile 25 %: phosphate buffer 75% PH = 4.5
5- acetonitrile 35 %: phosphate buffer 65% PH = 2.5
6- acetonitrile 30 %: phosphate buffer 65% PH = 2.5
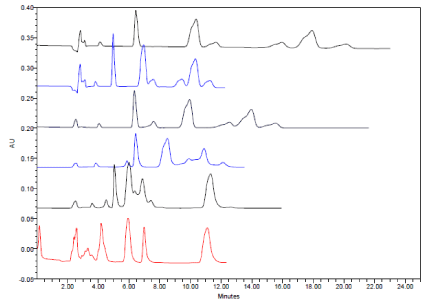
Typical HPLC chromatograms obtained from 20 μl injections of Amlodipine Besylate Amlodipine impurity A, B, Atorvastatin Calcium, Atorvastatin impurity A and C under chromatographic conditions of mobile phase consisted of acetonitrile 35%: aqueous buffer 65% (PH= 5.5) was pumped on Phenomenex kinetex 2.6 u C18 100A columns at a flow rate 1.2 ml/min and detected at 240 nm at constant temperature 40 °C.
(Ascending order)
1- potassium dihydrogen phosphate
2- sodium dihydrogen phosphate
3- Ammonium acetate
Typical HPLC chromatograms obtained from 20 μl injections of Amlodipine Besylate Amlodipine impurity A, B, Atorvastatin Calcium, Atorvastatin impurity A and C under chromatographic conditions of mobile phase consisted of methanol: phosphate buffer (PH= 5.5) was pumped on Phenomenex kinetex 2.6 u C18 100A columns at a flow rate 1.2 ml/min and detected at 240 nm at constant temperature 40 °C.
(Ascending order)
1- Methanol 65%
2- Methanol 55%
3- Methanol 45%
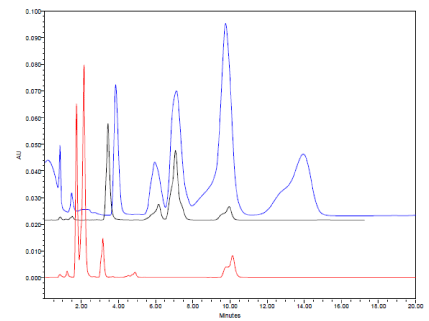
Typical HPLC chromatograms obtained from 20 μl injections of solutions of Amlodipine Besylate, Atorvastatin Calcium and Caduet tablet which were subjected to acidic condition under optimized chromatographic conditions.
(Descending order)
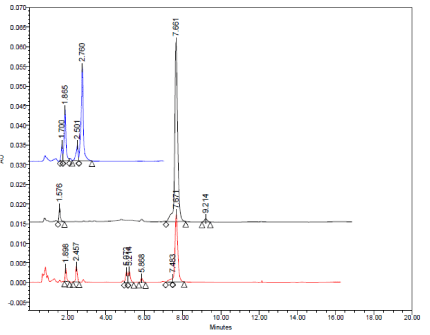
Typical HPLC chromatograms obtained from 20 μl injections of solutions of Amlodipine Besylate impurity D (pyridine analogue) under optimized chromatographic conditions.
Degradation pathway of amlodipine by acid, peroxide or light.
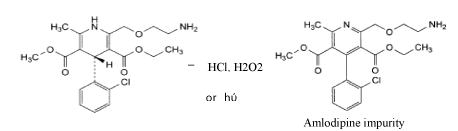
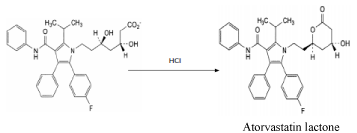
Degradation pathway of Atorvastatin by acid.
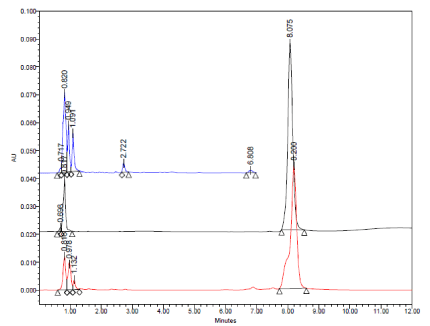
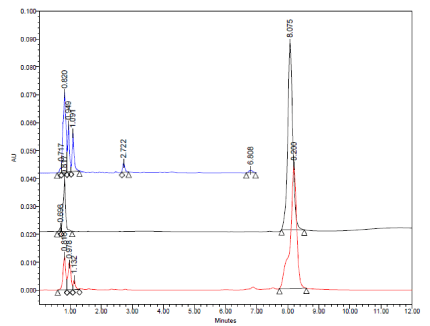
Typical HPLC chromatograms obtained from 20 μl injections of solutions of Amlodipine Besylate, Atorvastatin Calcium and caduet tablet which were subjected to alkaline condition under optimized chromatographic conditions. (Descending order).
Typical HPLC chromatograms obtained from 20 μl injections of solutions of Amlodipine Besylate, Atorvastatin Calcium and caduet tablet which were subjected to thermal condition under optimized chromatographic conditions. (Descending order).
Typical HPLC chromatograms obtained from 20 μl injections of solutions of Amlodipine Besylate, Atorvastatin Calcium and caduet tablet which were subjected to oxidative condition under optimized chromatographic conditions. (Descending order).
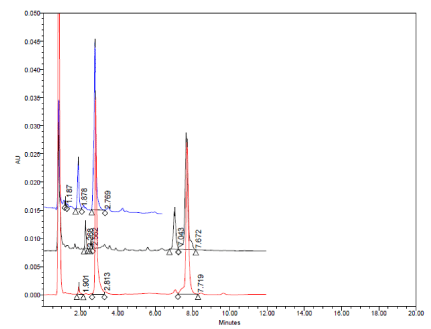
Typical HPLC chromatograms obtained from 20 μl injections of solutions of Amlodipine Besylate, Atorvastatin Calcium and caduet tablet which were subjected to photolytic degradation under optimized chromatographic conditions. (Descending order).